Abstract
Extensive previous studies on taxonomy, behavior/bionomics and control of Anopheles sinensis are reviewed and summarized. Recent molecular identification revealed that the population of An. sinensis complex includes An. sinensis, An. pullus, An. lesteri and at least two new species, and An. yatsushiroensis is synonmy of An. pullus. An. sinensis is the main vector specie of vivax malaria in Korea. Larvae of An. sinensis breed in wide range of habitats which are naturally-made clean water, stagnant or flowing; main habitats include rice fields, ditches, streams, irrigation cannals, marshes, ponds, ground pools, etc. Their host preferences are highly zoophilic. Human blood rate is very low (0.7-1.7%); nevertheless An. sinensis readily feeds on man when domestic animals are not found near by. They feed on hosts throughout the night from dusk to dawn with a peak period of 02:00-04:00 hours; they are slightly more exophagic (biting outdoors); much larger numbers come into the room when light is on. Main resting places are outdoors such as grasses, vegetable fields and rice fields. A mark-release-recapture study resulted that 37.1% was recaptured within 1 km, 29.4% at 1-3 km, 21.1% at 3-6 km, 10.3% at 6-9 km and 2.1% at 9-12 km distance. An. sinensis hibernate outdoors (mostly under part of dense grasses) during October-March. At the end of the hibernation period (March-April) they feed on cows at daytime. Until today any single measure to effectively control An. sinensis population has not been found. Indoor residual spray with a long-lasting insecticide can not reduce vector population densities, but shorten their life spans in some degree, so contributes to malaria control.
-
Key words: Anopheles sinensis, malaria, Korea
INTRODUCTION
In Korea, vivax malaria which had been prevalent throughout the country for many years was eradicated in 1979. Fifteen years later, malaria re-emerged in South Korea in 1993 and an outbreak has been occurring, with thousands of cases every year (
Chai, 1999;
Ree, 2000). Moreover, in North Korea hundred thousands of malaria cases have been found recently, being reported 204,428 cases and 300,000 cases in 2000 and 2001, respectively. Infection rate of the inhabitants was 32.9% in Kaepung-gun, Hwhanghaenam-do in 2001 (unpublished report).
The degree of epidemicity of malaria is decided by many factors, of which vector efficiency is one of the most important ones. The main vector species of vivax malaria in Korea is
Anopheles sinensis (
Ree et al., 1967;
Hong, 1977;
Lee et al., 2000;
Strickman et al., 2001). The knowledge of behavior and bionomics of the vector species is of vital importance to understand the epidemiological features, to find effective control measures, and to interrupt vector-human contact. Studies of
An. sinensis in Korea initiated by several Japanese workers in 1910s-1930s, providing very primitive informations. Thereafter, comprehensive studies were implemented by the entomological team of National Malaria Eradication Service (NMES) in 1960s and by various workers in 1990s. The author reviewed all the available study results of
An. sinensis, which would contribute for the control/eradication of recent outbreak vivax malaria not only in South Korea but also in North Korea.
Anopheles sinensis Wiedmann 1828 is a member of the
Anopheles hyrcanus group belonging to the Myzorhynchus series of the subgenus
Anopheles, which are distinguished from other series by the presence of pale bands (usually four) on the palpi and by the presence of a tuft of dark scales on the clypeus on each side in the female adult.
An. sinensis is distributed in Assam, Burma, Thailand, Malaysia, Indonesia, Kampuchea, Vietnam, Nam, China, Taiwan, Japan and Korea. Among 28 related species of the Hyrcanus group, five species was found in Korea.
An. sinensis was reported in Korea as the new species, named
An. yesoensis by Hasegawa (
1913), and it was revealed that it was synonym of
An. sinensis by Yamada (
1924). In 1936
An. sineroides Yamada, 1924. was found near Seoul, and
An. pullus, a new species was collected in Taejeon, Chungchongnam-do (
Yamada, 1937).
An. lesetri Baisa et Hu, 1936 was reported for the first time in Korea by Whang (
1964).
An. yatsushiroensis Miyazaki, 1951 was found by Hong and Ree (
1968). Besides five related species of the Hyrcanus group,
An. koreicus Yamada and Watanabe, 1918 and
An. lindesai japonicus Yamada, 1958 were listed in Korean mosquito fauna by Yamada (
1937) and Hong and Ree (
1968), respectively. Recently
An. yatsushiroensis was synonimized of
An. pullus by morphological observation (
Shin and Hong, 2001) and by molecular evidences (
Hwang et al., 2004). Recent molecular studies revealed that
An. lesteri from Korea,
An. anthropophagus from China and
An. lesteri from the Philippines were all same species (
Wilkerson et al., 2003), and
An. lesteri from Japan and
An. anthropophagus from China were same species (
Hwang et al., 2005). Ree et al. (
2005) reported an unknown
Anopheles species which was morphologically identical to
An. sinensis, and Li et al. (
2005) also found two unknown species. Rueda (
2005) designated these two species as new species,
An. belenrae sp. nov. and
An. kleini sp. nov. They were morphologically identical with
An. pullus and
An. sinensis, respectively. Morphological identification of
sinensis complex is extremely difficult, so that some numbers of
An. lesteri,
An. pullus (including the form
yatsushiroensis) and two species (at least) are mixed in the population of
An. sinensis in Korea.
TAXONOMY OF ANOPHELES HYRCANUS COMPLEX
Malaria Infection of An. sinensis
Within the range of distribution of An. sinensis its importance as a malaria vector would appear to be confined to China, Taiwan, Japan including the Ryuku Islands, and Korea. An. sinensis is not probably considered to be a malaria vector in Indonesia and Malaysia, and of little significance to human health in Thailand. In Japan, an infection rate of 0.9% was reported in Kyoto prefecture in 1903. In China various workers reported 0.006-2.58% of infection rates of An. sinensis to P. vivax. The infection rate of An. sinensis in Taiwan was 0.02% in 1947-1949.
In Korea, salivary gland dissections of
Anopheles mosquitoes for natural infection rate of vivax malaria were implemented in three different locations in 1960-1962 (
Table 1). Total 7,517 females of
An. sinensis were dissected and one positive was found, showing the infection rate of 0.01% in total of three locations (
Ree et al., 1967). In 1966-1967, 4,225
An. sinensis and 1,469,
An. yatsushiroensis were dissected for sporozoites and oocysts, and two and one positive cases were found from the former (0.05%) and the latter species (0.07%), respectively, as shown in
Table 2 (
Hong, 1977).
At 14 locations of malaria endemic areas, 4,866
An. sinensis, 673
An. pullus, 58
An. sineroides and 12
An. lesteri were collected in August - September 1996. They were pooled (50 ± 5) and nested-PCR were implemented for application of
P. vivax specific gene, and two positives from
An. sinensis pools were found showing at minimum 0.04% of infection rate (
Lee et al., 2000).
Anopheles mosquitoes which were collected during landing or biting humans in Paju city, Kyonggi-do in July 1996 were studied for vivax infections by ELISA assay of circumsporozoite antigen and 0.28% (1 positive/361 females) and 0.06% (1/1,559) of the infection rate were found at Taesong-dong and Camp Bonifas, respectively (
Strickman et al., 2001). None of 2,005
An. sinensis from other collecting sites were infected. None of 82
An. lesteri or 29
An. yatsushiroensis was positive for vivax malaria.
Broadly speaking, the breeding habitat of
An. sinensis is everywhere much the same. The larvae are found in rice fields, open grassy ponds, ground pools, swamps, marshes, shores of lakes, stream margins, ditches, and seepages. All these are normally fresh, shallow water habitats, either stagnant or flowing, usually with emergent vegetation and exposed to sunlight. Results of larval collections of
An. sinensis in May-September by dipping are given in
Table 3 (
NMES, 1968) and
Table 4 (
Self et al., 1971). Though numbers of the larvae collected in rice fields are smaller than those collected in parceley fields and marshes, the main breeding place of
An. sinensis is undoubtedly the rice field, taking into account the total area size of each breeding place throughout the whole country. Comparative studies between adult population densities and size of the rice field were carried out in 13 counties of Chollabuk-do in 1985-1990 (
Ree and Lee, 1993). Population densities of
An. sinensis had no correlation with sizes of the rice fields (r = 0.12), whereas those of
Cx. tritaeniorhynchus had a positive correlation (r = 0.62). These findings indicate that
An. sinensis had many other breeding habitats together with rice fields so that density of
An. sinensis population was not much influenced by the size of rice fields. Large or small temporary ground pools, particularly in rainy season, provide ideal breeding places for
An. sinensis. Streams are also one of the main breeding sources. The author collected 347 larvae of
An. sinensis in 30 minutes at water weed of a rapidly flowing stream in August 1992 (unpublished data).
Recently, Claborn et al. (
2002) reported that six primary habitats of
An. sinensis were rice fields, irrigation ditches, drainage ditches, stream pools, irrigation pools and marshes, most of which harbored similar densities of larvae. They also reported that environmental factors, such as pH, total dissolved solids, percent of surface covered with floating vegetation, and nitrate and phosphate concentrations were not correlated with larval densities.
The seasonal prevalence of
An. sinensis population has been studied by many workers in Korea, mainly applying two monitoring methods, either cow biting collections throughout the night during the period of 1960s - early 1970s (
Whang, 1964;
Paik et al., 1965;
NMES, 1966,
1968;
Hong, 1967;
Kim et al., 1978), or light trap collections in 1970s-1990s (
Kim et al., 1978;
Hong, 1983;
Sohn, 1984;
Lee and Ree, 1991;
Yoon et al., 1994;
Kim et al., 1995,
1997,
1999,
2000,
2001,
2003a,
2003b,
2004;
Shim et al., 1997;
Lee et al., 1999;
Strickman et al., 2000;
Ree et al., 2001,
Lee and Kim, 2001).
Cow biting collections with two hour intervals throughout the night were carried out at three different areas in 1964, and the peak appeared in August at Yangpyeong and Yeoju, Gyeonggi-do and in July at Okgu, Jeollabuk-do, as shown in
Table 5 (
Paik et al., 1965). Seasonal prevalence of this species was also studied in 1967 by means of cow biting collections with weekly intervals in Jeongup-gun, Jeollabuk-do and Cheongsong-gun, Gyeongsangbuk-do, showing the peak period at late July in both areas as shown in
Table 6 (
NMES, 1968). In Daegu, Gyeongsangbuk-do, the peak of
An. sinensis population were appeared at the 5th week of July in 1981 and at the 3rd week of July in 1982; population density was markedly different, being 5,556 mosquitoes collected in 1981 whereas 29,755 mosquitoes in 1982 (
Sohn, 1984). Most extensive studies on seasonal prevalence were done by Lee and Ree (
1991), who monitored seasonal trend of mosquitoes with weekly operation of light traps at 18 cities/guns of Jeollabuk-do in 1985-1990 (
Fig. 1), and also by Kim et al. (
2000) who weekly operated light traps at four different locations in the same area (Munsan, Gyeonggi-do) in 1994-1997 (
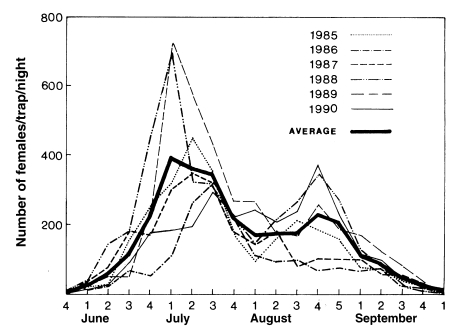
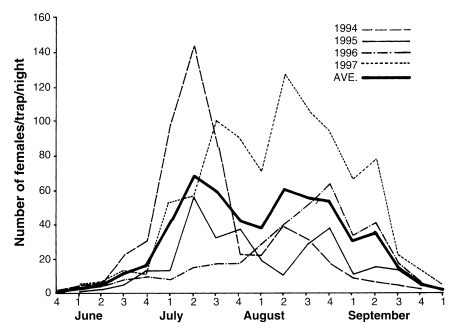
Fig. 2). It is noteworthy that the peak of the population density has appeared one to two weeks earlier since late 1980s, compared to the peak period of 1960s-1970s.
Summarizing all the study results of various workers mentioned above, seasonal fluctuations and population sizes of An. sinensis are markedly different from location to location and from year to year. In general, females of the new generation start appearing in late April - early May. Population sizes steadily increase from early - mid June, reach the peak during the perod of late June - mid July, follow by a significant decline in August, appear a small secondary peak in early September, and disappear in mid-late October.
Only a few studies on weather factors influencing population dynamics of
An. sinensis were carried out in Korea. Ree and Lee (
1993) reported that the summer air temperature was correlated in some degree with the adult population size, but other factors such as precipitation, sunshine hours, relative humidity and winter temperature were not correlated. Lee and Kim (
2001) reported that temperature and precipitation were the major factors influencing
An. sinensis populations.
After blood feeding, female mosquitoes rest in shaded and humid places, waiting for development of follicles during the period of the gonotrophic cycle. Gonotrophic period of
An. sinensis was 2.7 days in average (2-3 days) in July-August in north Gyeonggi-do (
Ree et al., 2001).
Indoor resting place collections of
An. sinensis were weekly done in 10 houses and 10 cowsheds in 5 different localities in June-September 1964-1967, and the results are summarized in
Table 7 (
Paik et al., 1965;
Won and Hong, 1968). Very small numbers were found in houses, being collected 4.0 females/room, 2.3 females/verandah, 2.4 females/kitchen and 42.3 females/stock place, whereas large numbers were collected in animal sheds, being collected 370.3 females/pigsty and 1,077.9 females/cowshed.
On the other hand, 8.3 females of
An. sinensis resting on walls of a bedroom (living room) were collected at night (21:30-22:30 hours), as shown in
Table 8 (
NMES, 1966). Majority (86.4%) of total mosquitoes were unfed, and only 4.1% was blood fed, which means that most of the females entered rooms rest on walls for a while before feeding, whereas 93.4% of
An. sinensis females resting on the wall of a cowshed during the night was fed ones (
Hong and Kim, 1990), which means that they rest after fed for a while, before flying outside. It is not clear why a majority of females resting in living rooms were unfed whereas a majority of females resting in cowsheds were fed; it would be resulted from their animal host preference. When they rest on the wall of cowsheds, they prefer low side of the wall (65.3%) rather than upper part (34.7%).
As shown in
Table 9, resting place collections in cowsheds in the morning revealed that most of
An. sinensis females after feeding flew out of sheds before sunlight, particularly in August, taking into account that numbers of resting mosquitoes were much smaller compared to numbers of biting mosquitoes on cows (Refer
Tables 5 and
6). Experimental hut collections with exit window traps showed that only 17.2% of the total
An. sinensis rested on walls of the hut, and the others (82.8%) were trapped into the exit window traps in June-July (
Table 10), which means that
An. sinensis is highly exophilic.
Outdoor resting mosquitoes were collected by sweeping an insect net at a wide range of habitats, such as grasses, rice fields, parsley fields, potato fields, bean fields and other vegetable fields, as shown in
Table 11 (
NMES, 1968). Though the highest number of the mosquitoes were collected in parsley fields, it is not a main resting place, as the size of parsley fields was very small, compared to the areas of grasses and rice fields that are the main resting places.
It was known that
An. sinensis was almost entirely zoophilic in Thailand; in comparative biting tests involving man and cow, almost none of them were attracted to man (
Harrison and Scanlon, 1975). In Japan, Sasa (
1951) showed that
An. sinensis was strongly attracted by big animals and usually very few fed on man.
In Korea precipitin tests of the blood fed females of
An. sinensis collected at outdoor resting places in Yangpyeong, Gyeonggi-do in 1962 showed that this mosquito fed exclusively bovines (54.8%) and pigs (42.5%); human blood rate was 1.7% (
Table 12;
Ree et al., 1967). In north Kyonggi-do in 1999 host blood analysis of
An. sinensis collected at outdoor resting places by ELISA also showed similar results, giving 0.7% for human, 89.8% for bovine, 3.3% for pig (
Ree et al., 2001). A significant difference between two localities was pig blood meals (42.5% vs 3.3%), which resulted from the availability of pigs for blood feeding.
Lee et al. (2001) also studied host preference of
An. sinensis in paju, Kyonggi-do in 2000, showing 0.8% of human blood rate (
Table 12). Though human blood rates of these host blood tests were very low, they readily fed on man in great numbers where domestic animals (cows and pigs) were not near by for feeding. Number of human biting mosquitoes were 30/man/night at Taesong-dong and 130/man/ night at Camp Bonifas located in DMZ where domestic animals were not found (
Strickman et al., 2001).
An. sinensis fed also dogs, chicken and cats in small numbers.
Cow biting collections of
An. sinensis were hourly carried out at three different locations in 1965-1967 (
Table 13;
Hong, 1977). Human biting collections throughout the night were also carried out at Okgu, Jeollabuk-do and Yangpyeong, Gyeonggi-do in July 1965 (
Table 15;
NMES, 1966) and at several different locations of northern Gyeonggi-do in July-August 1995-1996 (
Tables 14;
Shim et al., 1997;
Strickman et al., 2000). All of the study results showed that
An. sinensis fed throughout night from dusk to dawn, apparently feeding more actively from 24:00 to 04:00. Exceptionally, the peak of feeding time appeared at 05:00 in the study result of Strickman et al. (
2000).
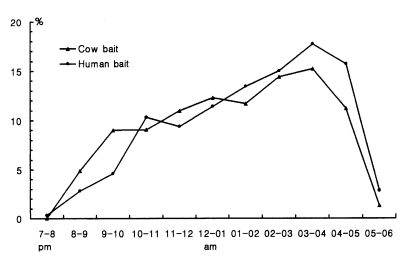
Figure 3 shows that feeding time on animal bait and human bait is not different. Feeding activity at outdoors and indoors was not much different when the room was dark as shown in
Table 15. However, when the light was on, much higher numbers of
An. sinensis came into the room and fed on man (212.0/man/night with light vs 86.7 without light in Okgu; 17.5 with light vs 5.0 without light at Yangpyeong).
Feeding activity of
An. sinensis were significantly different by month, showing that the peak time appeared in early night (2000-2300 hr) in May, June and September, whereas it appeared after midnight in July and August (
Table 13). These results strongly support that feeding activity of
An. sinensis largely relies upon meteorological factors, particularly air temperature, with the majority of individuals biting late at night during warm weather (>20℃) and early at night during cool weather.
It was observed that hibernated
An. sinensis fed on cow during daytime (12:00-17:00) when temperature was above 12℃ in March-April (
NMES, 1968). The biting activities of
An. sinensis were sensitibly influenced by wind speed and direction (
Whang, 1964), and negatively correlated with size of the moon (
Strickman et al., 2000).
For physiological age determination, ovaries were dissected for parous rate at three topographically different areas in 1964 and 1967 (
Table 16) (
Paik et al., 1965;
Hong, 1977). Parous rates were significantly different at three study areas. It was 50.6% (0.711 of proportion of daily survival) in Okgu, Jeollabuk-do, located in plain, malaria-free area, whereas it was 70.4% (0.890 of proportion of daily survival) in Yangpyeong, Gyeonggi-do, located in hilly, moderately endemic area of malaria, and 75.3% (0.911 of proportion of daily survival) in Cheongsong, Gyeongsangbuk-do, located in mountainous area where malaria was highly endemic. These results showed positive correlation between longevity of the vector species and degree of malaria endemicity, i.e. the longer life span of the vector mosquitoes, the more malaria cases. Recently in northern part of Gyeonggi-do, where outbreaks of malaria cases have occurred, parous rate of
An. sinensis was 70.5% (0.890 of daily survival ratio) at Gusan-dong, Goyang-si, Gyeonggi-do in 1999 (
Ree et al., 2001), which was very similar to 70.4% at Gaegun-myeon, Yongpyeong-gun, Gyeonggi-do in 1964. Contrary to the above result,
Lee et al. (2001) reported that parous rate was 48.8% (0.787 of daily survival ratio) in Paju-si in 2000 (
Table 17).
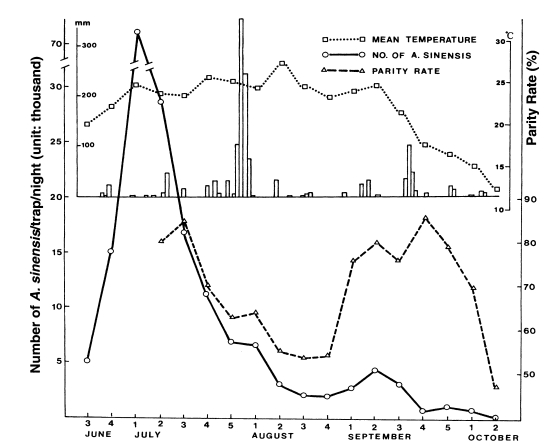
Ree et al. (2001) observed that adult population density of
An. sinensis and their parous rate (longevity) were positively correlated, showing that sharp decrease of the population density during late July-August resulted from the short life span of the vector mosquitoes (
Fig. 4).
Geographical expansion of malaria transmission is largely determined by flight/dispersal activities of the vector mosquitoes, when vivid human movement is not observed. Therefore, flight/dispersal range of An. sinensis, the vector species of vivax malaria, is a vital important factor for understanding recent malaria epidemics in border areas between North and South Korea.
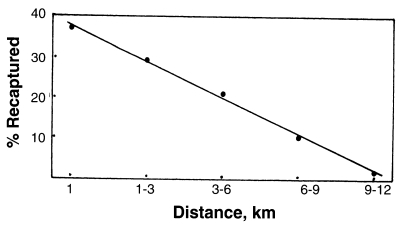
A mark-release recapture experiment of
An. sinensis was carried out in Paju city, Gyeonggi-do, Korea during the period of 7-28 September 1998 (
Cho et al., 2002). Total 12,772 unfed females were marked with fluorescent dye and released. Recaptures of marked females were made from following days of the release, by operating 13 light traps, each of which was set up in a cowshed at various distance from the release point. Light trap collections continued every night for 21 days. Among total 194 marked females recaptured (1.52% recapture rate), 37.1% were recaptured within 1 km from the release point, 29.4% at 1-3 km distance, 21.1% at 3-6 km, 10.3% at 6-9 km, and 2.1% (4 females) at 9-12 km. Correlation between flight distance and number of the recaptured
An. sinensis was observed, shown as
Fig. 5.
Studies on finding hibernation places of
An. sinensis have been rather extensively carried out by various workers (
Whang, 1961,
1964;
NMES, 1968;
Hong, 1977;
Ree et al., 1976;
Shim et al., 1987c,
1989).
Whang (1964) reported that among total 4,402 hibernating females 22
An. sinensis were found in culverts and 6
An. sinensis under bridges in November-December in 1959-1960. Two straw piles (one 2.5 × 3 × 3 m, and the other 2.7 × 3.5 × 1.4 m) were covered with specially-made extra-large mosquito nets in Jeongup, Jeollabuk-do from 11 March to 30 April 1966. A collector searched mosquitoes resting in the mosquito net every day (twice a day) and 6 females of
An. sinensis and one
Culex pipiens were collected (
NMES, 1968). An exit window trap was covered over an abandoned well (stone-piled with 10 m deep), and 108 rat holes at banks of rice fields were covered with specially designed cone-shape trap from February to May. The results of both trials were negative (
NMES, 1968). A vinyl tent was covered over grasses in December in Buan-gun, Jeollabuk-do, and collected 6 females of
An. sinensis (
Shim et al., 1987c). The same collection method was applied at Gosan, Cheju-do and 3 females of
An. sinensis were collected (
Shim et al., 1989). Stone piles were covered with a plastic tent and stones were removed one by one in December 1972 in Busan, and 15 females of
An. sinensis were found together with 1
An. pullus, 31
Cx. pipiens pallens and 4
Cx. orientalis (
Ree et al., 1976). A mobile vinyl tent was covered over heavy grasses of rice paddy banks at Samha-ri, Yangju-gun, Gyeonggi-do in January-March 1999. When temperature inside the tent raised high (>15℃) by sun light, hibernating mosquitoes flew out from grasses in a few minutes. At total 46 sites, 145 females of
An. sinensis complex were collected. Most of them were
An. pullus (
Hwang et al., 2004).
The first collection of
An. sinensis larvae of the first new generation of the season was on April 14 (one larva from ditch) in Cheongsong, Gyeongsangbuk-do and on April 15 (two 3rd instar larvae from rice fields) in Jeongup, Jeollabuk-do in 1967 (
NMES, 1968). These larval findings in mid April indicate that hibernated females fed on hosts in March and laid eggs in late March - early April. Because temperature at night was too low to feed, cow biting collections at daytime were carried out in Jeongup, Jeollabuk-do from early March to late April 1967.
An. sinensis started collecting from mid March. The results are shown in
Table 18 (
NMES, 1968). It is interesting to note that their feeding activity was observed during daytime even when temperature was 12-13℃.
Indoor resting place collections in cowsheds at daytime and cow biting collections at night showed that hibernating females appeared for feeding at night in small numbers in April - early May; 0.3 and 4.0 females per cow per night were collected in April and May respectively in Cheongsong, Gyeongsanbuk-do in 1967, and 3.3 females per cow per night were collected on cow bait in May in Asan, Chungcheongnam-do in 1965 (
Hong, 1977).
In conclusion, females of An. sinensis hibernate outdoors at various concealed places such as grasses, straw piles, stone piles and others. Successfully survived females during several months of overwintering period appear for feeding on cows in daytime in mid March-April. They start feeding on cows when temperature reaches above 12℃ at night.
CONTROL
Two different control measures are applied for malaria vector control, one larval control and the other adult control. Principal objective of the former is to reduce absolute population density and that of the latter is to cut off vector-human contact by selective killing of old-age mosquitoes and/or reducing longevity of the vector mosquitoes. Indoor residual spray with a long-lasting insecticide is the most classical methodology for the latter purpose.
In 1964 and 1965, Daesin-myeon with a population of 22,215 in 3,586 houses were selected for the treated area in Yeoju-gun, Gyeonggi-do and the neighbouring Gaegun-myeon, Yangpyeong-gun was selected for untreated (control) area. All surfaces of the houses and animal sheds in Daesin-myeon were sprayed with 2 g/m
2 of DDT in April-May each year of 1964 and 1965. Passive case detection for malaria cases was carried out in both areas and suspected fever cases were dosed with chloroquine (
NMES, 1966). Relative population densities of
An. sinensis were compared by cow biting collections throughout night with a week interval. The result is summarized in
Table 19, showing no significant difference of densities and seasonal trends of the vector mosquitoes between in the DDT-sprayed and control areas. In other words, total coverage of the houses and animal sheds with DDT could not suppress density of
An. sinensis population. Parous rate of
An. sinensis was 68.3% in the treated area and 74.2% in the control area, which was slightly lower in the treated area (
Table 20). Malaria cases were decreased in both the sprayed and unsprayed areas in the first year of the treatment, and then significantly decreased in the sprayed area in the second year of the treatment (
Table 21). The slide positive rate (SPR) of malaria before treatment (1963) were 12.9% and 30.4% in the treated and untreated areas, respectively, and SPR in 1964 (after 1st treatment) was 6.8% and 3.1%, respectively, which would probably be resulted from the passive case detection and drug administration in both areas. SPR in 1965 (after the 2nd treatment) was further decreased (1.1%) in the sprayed area, whereas increased (4.5%) in the unsprayed area. It can be explained that PCD (case finding and treatment) could significantly reduce the cases in a short-term period only where incidence rate was high, and when the incidence rate decreased to very low level PCD alone could not reduce cases further, but DDT spray, as an additional supplemental control measure, reduced the cases further.
Thermal foggings which have been widely and extensively applied in villages in Korea, and permethrin-treated mosquito nets which are successfully applied in tropical countries have not been evaluated for the effectiveness of An. sinensis control. Therefore such studies are required with high priority.
For larval control of
An. sinensis, susceptibility of
An. sinensis larvae to various insecticides including
Bacillus thuringiensis israelensis (B.t.i.) and
Bacillus sphaericus was tested by many researchers (
Self et al., 1974;
Shim and Kim, 1980,
1981;
Ree et al., 1981b;
Shim et al., 1987 a,
b,
1995;
Lee et al., 1996;
Lee and Yu, 1999;
Shin et al., 2003). These studies revealed that
An. sinensis has developed high level of resistance since 1980s to most of the organophosphorous and carbamate insecticides which had been extensively applied on rice fields for the control of agricultural pests. Ree et al. (
1981 a,
b;
1982) reported that the seasonal prevalence of
An. sinensis larvae in the rice field was rather stable, not being affected by insecticide pressure at all, whereas natural predators such as larvae of
Odonata and
Coleoptera drastically decreased by agricultural insecticide applications. Field trials in rice fields also showed that 14 insecticides tested were ineffective to control of
An. sinensis larval population except cartap and B.t.i. (
Ree et al., 1981a;
Yu et al., 1982a;
Shim et al., 1987a,
b,
d). Entomological studies were performed on the community structures of aquatic animals including mosquito larvae in organically-farmed rice fields (insecticides not applied) and conventionally-farmed rice fields (insecticides applied) in Boseong, Jeollanam-do in 1995-1996 (
Lee et al., 1997;
Lee, 1998). The abundance of
An. sinensis larvae was lower in the organically-farmed rice fields in which number of the Chinese muddy loaches (
Misgurnus mizolepis), predator of mosquito larvae was high, as compared to the conventionally-farmed rice fields.
For the development of biological control measures against larval population of
An. sinensis, laboratory and field tests were carried out to find effective biological control agents by Yu et al. (
1981,
1982a,
b,
1993), Ree and Lee (
1983), Yu (
1986), Shim et al. (
1987a,
d), Yu and Kim (
1993), and Kim et al. (
1994). Potential biological control agents as effective predators of
An. sinensis larvae were
Aphyocypris chinensis,
Aplochilus latipes,
Moroco oxycephalus and
Misgurnus anguillicaudatus among native fishes, and nymphs of
Orthetrum triagulare melania and
Sympetrum sp. in
Odonata, and larva of
Cybister japonicus,
lacophilus sp. and
Hydrophilus affinis in Coleoptera.
Analyzing all available data on biological control against An. sinensis larvae, it would not practically applicable in the rice field, not only in technical but in economical ponts of view, except in a very limited area or isolated habitats.
References
- 1. Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol 1999;37:129-143.
- 2. Cho SH, Lee HW, Shin EH, Lee HI, Lee WG, Kim CH, Kim JT, Lee JS, Lee WJ, Jung GG, Kim TS. A mark-release-recapture experiment with Anopheles sinensis in the northern part of Gyonggi-do, Korea. Korean J Parasitol 2002;40:139-148.
- 3. Claborn DM, Hshieh PB, Roberts DR, Klein TA, Zeichner BC, Andre RG. Environmental factors associated with larval habitats of malaria vectors in northern Kyonggi Province, Republic of Korea. J Am Mosq Control Assoc 2002;18(3):178-185.
- 4. Harrison BA, Scanlon JE. The subgenus Anopheles in Thailand. Contrib An. Entomol Instit 1975;12:1-307.
- 5. Hasegawa Y. Malaria in Korea. J Chosun Med Soc 1913;4:53-69. (in Japanese).
- 6. Hong HK. Bionomics of Anopheles sinensis Wiedmann in western plain area in Korea. Korean J Zool 1967;10:76-80. (in Korean).
- 7. Hong HK. Ecological studies on Korean mosquitoes mainly found in the human habitation. PhD Thesis. 1977, Tongkook University; p 57 (in Korean).
- 8. Hong HK. Ecological studies on the vector of Japanese encephalitis Culex tritaeniorhynchus in Korea. Korean J Epidemiol 1983;5:29-40. (in Korean).
- 9. Hong HK, Kim CM. Studies on resting behaviour of mosquitoes in rural villages in Incheon, Korea. J Nat Sci, Incheon Univ 1990;1:59-71. (in Korean).
- 10. Hong HK, Ree HI. Two unreported Anopheles mosquitoes in Korea. Korean J Zool 1968;11:16-18.
- 11. Hwang UW, Yong TS, Ree HI. Molecular evidence for synonymy of Anopheles yatsushiroensis and An. pullus. J Am Mosq Control Asso 2004;20:99-104.
- 12. Hwang UW, Tang LH, Kobayashi M, Yong TS, Ree HI. Molecular evidence supports that Anopheles anthropophagus from China and An. lesteri from Japan are the same species. J Am Mosq Control Assoc 2005;21:(in press).
- 13. Kim KH, Shin HK, Ree HI. Survey for the production of the occurrance of Japanese encephalitis epidemics in Korea (1978). Korean J Virol 1978;8:37-43. (in Korean).
- 14. Kim HC, Kim MS, Yu HS. Biological control of vector mosquitoes by the use of fish predators, Moroco oxycephalus and Misgurnus anguillicaudatus in the laboratory and semi-field rice paddy. Korean J Entomol 1994;24:269-284.
- 15. Kim HC, Lee KW, Robert LL, Sardelis MR, Chase FE. Seasonal prevalence of mosquitoes collected from light trap in Korea (1991-1992). Korean J Entomol 1995;25:225-234.
- 16. Kim HC, Lee KW, Jones JW, Korch GW. Seasonal prevalence of mosquitoes collected from light trap in Korea (1993-1994). Korean J Entomol 1997;27:21-28.
- 17. Kim HC, Lee KW, Klein TA, Strickman DA. Seasonal prevalence of mosquitoes collected from light trap in Korea (1995-1996). Korean J Entomol 1999;29:181-187.
- 18. Kim HC, Strickman CD, Lee KW. Seasonal prevalence and feeding activity of Anopheles sinensis (Diptera: Culicidae) in the northwestern part of Kyonggiprovince, Republic of Korea. Korean J Entomol 2000;30:193-199.
- 19. Kim HC, Lee KW, Miller WB, Strickman D. Seasonal prevalence of mosquitoes collected from light traps in Korea (1997-1998). Korean J Entomol 2001;31:7-13.
- 20. Kim HC, Lee KW, Richards RS, Schleich SS, Herman WE, Klein TA. Seasonal prevalence of mosquitoes collected from light traps in Korea (1999-2000). Korean J Entomol 2003a;33:9-16.
- 21. Kim HC, Friendly OS, Pike JG, Schuster AL, O'Guinn ML, Klein TA. Seasonal prevalence of mosquitoes collected from light traps in Korea, 2001. Korean J Entomol 2003b;33:189-199.
- 22. Kim HC, Chong ST, Pike JG. Seasonal prevalence of mosquitoes collected from light traps in the Republic of Korea, 2002. Entomol Res 2004;34:177-186.
- 23. Lee DK. Effect of two rice culture methods on the seasonal occurrence of mosquito larvae and other aquatic animals in rice fields of southwestern Korea. J Vector Ecol 1998;23:161-170.
- 24. Lee DK, Kim SJ. Seasonal prevalence of mosquitoes and weather factors influmencing population size of Anopheles sinensis (Diptera, Culicidae) in Busan, Korea. Korean J Entomol 2001;31:183-188.
- 25. Lee DK, Yu HS. Susceptibility of medically important larvae and larvivorous fishes to Abate and Abate-S in Korea. Korean J Appl Entomol 1999;38:165-169. (in Korean).
- 26. Lee DK, Jeon JH, Kang HS, Yu HS. Analysis of aquatic ecosystems in organic and conventional farming rice fields and mosquito larval populations. Korean J Entomol 1997;27:203-214. (in Korean).
- 27. Lee DK, Cho HC, Kwon HD, Lee CN, Ha ST, Bin JH, Jung GY. Seasonal prevalence of mosquitoes collected from light trap in Pusan, Korea (1993-1995). Korean J Hlth Sci 1999;9:79-82.
- 28. Lee HI, Lee JS, Shin EH, Lee WJ. Malaria transmission potential by Anopheles sinensis in the Republic of Korea. Korean J Parasitol 2001;39:185-192.
- 29. Lee JS, Lee WJ, Cho SH, Ree HI. Outbreak of vivax malaria in areas adjacent tot he demilitarized zone, South Korea, 1998. Am J Trop Med Hyg 2002;66:(in press).
- 30. Lee KW, Kim HC, Lee SH, Korch GW, Klein TA. Susceptibility and resistance to diagnostic doses of insecticides on vector and nuisance mosquitoes in Korea. Korean J Entomol 1996;26:249-256. (in Korean).
- 31. Lee SK, Ree HI. Studies on mosquitoe population dynamics in Chollabug-do, Korea (1985-1990) - 2. Factors influencing populaton sizes of Culex tritaeniorhynchus and Anopheles sinensis -. Korean J Entomol 1991;23:185-194.
- 32. Lee WJ, Lee HW, Shin EH, Yang YC, Kim NR, Hong ST, Lee JS. Vector determination of tertian malaria Plasmodium vivax by polymerase chain reaction. Korean J Entomol 2000;30:77-83. (in Korean).
- 33. Li C, Lee JS, Groebner JL, Kim HC, Klein TA, O'Guinn ML, Wilkerson RC. A newly recognized species in the Anopheles Hyrcanus complex and molecular identification of related species from the Republic of South Korea (Diptera: Culicidae). Zootaxa 2005;939:1-8.
- 34. NMES. Malaria pre-eradication programme in Korea. Progress Report, 1961-1965. 1966, National Malaria Eradication Service, Ministry of Health and social Affairs; p 74 (unpublished document).
- 35. NMES. Studies on the behaviour and possible control measures of the confirmed vector of Japanese encephalitis in Korea. 1968, National Malaria Eradication Service, the Ministry of Health and Social Affairs; p 81 (unpublished document).
- 36. Paik YH, Song JH, Ree HI, Hong HK. Epidemiological studies on malaria situation in Korea. I. On the binomics of Anopheles sinensis and its relation to malaria in Korea. New Med J 1965;8:53-59. (in Korean).
- 37. Ree HI. Unstable vivax malaria in Korea. Korean J Parasitol 2000;38:119-138.
- 38. Ree HI, Lee SK. Studies on mosquito population dynamics in Chollabug-do, Korea (1985-1990) II. Factors influencing population sizes of Culex tritaeniorhynchus and Anopheles sinensis. Korean J Entomol 1993;23:185-194.
- 39. Ree HI, Lee WJ. Laboratory studies on predation efficacy of sow Odonata nymphs and Coleoptera larvae against mosquito larvae. Korean J Entomol 1983;13:31-38.
- 40. Ree HI, Hong HK, Paik YH. Study on natural infection of Plasmodium vivax in Anopheles sinensis in Korea. Korean J Parasitol 1967;5:3-4. (in Korean).
- 41. Ree HI, Wada Y, Jolivet PHA, Hong HK, Self LS, Lee KW. Studies on over-wintering of Culex tritaeniorhynchus Giles in the Republic of Korea. Cah ORSTOM sér Ent Méd Parasitol 1976;14:105-109.
- 42. Ree HI, Hong HK, Shim JC, Lee JS, Cho HW, Kim CL. A study on seasonal prevalence of the populations of the mosquito larvae and other aquatic invertebrates in rice fields in Korea. Korean J Zool 1981a;24:151-161.
- 43. Ree HI, Shim JC, Hong HK, Lee JS, Cho HW, Kim CL. Studies on control effects of pesticides applications against the vector mosquito larvae in rice fields in Korea. Korean J Entomol 1981b;11:39-45.
- 44. Ree HI, Shim JC, Kim CL, Lee WJ. Studies on population dynamics of vector mosquito larvae and other aquatic animals including predators breeding in rice fields in 1982. Rep NIH Korea 1982;19:137-149.
- 45. Ree HI, Hwang UW, Lee IY, Kim TU. Daily survival and human blood index of Anopheles sinensis, the vector species of malaria in Korea. J Am Mosq Control Assoc 2001;17:67-72.
- 46. Ree HI, Yong TS, Hwang UW. Identification of four species of the Anopheles hyrcanus complex (Diptera: Culicidae) found in Korea using species-specific primers for Polymerase Chain Reaction Assay. Med Entomol Zool 2005;56:(in press).
- 47. Rueda LM. Two new species of Anopheles (Anopheles) Hyrcanus group (Diptera: Culicidae) from the Republic of South Korea. Zootaxa 2005;941:1-26.
- 48. Sasa M. Two years' observation on the seasonal activities and zoophilism of mosquitoes in Tokyo by animal trap method. Jap J Exp Med 1951;20:509-517.
- 49. Self LS, Shin HK, Kim KH, Lee KW, Chow CY, Hong HK. Ecological studies on Culex tritaeniorhynchus in the Republic of Korea in 1971. WHO/VBC/71. 1971, p 332 (unpublished WHO document).
- 50. Self LS, Shim JC, Jolivet P. Susceptibility of Culex tritaeniorhynchus and six other mosquitoes to insecticides in Korea. Cah ORSTOM sér Ent méd Parasitol 1974;12:81-92.
- 51. Shim JC, Kim CL. Studies on the susceptibility of public health insecticides against mosquitoes in Korea. Rep NIH Korea 1980;17:357-362. (in Korean).
- 52. Shim JC, Kim CL. On the susceptibility of insecticides against vector mosquitoes. Rep NIH Korea 1981;18:249-255. (in Korean).
- 53. Shim JC, Yoon YH, Kim CL, Lee WJ, Lee BI. Pesticides effect against the vector mosquitoes and aquatic animals in ice and parsley fields in the context of integrated control. Rep NIH Korea 1987a;24:477-492. (in Korean).
- 54. Shim JC, Ban SJ, Eym YB, Yu HS. Development of Research on bacterial pathogen (B.t.i. and B. sphaericus) and evaluation of effectiveness against disease vector mosquitoes in Korea. Rep NIH Korea 1987b;24:501-517. (in Korean).
- 55. Shim JC, Yoon YH, Kim CL, Lee WJ. Studies on the hibernation of vector mosquitoes in Korea. Rep NIH Korea 1987c;24:493-500. (in Korean).
- 56. Shim JC, Yoon YH, Kim CL, Lee WJ, Lee BI, Kim SC. Integrated control of vector mosquitoes in rice fields. Korean J Entomol 1987d;17:83-91. (in Korean).
- 57. Shim JC, Yoon YH, Kim CL, Lee WJ, Shin EH. Studies on the hibernation of vector mosquitoes in Korea. Report NIH, Korea 1989;26:213-222. (in Korean).
- 58. Shim JC, Hong HK, Koo SH, Lee DK. Susceptibilities of Anopheles sinensis larvae (Culicidae, Diptera) to various insecticides. Korean J Entomol 1995;25:69-76. (in Korean).
- 59. Shim JC, Shin EH, Yang DS, Lee WK. Seasonal prevalence and feeding time of mosquitoes (Diptera: Culicidae) at outbreak regions of domestic malaria (P. vivax) in Korea. Korean J Entomol 1997;27:265-277. (in Korean).
- 60. Shin EH, Hong HK. A new synonym of Anopheles (Anopheles) pullus Yamada, 1937 - A. (A.) yatsushiroensis Miyazaki, 1951. Korean J Entomol 2001;31:1-5.
- 61. Shin EH, Park YI, Lee HI, Lee WJ. Insecticide susceptibilities of Anopheles sinensis larvae from Paju-shi, Korea. Korean J Entomol 2003;33:33-37.
- 62. Sohn SR. Seasonal prevalence of mosquitoes collected with light trap - At a pigshed in the vicinity of Daegu city, Korea. Korean J Zool 1984;27:117-125. (in Korean).
- 63. Strickman D, Miller ME, Kim HC, Lee KW. Mosquito surveillance in the demilitarized zone, Republic of Korea, during an outbreak of Plasmodium vivax malaria in 1996 and 1997. J Am Mosq Cont Assoc 2000;16:100-113.
- 64. Strickman D, Miller ME, Lee KW, Kim HC, Wirtz RA, Perich M, Novakoski WL, Feighner BH, Roh CS. Successful entomological intervention against Anopheles sinensis limiting transmission of Plasmodium vivax to American soldiers in the Republic of Korea. Korean J Entomol 2001;31:189-195.
- 65. Whang CH. Hibernation of mosquitoes in Korea. Mosq News 1961;21:17-20.
- 66. Whang CH. Studies on bionomics of Anopheles mosquitos in Korea. Korean Med J 1964;9:49-74. (in Korean).
- 67. Wilkerson RC, Li C, Rueda LM, Kim HC, Klein TA, Song GH, Strickman D. Molecular confirmation of Anopheles (Anopheles) lesteri from the Republic of South Korea and its genetic identity with An. (Ano.) anthropophagus from China (Diptera: Culicidae). Zootaxa 2003;378:1-14.
- 68. Won PH, Hong HK. Studies on bionomics of Korean mosquitoes Part II Ecological studies on Anopheles mosquitoes in Korea. Dongkuk University thesis 1968;5:122-158. (in Korean).
- 69. Yamada M. A new species of Anopheles in Chosen (Korea). Keijo J Med 1937;8:237-260.
- 70. Yamada S. A revision of the adult anopheline mosquitoes of Japan: Systematic descriptions, their habits and their relations to human diseases, together with an account of three new species. Sci Rep Gov Inst Infect Dis Tokyo 1924;3:215-241.
- 71. Yoon JH, Park HC, Jung BG. Seasonal prevalence of mosquitoes collected with light trap - At a cow shed inthe vicinity of Taegu city, Korea. Korean J Entomol 1994;24:7-17. (in Korean).
- 72. Yu HS. Biological control of malaria vector, Anopheles sinensis Wiedemann by the release of larvivorous fish, Aplocheilus latipes in simulated rice paddies in Korea. Korean J Entomol 1986;16:93-99.
- 73. Yu HS, Kim HC. Integrated control of encephalitis vector (Culex tritaenior- hynchus) with native fishes (Aplocheilus latipes and Aphyocypris chinensis) and Bacillus thuringiensis (H-14) in marshes in Jindo Island of Korea. Korean J Entomol 1993;23:221-230.
- 74. Yu HS, Yun YH, Lee DK, Lee WJ. Biological control of mosquito larvae breeding in rice paddies in the presence of fish predator, Aphyocypris chinensis in Korea. Korean J Entomol 1981;11:29-37.
- 75. Yu HS, Lee DK, Lee WJ, Shim JC. Mosquito control evaluation of Bacillus thuringiensis var. islaelensis in the laboratory, simulated rice fields, and confined field trials in Marsh and sewage effluent in South Korea. Korean J Entomol 1982a;12:69-82.
- 76. Yu HS, Lee DK, Lee WJ. Mosquito control by the release of fish predator, Aphyocypris chinensis in natural mosquito breeding habitats of rice paddies and stream seepage in South Korea. Korean J Entomol 1982b;12:61-67.
- 77. Yu HS, Kim HC, Yang KH. Mosquito vector control by the use of native fish (Aphyocypris chinensis) and Bacillus thuringiensis (H-14) in natural rice fields and parsley fields in Korea. Korean J Entomol 1993;23:231-243.
Fig. 1Seasonal prevalence of
Anopheles sinensis adult populations in Jeollabuk-do in 1985-1990 (Average of 18 Si/Gun collections) (
Lee and Ree, 1991).
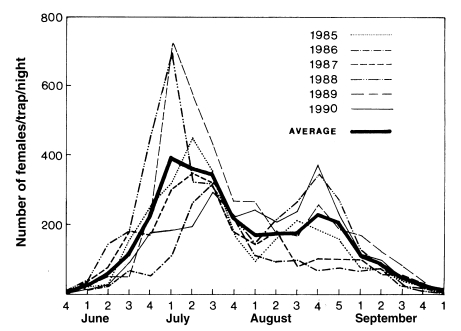
Fig. 2Seasonal prevalence of
Anopheles sinensis collected by New Jersey light traps at Munsan area, Gyeonggi-do in 1994-1997 (
Kim et al., 2000).

Fig. 3

Fig. 4Weekly occurrence of population density and parous rate of
Anopheles sinensis at Gusan-dong, Goyang-si, Gyeonggi-do, in 1999. Mean temperature by week and daily rainfall are also shown (
Ree et al., 2001).
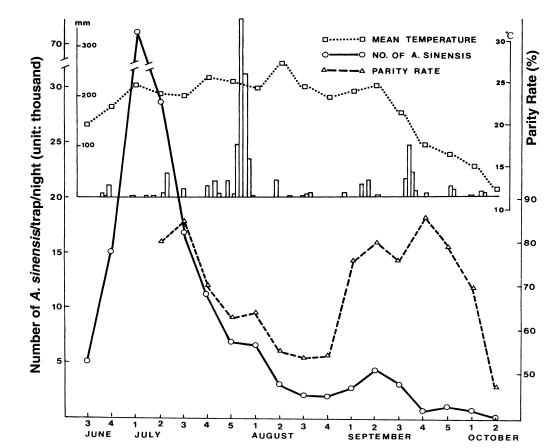
Fig. 5Correlation between flight distance (km) and per cent of
An. sinensis recaptured (
Cho et al., 2002; the figure drawn by Ree).
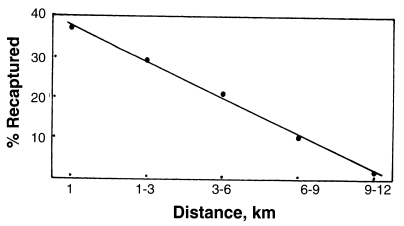
Table 1.Salivary gland dissections of
Anopheles mosquitoes for natural infection of vivax malaria in Korea in 1960-1962 (
Ree et al., 1967)
Table 1.
|
Locality |
A. sinensis
|
A. sineroides
|
A. koreicus
|
|
Yeongju |
1,589 |
6 |
287 |
|
Andong |
842 |
339 |
80 |
|
Yangpyeong |
4,906 (1)a)
|
2 |
5 |
|
Total |
7,337 (1)b)
|
347 |
372 |
Table 2.Salivary gland dissections of
Anopheles mosquitoes for natural infection of vivax malaria in 1966-1967 (
Hong, 1977)
Table 2.
|
Area (Year) |
An. sinensis
|
An. yatsushiroensis
|
|
No. dissected |
No. positives |
No. dissected |
No. positives |
|
Andong (1966) |
207 |
0 |
89 |
1 (1.12)a)
|
|
Cheongsong (1967) |
4,018 |
2 (0.05) |
1,380 |
0 |
|
Total |
4,225 |
2 (0.05) |
1,469 |
1 (0.07) |
Table 3.Collections of
An. sinensis larvae by dipping in May-September, 167 (
NMES, 1968)
Table 3.
|
Location |
Number of larvae per man hour
|
|
Rice field |
Ditch |
Parsley field |
Pond |
Ground Pool |
|
Jeongup-gun |
33.1 (24)a)
|
41.0 (2) |
- |
26 (1) |
5 (1) |
|
Cheongsong-gun |
77.0 (21) |
4.4 (11) |
74.8 (4) |
11.1 (13) |
14.6 (14) |
|
Average |
55.1 |
22.7 |
74.8 |
18.6 |
9.8 |
Table 4.Number of 3rd-4th instar larvae of
An. sinensis collected by dipping in 1971 (
Self et al., 1971)
Table 4.
|
Locality |
Habitat |
Hr.a)
|
No. of larvae per man per hour
|
|
May |
June |
July |
Aug. |
Sept. |
Ave. |
|
Busan |
Rice field |
40 |
0.3 |
2 |
9 |
4 |
- |
3.8 |
|
Marsh |
19 |
0 |
45 |
1 |
3 |
- |
12.3 |
|
Parsley field |
59 |
0.7 |
1 |
16 |
8 |
- |
6.4 |
|
Othersb)
|
52 |
0.6 |
0.2 |
0.5 |
4 |
- |
1.3 |
|
Sintaein |
Rice field |
163 |
3 |
8 |
13 |
3 |
0.3 |
5.5 |
|
Parsley field |
66 |
4 |
8 |
15 |
6 |
0 |
6.6 |
|
Others |
40 |
2 |
2 |
65 |
1 |
0 |
14.0 |
|
Seoul |
Rice field |
48 |
0.3 |
1 |
3 |
18 |
- |
5.6 |
|
Swamp |
156 |
0.1 |
1 |
8 |
2 |
17 |
5.6 |
|
Othersb)
|
53 |
0 |
0.8 |
4 |
0 |
- |
1.2 |
|
Average |
Rice field |
103 |
1.2 |
3.7 |
8.3 |
8.3 |
0.3 |
4.4 |
|
Marsh/swamp |
115 |
0.1 |
23 |
4.5 |
2.5 |
17 |
9.4 |
|
Parsley field |
77 |
2.4 |
4.5 |
15.5 |
7.0 |
0 |
5.9 |
|
Othersb)
|
53 |
0.9 |
1.0 |
23.2 |
1.7 |
0 |
5.4 |
Table 5.Cow biting collections
a) of
An. sinensis at Yangpyeong and Yeoju, Gyeonggi-do and Okgu, Jeollabuk-do in 1964 (
Paik et al., 1965)
Table 5.
|
Area |
Month |
Time
|
Total (5 hrs) |
Average (per hr) |
|
20:00-21:00 |
22:00-23:00 |
24:00-01:00 |
02:00-03:00 |
04:00-05:00 |
|
Yangpyeong |
May |
2 |
4 |
2 |
2 |
1 |
11 |
2.2 |
|
Jun. |
125 |
83 |
79 |
50 |
25 |
310 |
62.0 |
|
Jul. |
97 |
85 |
134 |
135 |
93 |
544 |
108.8 |
|
Aug. |
91 |
144 |
144 |
138 |
117 |
633 |
126.6 |
|
Sep. |
71 |
26 |
25 |
44 |
41 |
207 |
41.1 |
|
Yeoju |
Jun. |
73 |
52 |
28 |
36 |
24 |
213 |
42.6 |
|
Jul. |
134 |
123 |
124 |
139 |
76 |
592 |
118.4 |
|
Aug. |
113 |
171 |
156 |
127 |
71 |
637 |
127.4 |
|
Sep. |
54 |
43 |
45 |
32 |
35 |
208 |
41.6 |
|
Okgu |
May |
1 |
1 |
0 |
0 |
0 |
2 |
0.4 |
|
Jun. |
38 |
64 |
47 |
50 |
21 |
220 |
44.0 |
|
Jul. |
236 |
125 |
145 |
124 |
103 |
567 |
113.4 |
|
Aug. |
75 |
106 |
51 |
153 |
143 |
493 |
98.6 |
|
Sep. |
28 |
40 |
22 |
21 |
5 |
103 |
20.6 |
Table 6.Seasonal prevalence of
An. sinensis by means of cow biting collections at Jeongup, Jeollabuk-do and Cheongsong, Gyeongsangbuk-do in 1967 (cow/man/8 hours) (
NMES, 1968)
Table 6.
|
Month |
No. of week |
Number/cow/man/night (8hr)
|
|
Jeongup |
Cheongsong |
|
May |
1 |
4 |
1 |
|
2 |
20 |
3 |
|
3 |
10 |
8 |
|
4 |
170 |
4 |
|
June |
1 |
94 |
126 |
|
2 |
234 |
46 |
|
3 |
254 |
220 |
|
4 |
1,066 |
58 |
|
July |
1 |
1,239 |
70 |
|
2 |
628 |
220 |
|
3 |
1,028 |
824 |
|
4 |
1,394 |
848 |
|
August |
1 |
952 |
236 |
|
2 |
112 |
352 |
|
3 |
185 |
84 |
|
4 |
76 |
244 |
|
5 |
82 |
136 |
|
September |
1 |
540 |
640 |
|
2 |
88 |
24 |
Table 7.
Table 7.
|
Resting place |
Okgu (1964) |
Yangpyeong (1964) |
Asan (1965) |
Andong (1966) |
Cheongsong (1967) |
Average |
|
Room |
1.5 |
0.3 |
5.3 |
2 |
11 |
4.0 |
|
Verandah |
0.9 |
1.2 |
8.3 |
0.4 |
0.6 |
2.3 |
|
Kitchen |
0 |
0.5 |
8.5 |
0.8 |
2 |
2.4 |
|
Stock place |
48 |
5.5 |
42 |
65 |
51 |
42.3 |
|
Toilet |
3.7 |
0.0 |
7.1 |
6 |
4.2 |
4.2 |
|
Under eaves |
1.2 |
1.0 |
6.1 |
2 |
2.2 |
2.5 |
|
Hen |
7.5 |
- |
3.5 |
22 |
2.4 |
8.9 |
|
Pigsty |
734 |
- |
6.5 |
- |
- |
370.3 |
|
Cowshed |
1,333 |
162 |
1,610 |
964 |
1,320 |
1,077.8 |
Table 8.Resting place collections in bedrooms at night (21:30-22:30) at Yeongju, Gyeongsangbuk-do in June-July 1963, and Jeongup, Jeollabuk-do in July 1967 (
NMES, 1968)
Table 8.
|
Locality |
No. of rooms collected |
Unfed |
Fed |
Gravid |
Total |
No./room |
|
Yeongju |
417 |
2,999 |
139 |
337 |
3,475 |
8.3 |
|
Jeongup |
27 |
194 |
14 |
11 |
219 |
8.1 |
|
Total/Ave. |
444 |
3,193 (86.4) |
153 (4.1) |
348 (9.4) |
3,694 (100)a)
|
8.3 |
Table 9.Resting place collections at
An. sinensis in cowsheds at daytime (09:00-10:00) by month in Jeongup, Jeollabuk-do and Cheongsong, Gyeongsangbuk-do in 1967 (
NMES, 1968)
Table 9.
|
Month |
Number/cowshed
|
|
Jeongup |
Cheongsong |
Average |
|
April |
0.9 |
0.2 |
0.6 |
|
May |
44.4 |
18.8 |
31.6 |
|
June |
267.5 |
124 |
195.8 |
|
July |
153.4 |
270 |
211.7 |
|
August |
9.0 |
17.1 |
13.1 |
|
September |
34.5 |
6.0 |
47.3 |
|
Total |
509.7 |
490.1 |
500.1 |
Table 10.
An. sinensis collections in an experimental hut attached with exit window traps at Yeongju, Gyeongsangbuk-do in June-July, 1963 (unpublished)
Table 10.
|
Time |
Number |
% |
|
No. in exit window traps at: |
|
|
|
20:00-22:00 |
79 |
5.2 |
|
22:00-24:00 |
142 |
9.4 |
|
24:00-02:00 |
251 |
16.7 |
|
02:00-04:00 |
356 |
23.3 |
|
04:00-06:00 |
419 |
27.0 |
|
No. remained in the hut |
259 |
17.2 |
|
Total |
1,506 |
100 |
Table 11.Outdoor resting place collections
a) of
An. sinensis by sweeping at Cheongsong, Gyeongsangbuk-do and Jeongup, Jeollabuk-do in 1967 (
NMES, 1968)
Table 11.
|
Area |
Month |
Grasses |
Parsley field |
Bamboo bush |
Rice field |
Potato field |
Bean field |
Vegetable fields |
Total |
|
Cheongsong |
May |
0 |
7 |
- |
0 |
- |
- |
- |
7 |
|
Jun. |
4 |
265 |
- |
124 |
18 |
- |
- |
411 |
|
Jul. |
128 |
434 |
- |
- |
4 |
15 |
- |
581 |
|
Aug. |
120 |
132 |
- |
97 |
8 |
8 |
- |
365 |
|
Total |
252 |
831 |
- |
221 |
30 |
23 |
- |
1,357 |
|
Jeongup |
Jun. |
3 |
- |
- |
39 |
18 |
- |
3 |
63 |
|
Jul. |
14 |
- |
60 |
10 |
12 |
- |
193 |
289 |
|
Aug. |
3 |
- |
12 |
49 |
- |
11 |
- |
75 |
|
Total |
20 |
- |
72 |
98 |
30 |
11 |
196 |
427 |
Table 12.
Table 12.
|
Locality |
No. tested |
No. of host blood
|
|
Human |
Cow |
Pig |
Dog |
Chicken |
Cat |
Cow/Pig |
Unknown |
|
Yeoju (1962) |
301 |
5 (1.7)a)
|
165 (54.8) |
128 (42.5) |
0 |
0 |
- |
1 (0.3) |
2 (0.7) |
|
Goyang (1999) |
305 |
2 (0.7) |
274 (89.8) |
10 (3.3) |
2 (0.7) |
5 (1.6) |
- |
2 (0.7) |
11 (3.6) |
|
Paju (2000) |
241 |
2 (0.8) |
155 (61.8) |
55 (21.9) |
33 (13.1) |
2 (0.8) |
4 (1.6) |
0 |
35 (13.9) |
Table 13.Cow biting collections
a) of
An. sinensis at Asan, Chungcheongnam-do in 1965, Andong, Gyeongsangbuk-do in 1966 and Cheongsong, Gyeongsangbuk-do in 1967 (
Hong, 1977)
Table 13.
|
Area |
Month |
20:00-21:00 |
21:00-22:00 |
22:00-23:00 |
23:00-24:00 |
24:00-01:00 |
01:00-02:00 |
02:00-03:00 |
03:00-04:00 |
04:00-05:00 |
05:00-06:00 |
Total |
No./Cow/hour |
|
Asan |
May |
1 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
3 |
0.3 |
|
Jun. |
38 |
160 |
65 |
28 |
39 |
26 |
50 |
20 |
21 |
0 |
477 |
47.7 |
|
Jul. |
65 |
125 |
90 |
202 |
152 |
160 |
124 |
110 |
103 |
2 |
1,133 |
113.3 |
|
Aug. |
40 |
120 |
106 |
29 |
51 |
68 |
153 |
263 |
133 |
10 |
973 |
97.3 |
|
Sep. |
22 |
26 |
40 |
23 |
22 |
25 |
21 |
12 |
5 |
3 |
199 |
19.9 |
|
Andong |
May |
5 |
1 |
4 |
4 |
- |
3 |
1 |
- |
1 |
- |
19 |
2.7 |
|
Jun. |
16 |
33 |
31 |
14 |
13 |
13 |
7 |
13 |
10 |
- |
150 |
16.7 |
|
Jul. |
48 |
66 |
42 |
103 |
109 |
82 |
230 |
137 |
103 |
1 |
920 |
92.0 |
|
Aug. |
24 |
34 |
52 |
74 |
103 |
84 |
87 |
121 |
85 |
10 |
674 |
67.4 |
|
Sep. |
20 |
28 |
31 |
35 |
20 |
40 |
43 |
53 |
31 |
21 |
322 |
32.2 |
|
Cheongsong |
May |
1 |
2 |
1 |
2 |
1 |
1 |
0 |
0 |
0 |
- |
8 |
0.9 |
|
Jun. |
39 |
50 |
41 |
33 |
28 |
20 |
15 |
17 |
10 |
- |
253 |
28.1 |
|
Jul. |
32 |
52 |
89 |
81 |
120 |
106 |
73 |
85 |
78 |
13 |
729 |
72.9 |
|
Aug. |
29 |
53 |
50 |
46 |
41 |
56 |
44 |
50 |
46 |
18 |
433 |
43.3 |
|
Sep. |
66 |
30 |
53 |
30 |
16 |
15 |
17 |
24 |
21 |
11 |
283 |
28.3 |
Table 14.Human biting collections of An. sinensis at northern part of Gyeonggi-do in July-August in 1995 and 1996
Table 14.
|
Time |
1995a)
|
1996b)
|
|
No. |
% |
No. |
% |
|
18:00-19:00 |
- |
|
6 |
0.1 |
|
19:00-20:00 |
2.9 |
0.3 |
12 |
0.3 |
|
20:00-21:00 |
17.4 |
2.1 |
108 |
2.7 |
|
21:00-22:00 |
23.8 |
2.8 |
222 |
5.5 |
|
22:00-23:00 |
51.6 |
6.2 |
285 |
7.1 |
|
23:00-24:00 |
80.8 |
9.7 |
391 |
9.7 |
|
24:00-01:00 |
83.0 |
9.9 |
379 |
9.4 |
|
01:00-02:00 |
124.9 |
14.9 |
358 |
8.9 |
|
02:00-03:00 |
169.3 |
20.2 |
410 |
10.1 |
|
03:00-04:00 |
153.6 |
18.4 |
785 |
19.5 |
|
04:00-05:00 |
115.9 |
13.9 |
850 |
21.1 |
|
05:00-06:00 |
12.9 |
1.5 |
229 |
5.7 |
|
Total |
836.1 |
100 |
4,029 |
100 |
Table 15.Human biting collections at Okgu, Jeollabuk-do in 1964 and Yangpyeong, Gyeonggi-do in July 1965 (
NMES, 1966)
Table 15.
|
Time |
No. of mosquitoes/man/hour
|
|
Okgua)
|
Yangpyeongb)
|
|
Room
|
Outdoor |
Average |
Room
|
Outdoor |
Average |
|
Light |
No light |
Light |
No light |
|
20:00-21:00 |
7.3 |
3 |
5 |
5.1 |
0.6 |
0.5 |
0.5 |
0.5 |
|
21:00-22:00 |
9.7 |
4 |
9 |
7.6 |
1.6 |
0.3 |
0.8 |
0.9 |
|
22:00-23:00 |
14.3 |
6 |
10 |
10.1 |
1.6 |
0.5 |
0.3 |
0.8 |
|
23:00-24:00 |
17.7 |
7 |
12 |
12.2 |
2.5 |
0.9 |
1 |
1.5 |
|
24:00-01:00 |
36.3 |
12.7 |
12.7 |
20.6 |
2.4 |
0.9 |
0.6 |
1.3 |
|
01:00-02:00 |
37.3 |
15.7 |
15.3 |
22.8 |
2.5 |
0.5 |
0.7 |
1.2 |
|
02:00-03:00 |
30.7 |
11.7 |
18.3 |
20.2 |
2.8 |
0.5 |
0.4 |
1.2 |
|
03:00-04:00 |
29.3 |
14.7 |
19 |
21.0 |
2.8 |
0.8 |
0.5 |
1.4 |
|
04:00-05:00 |
25.7 |
9.7 |
14.3 |
16.6 |
0.8 |
0.3 |
0.3 |
0.5 |
|
05:00-06:00 |
3.7 |
2.3 |
0.7 |
2.2 |
- |
- |
- |
- |
|
Total |
212.0 |
86.7 |
116.3 |
138.4 |
17.5 |
5.0 |
5.1 |
9.2 |
|
No. per hr |
21.2 |
8.7 |
11.6 |
13.8 |
1.8 |
0.5 |
0.5 |
0.9 |
Table 16.Parous rate of
An. sinensis at Okgu, Jeollabuk-do and Yangpyeong, Gyeonggi-do in 1964 (
Paik et al., 1965) and Cheongsong, Gyeongsangbuk-do in 1967 (
Hong, 1977)
Table 16.
|
Area |
Month |
No. dissected |
Parous rate (%) |
Daily survival ratio |
|
Okgu, Jeollabuk-do (Malaria-free area) |
June |
103 |
27.2 |
|
|
July |
|
773 |
53.8 |
|
Aug. |
142 |
50.0 |
|
|
Total |
1,018 |
50.6 |
0.711 |
|
Yangpyeong, Gyeonggi-do (Endemic area) |
June |
|
173 |
66.5 |
|
July |
|
474 |
68.4 |
|
Aug. |
104 |
86.5 |
|
|
Total |
751 |
70.4 |
0.890 |
|
Cheongsong (High-endemic area) |
June |
677 |
71.9 |
|
|
July |
|
218 |
74.3 |
|
Aug. |
|
124 |
87.9 |
|
Total |
1,019 |
75.3 |
0.911 |
Table 17.Parous rate of An. sinensis at two localities in Gyeonggi-do in 1999-2000
Table 17.
|
Area |
Month |
No. dissected |
Parous rate (%) |
Daily survival ratio |
|
Goyanga)
|
July |
825 |
75.2 |
|
|
Aug. |
639 |
56.5 |
|
|
Sep. |
801 |
78.5 |
|
|
Oct. |
115 |
60.0 |
|
|
Total |
2,380 |
70.5 |
0.890 |
|
Pajub)
|
June |
145 |
35.2 |
|
|
July |
111 |
55.0 |
|
|
Aug. |
74 |
66.2 |
|
|
Total |
330 |
48.8 |
0.787 |
Table 18.Dry ice/mosquito net and cow biting collections of hibernating
An. sinensis at daytime in Jeongup, Jeollabuk-do in March - April 1967 (
NMES, 1968)
Table 18.
|
Date |
Time |
Temp. (°C) |
Dry ice |
Cow biting |
|
12 March |
12:00-13:00 |
13.5 |
3 |
- |
|
13:00-14:00 |
14.0 |
1 |
- |
|
15:00-16:00 |
15.0 |
1 |
- |
|
13 March |
14:00-15:00 |
14.0 |
3 |
0 |
|
15:00-16:00 |
15.5 |
1 |
7 |
|
14 March |
14:00-15:00 |
17.0 |
2 |
- |
|
15:00-16:00 |
17.0 |
3 |
- |
|
16:00-17:00 |
16.0 |
4 |
- |
|
15 March |
14:00-15:00 |
13.0 |
1 |
- |
|
16:00-17:00 |
14.5 |
1 |
- |
|
18 March |
11:00-13:00 |
12.0 |
1 |
- |
|
20 March |
10:00-11:00 |
12.0 |
1 |
0 |
|
11:00-12:00 |
12.0 |
1 |
1 |
|
14:00-15:00 |
16.5 |
2 |
2 |
|
15:00-16:00 |
16.0 |
1 |
2 |
|
16:00-17:00 |
14.5 |
1 |
11 |
|
6 April |
17:00-18:00 |
15.0 |
3 |
- |
|
7 Aprila)
|
15:00-17:00 |
14-16 |
- |
6a)
|
|
9 Aprila)
|
- |
14-17.5 |
- |
10a)
|
|
13 Aprila)
|
- |
12-16 |
- |
5a)
|
|
18 Aprila)
|
- |
13-16 |
- |
1a)
|
|
22 April |
18:30-19:00 |
13.0 |
4 |
- |
|
27 April |
19:00-19:30 |
12.0 |
2 |
- |
|
Total |
|
|
36 |
45 |
Table 19.Comparison of population densities of
An. sinensis between DDT-sprayed (Daesin-myeon) and control (Gaegun-myeon) areas in Gyeonggi-do, by means of cow biting collection at 2 hour intervals in June-September in 1964-1965. Total coverage of DDT indoor residual spray was completed in April-May each year (
NMES, 1966)
Table 19.
|
Area |
No. of An. sinensisa)
|
|
June |
July |
Aug. |
Sep. |
Total |
|
Sprayed (Daesin) |
42.6 |
118.4 |
127.4 |
41.6 |
330 |
|
Unsprayed (Gaegun) |
62 |
108.8 |
126.6 |
41.4 |
340.8 |
Table 20.Comparison of parous rate of
An. sinensis between DDT-sprayed and control aeras in June-September 1965 (
NMES, 1966)
Table 20.
|
Area |
No. dissected |
No. of parous |
Parous rate (%) |
|
Sprayed (Yeoju) |
1,041 |
711 |
68.3 |
|
Control (Yangpyeong) |
947 |
703 |
74.2 |
Table 21.Comparison of malaria cases between DDT sprayed and unsprayed areas in 1963-1965
Table 21.
|
Area |
Population |
No. of houses |
Pre-spray(1963)
|
Post-spray
|
1st spray (1964)
|
2nd spray (1965)
|
|
Slidesa)
|
SPRb)
|
Slidesa)
|
SPRb)
|
Slidesa)
|
SPRb)
|
|
Sprayed area (Yeoju) |
22,215 |
3,588 |
518 |
12.9 |
1,313 |
6.8 |
3,243 |
1.1 |
|
Unsprayed area (Yangpyeong) |
15,433 |
2,504 |
553 |
30.4 |
4,364 |
3.1 |
1,474 |
4.5 |