Abstract
The autoinfective filariform larva of Strongyloides stercoralis causes hyperinfection in immunosuppressed hosts. Here we report on the case of a male patient who was admitted to the emergency room at Gwangju Veterans Hospital with a complaint of dyspnea, and who was receiving corticosteroid therapy for asthma. Many slender larvae of S. stercoralis with a notched tail were detected in Papanicolaou stained sputum. They measured 269 ± 21.2 µm in length and 11 ± 0.6 µm in width. The esophagus extended nearly half of the body length. The larvae were identified putatively as autoinfective third-stage filariform larvae, and their presence was fatal. The autoinfective filariform larva of S. stercoralis has not been previously reported in Korea.
-
Key words: Strongyloides stercoralis, filariform larva, hyperinfection, autoinfection, notched tail
INTRODUCTION
Humans are infected percutaneously by the third-stage filariform larvae of
Strongyloides stercoralis. After penetrating the skin, they enter cutaneous vessels, are carried to the lung, migrate up the trachea to the glottis, and are then swallowed with sputum and reach the duodenum and proximal jejunum, their preferred sites of residence. The parasitic females of
S. stercoralis live buried in the crypts of the small intestine, and produce eggs that rapidly develop into first-stage rhabditoid larvae in the intestinal mucosa. Thus, diagnoses of strongyloidiasis are usually made by a finding of these larvae in feces (
Grove, 1996). However, in recent years,
S. stercoralis has been increasingly detected in cytologic specimens due to the higher registered numbers of immunosuppressed patients. During hyperinfection, larvae can be detected in a variety of body fluids (
Lai et al., 2002;
Steiner et al., 2002;
Premanand et al., 2003;
Hong et al., 2004), and are most frequently observed in sputum smears, which reflects respiratory tract involvement in both the normal life cycle and in cases of disseminated autoinfection (
Humphreys and Hieger, 1979;
Chaudhuri et al., 1980;
Harris et al., 1980;
Wang et al., 1980).
Microscopically, there are two common types of
S. stercoralis larvae, rhabditoid (L1) and filariform (L3). The latter can be distinguished from the former by their larger size, a relatively long esophagus and a unique tail with a notched appearance (
Grove, 1996). However, Schad et al. (
1993) described two morphologically different types of filariform larvae; long, thin larva (infective form third-stage larva; L3i), and short, stout larva (autoinfective form third-stage larva; L3a). L3i is typical filariform larva that is capable of percutaneous infection and observed in stool cultures, whereas L3a is a small filariform larva that is related to autoinfection and observed in immunocompromised and immunologically naive hosts (
Nolan et al., 1993;
Schad et al., 1993).
Here, we describe a hyperinfected case in which several autoinfective filariform larvae of S. stercoralis were detected in sputum from an immunocompromised patient.
CASE RECORD
A 71-year-old Korean man, living at Mokpo-si, Jeollanam-do, was admitted to the emergency room at the Gwangju Veterans Hospital with a complaint of dyspnea on February 17, 2003. According his history, he had been admitted on several occasions for chronic obstructive pulmonary disease. In addition, he had been treated for asthma at several private clinics for more than 20 years, and sometimes, was prescribed corticosteroid therapy. During an admission six years prior to this admission, iatrogenic Cushing syndrome was suspected. The patient was also known to have chronic arthritis and non-insulin dependant diabetes mellitus; he had a long history of cigarette smoking and alcohol consumption.
A physical examination revealed multiple ulcerated erythematous patches on the buttocks suspected as candidiasis, and lung auscultation disclosed coarse breathing sound with crackles in both lung fields. Because of extensive oral ulceration, the patient complained of poor oral intake during admission. A chest roentgenogram revealed pulmonary edema and cardiomegaly. Laboratory data showed the following values: hemoglobin, 11.7 g/dl, hematocrit, 34.4%, leukocyte count, 15,000/ml (89.6% neutrophils, 4.4% lymphocytes, 4.8% monocytes, and 1.1% eosinophils) and an erythrocyte sedimentation rate of 46 mm/hr. His total serum protein concentration was 5 g/dl with an albumin level of 3.1 g/dl. Other abnormal laboratory results included anemia, hyponatremia and hyperglycemia. No evidence of parasitic infection was found by routine stool examination (cellophane thick smear method).
Expectorated sputum was submitted for cytologic evaluation. Cytologic smears were prepared on glass slides, fixed immediately in 95% ethyl alcohol, and stained using a routine Papanicolaou method. The microscopic examination revealed abundant inflammatory cells, epithelial cells, and numerous larval nematodes of
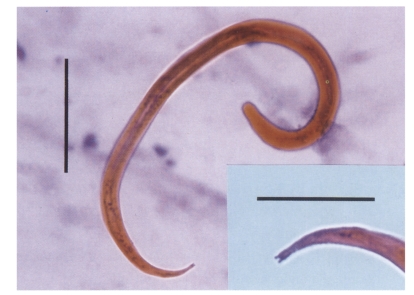
S. stercoralis. The larvae were elongated and slender. They measured 234 to 317 µm (269 ± 21.2 µm) in length and 10 to 13 µm (11 ± 0.6 µm) in width, and microscopically the esophagus was the most prominent organ. It measured 111 to 145 µm and occupied 42 to 52% of the body length. All measurements were done with a computer-aided image analysis system (analySIS, SIS GmbH, Germany). The buccal cavity was closed and a notched tail was evidently demonstrated by selective focusing with oil immersion (
Fig. 1). These larvae were identified as the filariform larvae of
S. stercoralis despite their low body lengths.
The patient was treated for suspected bacterial pneumonia due to a laboratory report of Streptococcus pneumoniae in sputum culture. No improvement was recorded. On the 5th hospital day he developed respiratory failure requiring intubation and mechanical ventilation. No specific treatment for strongyloidiasis was initiated because the patient died a day after the larval nematodes were identified.
DISCUSSION
Sputum cytology is a widely used simple, noninvasive, and cost-effective screening procedure, and may provide diagnostic evidence of infectious conditions due to viruses, fungi, or parasites (
Yassin and Garret, 1980). The larvae of
S. stercoralis in the lungs may be identified by expectorated sputum examination. Papanicolaou staining has been used for the diagnosis of pulmonary and gastrointestinal strongyloidiasis (
Humphreys and Hieger, 1979;
Yassin and Garret, 1980), because the parasite is easily seen in specimens stained. And, in the present case, a diagnosis of pulmonary strongyloidiasis was made by this method, whereas the parasite was not observed in Giemsa or acid-fast stained sputum smears.
According to Schad et al. (
1993),
S. stercoralis has two morphologically distinct third-stage filariform larvae in its life cycle, namely, the infective larva (L3i), which is responsible for initiating infection, and the autoinfective larva (L3a), which causes chronic infection. The L3a larva was first described in primary infections of immunologically naive animals. Based on studies in experimentally infected dogs, it was described that L3a larvae have larger diameters, shorter lengths, and a more strongyliform esophagus than the free-living infective larva (L3i). The L3a larvae are, therefore, distinguishable from the culture-derived or free-living L3i larva, which is capable of percutaneous infection (
Nolan et al., 1993;
Schad et al., 1993). Immune responses to these larvae were found to be different in a mouse model (
Brigandi et al., 1997). L3a larvae have also been reported in other neonatal hosts (< 1 month of age) (
Nolan et al., 1999a), in adult naive hosts (
Nolan et al., 1999b,
2002), and in hosts treated with corticosteroids (
Nolan et al., 1993) or other immunosuppressive drugs (
Nolan and Schad, 1996).
In humans, Humphreys and Hieger (
1979) reported short and slender filariform larvae, measuring approximately 290 µm in length and 10 µm in width with an esophagus extending for about one-half of the body length and a notched tail. Gocek et al. (
1985) also observed similar larvae measuring approximately 270 to 330 µm in length with a notched tail and an esophagus occupying almost half of larval length. In the present case, a sputum preparation also showed some slender larvae measuring approximately 270 µm in length and 11 µm in width. They had the typical features of filariform stage including a closed buccal cavity, an esophagus occupying almost half the larval length, and a notched tail. Based on caudal appearance, they were easily diagnosed as the third-stage filariform larvae of
S. stercoralis. However, they more resembled autoinfective filariform larva (L3a) than typical infective filariform larva (L3i) with respect to their body lengths and esophageal characteristics. In Korea, total 38 cases of human strongyloidiasis have been recorded in the literature since 1945. Of the reported cases, filariform larvae were detected in sputum from some hyperinfective patients (
Hong et al., 1988;
Yun et al., 2001;
Kim et al., 2002). But, there were no fine distinctions between L3i and L3a excluding the present case.
The preferred location for
S. stercoralis development is the duodenum and upper jejunum. However, uncommonly they invade the stomach mucosa (
Kim et al., 2003), and rarely, except in immunosuppressed hosts, the larvae enter, mature, and produce eggs that hatch in respiratory epithelium. If biopsies of the lung have not been performed, it should be considered that this observation might have arisen due to specimen contamination with gastric or duodenal contents. Thus, the presence of vomiting is of importance during sputum collection period. In the present case, nausea and vomiting were absent at the time of the cytologic screening.
Usually gastrointestinal problems dominate strongyloidiasis infection clinically. The pulmonary disease caused by
S. stercoralis is generally mild and is characterized by transient symptoms of asthma or pneumonia. Our patient was unique in that his chief complaints were pulmonary symptoms without obvious gastrointestinal manifestations. It is well known that the larvae may disseminate, and cause serious disease or death in immunocompromised conditions. In the present case, the patient had received corticosteroid over a protracted period, and had suffered from asthma, arthritis, and diabetes mellitus, and he had also been a heavy drinker and smoker over many years. Radiologic findings also showed change due to chronic obstructive pulmonary disease in both lungs. He was also malnourished due to poor oral intake caused by an acute oral ulcer. A lack of eosinophilia was observed in this patient, which has been suggested to be a poor prognostic sign in disseminated strongyloidiasis (
Igra-Siegman et al., 1981), and which may also have contributed to the misdiagnosis. In retrospect, mild eosinophilia had been observed at his initial hospital visit in 1989 and during his second admission in 1997.
A diagnosis of S. stercoralis autoinfection can be made by recognizing the autoinfective filariform larvae in various submitted specimens. Moreover, the presence of autoinfective larvae might be indicative of a potentially fatal condition, because they are the causative agents of severe hyperinfective strongyloidiasis. Early diagnosis and timely therapy in case of hyperinfection syndrome can have a marked impact on disease outcome. Therefore, it is extremely important that the diagnostic laboratory reports a finding of dangerous autoinfective filariform larvae to the physician as soon as possible. Our case emphasizes that cytologists should be aware of the possibility of detecting autoinfective filariform larvae in specimens from any patient at risk of disseminated disease.
References
- 1. Brigandi RA, Rotman HL, Nolan TJ, Schad GA, Abraham D. Chronicity in Strongyloides stercoralis infections: dichotomy of the protective immune response to infective and autoinfective larvae in a mouse model. Am J Trop Med Hyg 1997;56:640-646.
- 2. Chaudhuri B, Nanos S, Soco JN, McGrew EA. Disseminated Strongyloides stercoralis infestation detected by sputum cytology. Acta Cytol 1980;24:360-362.
- 3. Gocek LA, Siekkinen PJ, Lankerani MR. Unsuspected strongyloides coexisting with adenocarcinoma of the lung. Acta Cytol 1985;29:628-631.
- 4. Grove DI. Human strongyloidiasis. Adv Parasitol 1996;38:251-309.
- 5. Harris RA Jr, Musher DM, Fainstein V, Young EJ, Clarridge J. Disseminated strongyloidiasis. Diagnosis made by sputum examination. JAMA 1980;244:65-66.
- 6. Hong IS, Zaidi SY, McEvoy P, Neafie RC. Diagnosis of Strongyloides stercoralis in a peritoneal effusion from anHIV-seropositive man. A case report. Acta Cytol 2004;48:211-214.
- 7. Hong SJ, Shin JS, Kim SY. A case of strongloidiasiswith hyperinfection syndrome. Korean J Parasitol 1988;26:221-226. (in Korean).
- 8. Humphreys K, Hieger LR. Strongyloides stercoralis in routine Papanicolaou-stained sputum smears. Acta Cytol 1979;23:471-476.
- 9. Igra-Siegman Y, Kapila R, Sen P, Kaminski ZC, Louria DB. Syndrome of hyperinfection with Strongyloides stercoralis. Rev Infect Dis 1981;3:397-407.
- 10. Kim J, Joo HS, Kim DH, Lim H, Kang YH, Kim MS. A case of gastric strongyloidiasis in a Korean patient. Korean J Parasitol 2003;41:63-67.
- 11. Kim NR, Kim DS, Han J, Choe DC. Fatal strongyloidiasis with residual cutaneous larvae: an autopsy case report. Korean J Pathol 2002;36:266-270. (in Korean).
- 12. Lai CP, Hsu YH, Wang JH, Lin CM. Strongyloides stercoralis infection with bloody pericardial effusion in a non-immunosuppressed patient. Circ J 2002;66:613-614.
- 13. Nolan TJ, Bhopale VM, Rotman HL, Abraham D, Schad GA. Strongyloides stercoralis: high worm population density leads to autoinfection in the jird (Meriones unguiculatus). Exp Parasitol 2002;100:173-178.
- 14. Nolan TJ, Bhopale VM, Schad GA. Hyperinfective strongyloidiasis: Strongyloides stercoralis undergoes an autoinfective burst in neonatal gerbils. J Parasitol 1999a;85:286-289.
- 15. Nolan TJ, Bhopale VM, Schad GA. Strongyloides stercoralis: oral transfer of parasitic adult worms produces infection in mice and infection with subsequent autoinfection in gerbils. Int J Parasitol 1999b;29:1047-1051.
- 16. Nolan TJ, Megyeri Z, Bhopale VM, Schad GA. Strongyloides stercoralis: the first rodent model for uncomplicated and hyperinfective strongyloidiasis, the Mongolian gerbil (Meriones unguiculatus). J Infect Dis 1993;168:1479-1484.
- 17. Nolan TJ, Schad GA. Tacrolimus allows autoinfective development of the parasitic nematode Strongyloides stercoralis. Transplantation 1996;62:1038-1038.
- 18. Premanand R, Prasad GV, Mohan A, Gururajkumar A, Reddy MK. Eosinophilic pleural effusion and presence of filariform larva of Strongyloides stercoralis in a patient with metastatic squamous cell carcinoma deposits in the pleura. Indian J Chest Dis Allied Sci 2003;45:121-124.
- 19. Schad GA, Smith G, Megyeri Z, Bhopale VM, Niamatali S, Maze R. Strongyloides stercoralis: an initial autoinfective burst amplifies primary infection. Am J Trop Med Hyg 1993;48:716-725.
- 20. Steiner B, Riebold D, Wolff D, Freund M, Reisinger EC. Strongyloides stercoralis eggs in a urethral smear after bone marrow transplantation. Clin Infect Dis 2002;34:1280-1281.
- 21. Wang T, Reyes CV, Kathuria S, Strinden C. Diagnosis of Strongyloides stercoralis in sputum cytology. Acta Cytol 1980;24:40-43.
- 22. Yassin SM, Garret M. Parasites in cytodiagnosis: a case report of Strongyloides stercoralis in Papanicolaou smears of gastric aspirate, with a review of the literature. Acta Cytol 1980;24:539-544.
- 23. Yun HR, Yoo DH, Lee HS, Kim TH, Ahn MH, Min DY, Park MH, Kim SY. Fatal strongyloides hyperinfection in a patient with rheumatoid arthritis. Clin Exp Rheumatol 2001;19:224-224.
Fig. 1The autoinfective filariform larva of Strongyloides stercoralis has a short body and typically a notched tail under the light microscope. Bar represents 50 µm (x 400). Insert: magnification of the notched tail of the same larva (bar = 20 µm, x 1,000).