Abstract
To evaluate the role of nitric oxide (NO) in IFN-γ production and apoptosis of splenocytes in genetically different strains of mice with toxoplasmosis, BALB/c (a toxoplasmosis resistant strain) and C57BL/6 (a toxoplasmosis susceptible strain) mice were infected with Toxoplasma gondii cysts orally and subsequently injected intraperitoneally with aminoguanidine, an iNOS inhibitor (AG; 35 mg/kg per mouse daily for 14 days). When BALB/c or C57BL/6 mice were infected with T. gondii without AG treatment, number of brain cysts, NO and IFN-γ production by splenocytes, and percentages of apoptotic splenocytes were increased compared to uninfected control mice without AG treatment. AG treatment increased the number of brain cysts, and reduced NO and IFN-γ production in T. gondii-infected C57BL/6 mice. In contrast, in T. gondii-infected BABL/c mice, the number of brain cysts, and NO and IFN-γ production of splenocytes was not altered by treatment with AG. However, the percentages of apoptotic splenocytes in T. gondii-infected BALB/c or C57BL/6 mice were not affected by AG treatment. These results suggest that NO modulates IFN-γ production in T. gondii-infected C57BL/6 mice, and that NO is involved in mediating a protective response in toxoplasmosis susceptible, but not resistant, mice strain during acute infection.
-
Key words: Toxoplasma gondii, nitric oxide, interferon-γ, apoptosis
INTRODUCTION
Toxoplasma gondii is an obligate intracellular protozoan parasite with a global distribution among humans and animals. Although the infection of immunocompetent humans is usually asymptomatic infection persists throughout life. Moreover,
T. gondii may lead to life-threatening disease in fetuses or newborns from primarily infected mothers or in immunocompromised patients after dormant parasites have been reactivated (
Kasper, 2001). Thus, an understanding of the immunopathology of toxoplasmosis is crucial for controlling the progression of this disease.
Type 1 cytokines, such as interferon-γ (IFN-γ), are essential for protective immunity to
T. gondii (
Denkers 1999;
Miller et al., 1999;
Gavrilescu and Denkers, 2001). IFN-γ has been shown to be crucial both for the early control of tachyzoite expansion and for preventing the reactivation of dormant parasite stages. Nevertheless, it is also clear that the
T. gondii-induced overproduction of IFN-γ and of other proinflammatory cytokines result in pathology and death (
Liesenfeld et al., 1999). Another significant molecular species in this context, nitric oxide (NO) is produced in mammalian cells from L-arginine by a family of enzymes known as NO synthases (NOS). The biological activities of NO include vasodilatation, the inhibition of platelet aggregation, neurotransmission, neural plasticity, and the modulation of inflammatory and immunological functions (
Ignarro, 2000). Moreover, the NO induced by a combination of IFN-γ and tumor necrosis factor-α (TNF-α) through the activation of inducible NOS (iNOS), is a critical mediator of intestinal pathology, and contributes to early mortality in genetically susceptible mice (
Liesenfeld et al., 1999;
Miller et al., 1999).
Apoptosis, the natural mechanism of cell death, is characterized by a set of specific alterations in cell morphology (
Heussler et al., 2001;
Sinai et al., 2004). The induction of immune cell apoptosis can be modulated by several factors, including the CD95 receptorligand system (Fas and Fas-L) (
Hu et al., 1999), and by cytokines like TNF-α and IFN-γ (
Liesenfeld et al., 1997;
Shen et al., 2001). However, few reports have addressed the relationship between NO and apoptosis in murine toxoplasmosis.
IFN-γ, NO, and apoptosis are important regulators of immune response to T. gondii infection, and although these factors may be interlinked, in the past they have been invariably assessed in isolation in vitro. Thus, little is known about the relationships between NO, IFN-γ, and apoptosis in T. gondii infection, especially in vivo. To evaluate and compare the roles of NO in IFN-γ production and apoptosis of splenocytes in genetically different strains of mice, we treated BALB/c and C57BL/6 mice with T. gondii cysts and aminoguanidine (AG), an iNOS inhibitor. The following parameters were evaluated over four weeks: survival rate (days), brain parasitic burden, NO and IFN-γ production, and splenocyte apoptosis.
MATERIALS AND METHODS
Mice and parasite strains
Female inbred BALB/c (a toxoplasmosis resistant strain) and C57BL/6 (a toxoplasmosis susceptible strain) were obtained from the Korean Research Institute of Bioscience and Biotechnology, Daejeon, Korea. All mice were 8~10 weeks of age and were documented as being specific-pathogen-free. Animal studies were carried out in a pathogen-free barrier zone at the College of Medicine, Chungnam National University.
Cysts of the Me49 strain were harvested from the brains of BALB/c mice, which had been inoculated orally with 20 cysts 2~3 months before experiment. For experimental infections, BALB/c and C57BL/6 mice were infected orally with five brain tissue cysts in PBS (a total volume of 0.2 ml) per animal. Control group of BALB/c or C57BL/6 mice were inoculated orally with normal mouse brain suspensions.
Treatment of T. gondii-infected mice with iNOS inhibitor aminoguanidine
To investigate and compare the roles of NO on IFN-γ production and apoptosis of splenocytes in
T. gondii infected mice, the animals were injected intraperitoneally with or without aminoguanidine hemisulphate (AG; Sigma Chemical Co., St Louis, MO, USA), a structural analogue of L-arginine reported to inhibit NO production (
Nilsson, 1999), at a dose of 35 mg/kg in 0.2 ml of sterile, endotoxin-free saline, daily, for 14 consecutive days, starting at the time of
T. gondii infection. All control mice (non-infected) were injected in the same manner with 0.2 ml of saline with or without AG. Preliminary studies demonstrated that the treatment of uninfected mice with AG did not induce illness or death. Mice were killed on days 0, 4, 7, 14, and 28 postinfection (PI), and survival, parasite burden, NO and IFN-γ production, and splenocyte apoptosis were evaluated.
In T. gondii-infected C57BL/6 and BALB/c mice with or without AG treatment, survival rates were monitored for four weeks. In addition, brain cyst numbers in the surviving animals were assessed two and four weeks after infection.
Preparation and culture of splenocytes
Mouse spleens were homogenized, RBCs were lysed (Red Blood Cell Lysis buffer; Sigma), and splenocytes were resuspended in complete RPMI 1640 medium (Sigma) containing 10% FBS, 1 mM sodium pyruvate, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. Splenocytes (200 µl of 4 × 106 cells/ml) were cultured in 96-well flat-bottom tissue culture plates for 72 hr at 37℃ in 5% CO2 incubator. Cell-free supernatants were harvested and stored at -20℃ until assayed for IFN-γ and NO.
Apoptosis analysis by flow cytometry
Apoptosis was determined using an Annexin VFITC Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA). In brief, 1 × 105 splenocytes were suspended in 100 µl of binding buffer [0.01 M HEPES (pH 7.4), 0.14 M NaCl, 2.5 mM CaCl2] containing 5 µl of FITC-conjugated Annexin V and 5 µl of propidium iodide. After mixing, the cells were incubated for 15 min in the dark at room temperature, 400 µl of binding buffer was then added, and the cells were subjected to flow cytometer (FACScan; Becton Dickinson, San Jose, CA, USA). Data was analyzed using the Cell-Quest program (Becton Dickinson).
Measurement of NO in splenocyte culture supernatants
Nitrite (NO
2-) levels, as determined by the Griess assay (
Hayashi et al., 1996), were equated with the levels of reactive nitrogen intermediates in the splenocyte culture supernatants. Briefly, 100 µl of supernatant was mixed with 600 µl of Griess reagent [1:1 mixture of 1.5% sulfanilamide in 1 N HCl and 0.15% N-(1-naphthyl)ethylenediamide in distilled water]. After incubating for 30 min at room temperature absorbance was read at 540 nm, and the nitrite concentrations were measured by standard curve prepared using NaNO
2.
IFN-γ levels were determined using cytokine detection ELISA kit (Genzyme, Cambridge, MA, USA) according to the manufacturer's protocol. Each well of a 96-well microtiter plate was coated with 100 µl of hamster anti-mouse IFN-γ diluted in 0.05 M carbonate buffer (pH 9.5), and incubated overnight at 4℃. The plates were washed three times, and blocked with 1% BSA/PBS. Culture supernatants or recombinant mouse IFN-γ standards were then applied to the plates, and incubated for 1 hr at 37℃. After washing, biotinylated goat anti-mouse IFN-γ was added, and the plates were incubated for an additional 1 hr at 37℃. One hundred µl of HRP-conjugated streptavidin was then added to each microtiter well. Binding was visualized using tetramethylbenzidine containing H2O2. The reaction was stopped with 2 N H2SO4, and absorbance was read at 450 nm on a Titertek Multiscan plate reader.
RT-PCR for IFN-γ and iNOS mRNA expression in mouse spleens
IFN-γ and iNOS mRNA expressions in mouse spleens were determined as described by Deckert-Schluter et al. (
1995). Total RNA was isolated from tissue samples using an RNAgent kit (Promega, Madison, WI, USA). A preparation of cDNA was produced using a starting mixture containing 5 µg of total RNA, 8 µl of 5× RT buffer, 4 µl of dNTPs, 25 µM oligo(dT)20, and 1 µl of AMV reverse transcriptase. PCR was performed using 2-13 µl cDNA reaction mixtures containing 10× polymerase buffer, 250 µM dNTPs, 0.4 µM of 3'- and 5'-primers, and 2.5 U
Taq polymerase. Amplification was performed over 35 cycles; 94℃ for 1 min, 57℃ for 1 min, and 72℃ for 1 min in a Thermal cycler. The sequences of the cytokine-specific primer pairs used were as follows: HPRT, 5'-GTTGGATACAGGCCACTTTGTTG (sense) and 5'-GAGGGTAGGCTGGCCTATGGCT (anti sense); IFN-γ, 5'-AACGCTACACACTGCATCTTGG and 5'-GACTTCAAAGAGTCTGAGG; iNOS, 5'-TATGGAGACTGTCCCAG and 5'-GGATCTGAATGTGATGTTTG. The PCR products obtained were separated by electrophoresis on 2% agarose gels, and mRNA levels were quantified using an imaging densitometer (Bio-RAD, Hercules, CA, USA).
Results are expressed as means ± standard deviation (SD) for each group. Statistical evaluations of differences in survival days, parasite loads in the brain, apoptosis percent, and of NO, IFN-γ, and iNOS levels were performed using the two-tailed Mann-Whitney U test and the Student's t-test. Differences between groups were considered significant when P values were < 0.05.
RESULTS
Treatment with iNOS inhibitor AG decreased survival times of C57BL/6, but not BALB/c, mice infected with T. gondii
As shown in
Table 1, uninfected mice treated with or without AG showed no mortality during the experimental period. In addition, both
T. gondii-infected strains survived throughout the 28-day experimental period. However, mean survival days in
T. gondii-infected C57BL/6 mice were reduced by AG treatment. In contrast, mean survival days in
T. gondii-infected BABL/c mice were not affected by AG treatment.
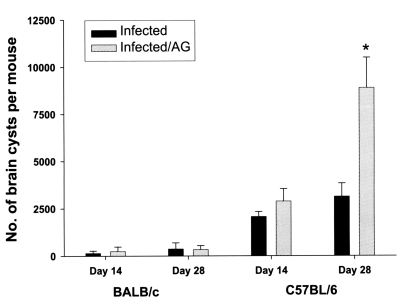
As shown in
Fig. 1, increased number of brain cysts were found at 2 and 4 week, respectively, after
T. gondii infection in BALB/c or C57BL/6 mice, although the higher number of cysts in the brain tissues were observed in
T. gondii-infected C57BL/6 mice rather than BABL/c mice. AG treatment markedly increased the number of brain cysts in
T. gondii-infected C57BL/6, but not BALB/c, mice.
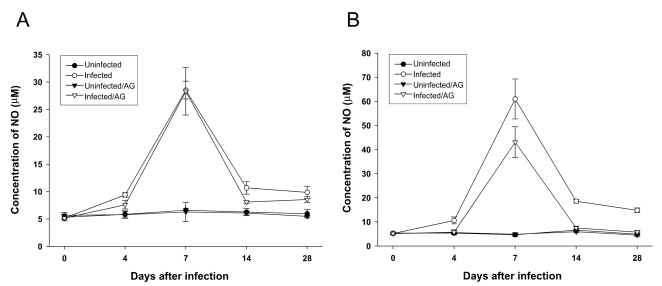
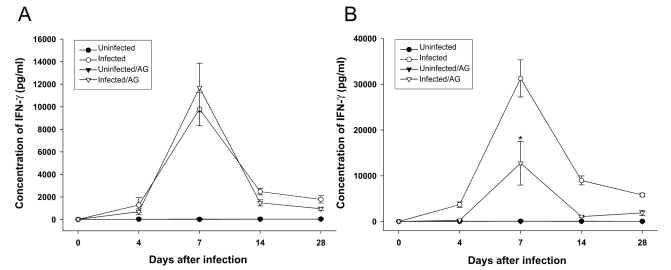
As shown in
Fig. 2,
T. gondii infection in BALB/c or C57BL/6 mice induced NO production at day 7 postinfection. AG treatment partially, but not significantly, inhibited the NO production induced by
T. gondii infection in C57BL/6, but not BALB/c, mice. Similarly, IFN-γ production by splenocytes from BALB/c or C57BL/6 mice was augmented at day 7 of
T. gondii infection (
Fig. 3). AG treatment resulted in the significant reduction of IFN-γ production in
T. gondii-infected C57BL/c, but not BALB/c, mice. There was no or little production of NO or IFN-γ by spleno-cytes from uninfected mice with or withour AG treatment.
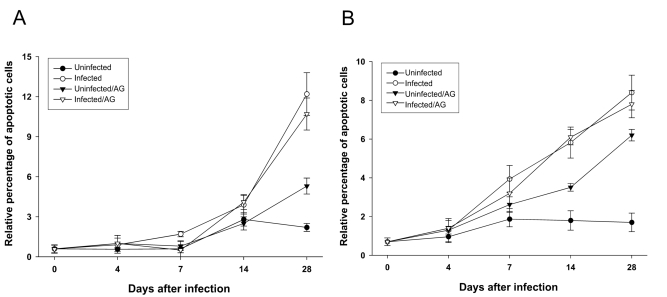
As shown in
Fig. 4, when BALB/c or C57BL/6 mice were infected with
T. gondii, percentages of apoptotic splenocytes were increased after 7 days of infection compared to uninfected mice without AG treament. However, AG treatment did not influence on the splenocyte apoptosis induced by
T. gondii infection in both mice.
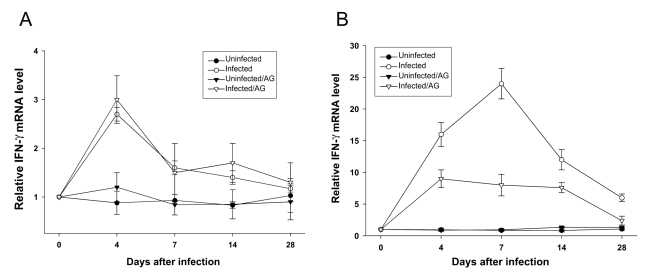
As shown in
Fig. 5,
T. gondii infection in BALB/c or C57BL/6 mice induced elevation of IFN-γ mRNA expression in the spleen after 4 days of infection, although the induction of IFN-γ mRNA expression in C57BL/6 mice was about ten fold greater than that in BALB/c mice. The high expression of IFN-γ mRNA in C57BL/c mice was significantly decreased by AG treatment. In contrast,
T. gondii-induced expression of IFN-γ mRNA expression in BALB/c mice was not inhibited by AG treatment.
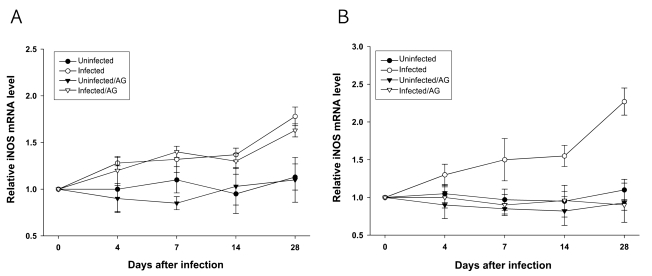
In addition, as shown in
Fig. 6,
T. gondii infection in BALB/c or C57BL/6 mice induced elevation of iNOS mRNA expression in the spleen after 4 days of infection. AG treatment completely suppressed
T. gondii-elicited iNOS mRNA expression in C57BL/6 mice. However,
T. gondii-induced expression of iNOS mRNA in BALB/c mice was not affected by AG treatment. The expression of splenic HPRT mRNA in both mice was constant during the experimental periods (data not shown).
DISCUSSION
The present study demonstrates the effects of iNOS inhibitor on IFN-γ production and apoptosis induction in genetically dissimilar murine toxoplasmosis mice models. C57BL/6 mice, a susceptible strain of toxoplasmosis, injected with T. gondii in combination with iNOS inhibitor AG showed significantly reduced survival, decreased IFN-γ and NO production levels, and increased parasite numbers in brain compared to infected mice without AG treatment. However, in T. gondii-infected BALB/c mice, treatment with AG did not show any significant changes on survival, parasite burden, or IFN-γ production and NO production. During acute infection, apoptosis of splenocytes in both strains of mice was not significantly affected by AG treatment. These results suggest that NO is involved in mediating a protective responses to T. gondii in susceptible, but not resistant, strains of mice during acute infection.
It has become increasingly apparent in recent years that reactive nitrogen intermediates, including NO, are potentially important regulators of the immune system. Inflammatory cells, including monocytes and resident macrophages, produce NO when incubated with bacteria, bacterial lipopolysaccharide, or cytokines. In the present study, we used the specific nitric oxide synthase inhibitor AG, which blocks the formation of NO by macrophages and spleen cells (
Xing et al., 1998). It has been reported that iNOS-deficient knockout mice succumb to
T. gondii infection within 4 weeks after infection, although they survive acute infection and control parasite growth at the site of inoculation (
Sharton-Kernsten et al., 1997). However, the
T. gondii resistant strain BALB/c did not apparently require reactive nitrogen intermediates during the acute infection period (
Schluter et al., 1999).
Cytokines are an essential component of host immunity to
T. gondii. Numerous studies have demonstrated unequivocally the protective role of IFN-γ in acute and chronic toxoplasmosis (
Denkers 1999;
Miller at al., 1999;
Gavrikescu and Denkers, 2001). It is evident that iNOS can be induced during immune and inflammatory responses to bacterial lipopolysaccharide, cytokines (TNF-α, IL-1, IFN-γ, -α and -β), and chemokines. In this study, IFN-γ and NO production levels were increased more in C57BL/6 mice than in BALB/c mice after
T. gondii infection. These results concur with those of previous studies, which showed that IFN-γ mRNA expression after infection is increased more in susceptible strains of mice (
Lee and Kasper, 2004), and that the overproduction of type 1 cytokines, in particular IFN-γ, may lead to immune hyperreactivity in susceptible hosts (
Mordue et al., 2001). In addition, Sharton-Kernsten et al. (
1997) and Hayashi et al. (
1996) reported that inducible NO is essential in C57BL/6 mice for protection against
T. gondii, and that C57BL/6 mice treated with the RNI inhibitor AG display enhanced disease susceptibility. However,
T. gondii resisting strain BALB/c did not show any significant detrimental features after treating with the NO inhibitor AG during acute infection. The reason for this discrepancy is not well known. One of the reasons is that IFN-γ is crucial for genetic resistance of BALB/c mice against toxoplasmosis (
Suzuki et al., 2000).
Apoptosis is a form of programmed cell death that plays a key role in both normal development and oncogenesis. Several studies have demonstrated that NO is involved in the induction of apoptosis in various cell types, including neutrophils. Thus, treatment of animals with NOS inhibitors may reduce the apoptosis rate of migrated neutrophils, thereby extending their life span and allowing them to linger at the inflammatory site.
The above results illustrate different features of these genetically dissimilar mouse strains after the administration of AG during the acute T. gondii infection period. The ability of C57BL/6 mice to resist T. gondii after blockage of NO synthase was seriously diminished. However, AG treatment did not influence on the protective immunity in T. gondii-resistant BALB/c mouse strain. Further work is required to understand the reasons for this discrepancy.
Notes
-
This study was supported by a grant from the Korea Science and Engineering Foundation [R05-2002-000-00808-0(2002)]
References
- 1. Deckert-Schluter M, Albrecht S, Hof H, Wiestler OD, Schluter D. Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenic strains of mice. Immunology 1995;85:408-418.
- 2. Denkers EY. T lymphocyte-dependent effector mechanisms of immunity to Toxoplasma gondii. Microbes Infect 1999;1:699-708.
- 3. Gavrilescu LC, Denkers EY. IFN-γ overproduction and high level apoptosis are associated with high but not low virulence Toxoplasma gondii infection. J Immunol 2001;167:902-909.
- 4. Hayashi S, Chan CC, Gazzinelli R, Roberge FG. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol 1996;156:1476-1481.
- 5. Heussler VT, Kuenzi P, Rottenberg S. Inhibition of apoptosis by intracellular protozoan parasites. Int J Parasitol 2001;31:1166-1176.
- 6. Hu MS, Schwartzman JD, Yeaman GR, Collins J, Seguin R, Khan IA, Kasper LH. Fas-FasL interaction involved in pathogenesis of ocular toxoplasmosis in mice. Infect Immun 1999;67:928-935.
- 7. Ignarro LJ. Nitric oxide: biology and pathology. 2000, New York, USA. Academic Press.
- 8. James SL. Role of nitric oxide in parasitic infections. Microbiol Rev 1995;59:533-547.
- 9. Kasper LH. Toxoplasma infection. Harrison's Principles of Internal Medicine. 2001, 15th. New York, USA. McGraw-Hill Company. pp 1222-1227.
- 10. Lee YH, Kasper LH. Immune responses of different strains after challenge with equivalent lethal doses of Toxoplasma gondii. Parasite 2004;11:89-97.
- 11. Liesenfeld O, Kang H, Park D, et al. TNF-α, nitric oxide and IFN-γ are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol 1999;21:365-376.
- 12. Liesenfeld O, Kosek JC, Suzuki Y. Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun 1997;65:4682-4689.
- 13. Martins GA, Vieira LQ, Cunha FQ, Silva JS. Gammainterferon modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect Immun 1999;67:3864-3871.
- 14. Miller CM, Smith NC, Johnson AM. Cytokine, nitric oxide, heat shock proteins and virulence in Toxoplasma. Parasitol Today 1999;15:418-422.
- 15. Mordue DG, Monroy F, La Regina , Dinarello CA, Sibley LD. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 2001;167:4574-4584.
- 16. Nilsson BO. Biological effects of aminoguanidine: An update. Inflamm Res 1999;48:509-515.
- 17. Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 1997;185:1261-1273.
- 18. Schluter D, Deckert-Schluter M, Lorenz E, Meyer T, Rollinghoff M, Bogdan C. Inhibition of inducible nitric oxide synthase exacerbates chronic cerebral toxoplasmosis in Toxoplasma gondii-susceptible C57BL/6 mice but does not reactivate the latent disease in T. gondii-resistant BALB/c mice. J Immunol 1999;162:3512-3518.
- 19. Shen DF, Matteson DM, Tuaillon N, Suedekum BK, Buggage RR, Chan CC. Involvement of apoptosis and interferon-γ in murine toxoplasmosis. Invest Ophthalmol Vis Sci 2001;42:2031-2036.
- 20. Sinai AP, Payne TM, Carmen JC, Hardi L, Watson SJ, Molestina RE. Mechanisms underlying the manipulation of host apoptotic pathways by Toxoplasma gondii. Int J Parasitol 2004;34:381-391.
- 21. Suzuki Y, Kang H, Parmley S, Lim S, Park D. Induction of tumor necrosis factor-α and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-γ in genetically resistant BALB/c mice. Microbes Infect 2000;2:455-462.
- 22. Xing DK, Canthaboo C, Corbel JM. Nitric oxide induction in murine macrophages and spleen cells by wholecell Bordetella pertussis vaccine. Vaccine 1998;16:16-23.
Fig. 1Parasite burdens of T. gondii-infected mice with or without aminoguanidine (AG). Mice were sacrificed at 2 and 4 weeks after infection, and the brain cysts were counted by microscopy. The data are represented as mean ± standard deviation (n = 5). *P < 0.01.

Fig. 2Concentration of NO in culture supernatants of splenocytes from T. gondii-infected BALB/c (A) or C57BL/6 (B) mice with or without AG treatment. Nitrite (NO2-) levels were determined in 72 hr splenocyte culture supernatants using the Griess reaction. Data are represented as mean ± SD (n = 5).

Fig. 3Concentration of IFN-γ by splenocytes in T. gondii-infected BALB/c (A) and C57BL/6 (B) with or without AG treatment. IFN-γ levels were measured in 72 hr splenocyte culture supernatants by ELISA. The data are represented as mean ± SD (n = 5). *, P < 0.05.

Fig. 4Percentage of apoptotic death of splenocytes from T. gondii-infected BALB/c (A) and C57BL/6 (B) mice with or without AG treatment. The data shown represent mean ± SD (n = 5).

Fig. 5Relative levels of IFN-γ mRNA expression of splenocytes from T. gondii-infected BALB/c (A) and C57BL/6 (B) mice with or without AG treatment. Total RNA was extracted from mice and analyzed for IFN-γ mRNA expression by
RT-PCR. Data are presented as mean ± SD (n = 5).

Fig. 6Relative levels of iNOS mRNA in splenocytes from T. gondii-infected BALB/c (A) and C57BL/6 (B) mice with or
without AG treatment. Total RNA was extracted from mice and analyzed for iNOS-γ mRNA expression by RT-PCR.
Data are presented as mean ± SD (n = 5).

Table 1.Survival days of BALB/c or C57BL/6 mice infected with brain tissue cysts of Toxoplasma gondii with or without aminoguanidine (AG)
Table 1.
|
Groupsa)
|
Survival days of groupsb) (range)
|
|
BALB/c mice |
C57BL/6 mice |
|
Uninfected |
28.0 ± 0.0 (28-28) |
28.0 ± 0.0 (28-28) |
|
Infected |
28.0 ± 0.0 (28-28) |
28.0 ± 0.0 (28-28) |
|
Uninfected/AG |
28.0 ± 0.0 (28-28) |
28.0 ± 0.0 (28-28) |
|
Infected/AG |
28.0 ± 0.0 (28-28) |
19.0 ± 6.5 (13-28)c)
|