Abstract
More than 1,500 clams of Corbicula fluminea, the most favorable food source of freshwater bivalves in Korea, were collected from 5 localities to examine cercarial and metacercarial infection with Echinostoma cinetorchis. Although 3 clams infected with suspicious E. cinetorchis metacercariae out of 200 specimens collected at Kangjin, Chollanam-do were detected, no cercarial and metacercarial infections with E. cinetorchis were observed in field-collected Corbicula specimens. In the susceptibility experiments with laboratory-reared clams, those infected with miracidia of E. cinetorchis did not release their cercariae up to 60 days after infection. To confirm the identity of second intermediate host of E. cinetorchis experimentally, a total of 30 clams were exposed to the cercariae from Segmentina hemisphaerula that had been infected with miracidia of E. cinetorchis. The clams were susceptible to cercariae of E. cinetorchis with an infection rate of 93.3%. Metacercariae from clams taken more than 7 days after cercarial exposure were fed to rats (S/D strain), and adult worms of E. cinetorchis, characterized by 37-38 collar spines on the head crown, were recovered from the ileocecal regions. This is the first report of C. fluminea as a possible second intermediate host of E. cinetorchis.
-
Key words: Corbicula fluminea, Echinostoma cinetorchis, susceptibility, metacercaria
Human intestinal fluke,
Echinostoma cinetorchis (Trematoda: Echinostomatidae), was first described as a new species by Ando and Ozaki (
1923). This trematode is characterized morphologically by a head crown with 37-38 collar spines and, in particular, 6 spines on the ventral lobe (
Ando and Ozaki, 1923;
Seo et al., 1980;
Lee et al., 1992).
Life cycle studies on
E. cinestorchis were carried out in Korea mostly after Seo and his colleagues reported a human case of echinostomiasis cinetorchis in 1980 (
Seo et al., 1984;
Lee et al., 1988a;
Ann et al., 1989;
Lee et al., 1990). Six cases of human infection by this trematode have been reported since the initial report (
Seo et. al., 1980;
Ryang et al., 1986;
Lee et al., 1988b;
Ryang, 1990;
Son et al., 1994). Of Korean freshwater snail species,
Hippeutis contorti,
Segmentina hemisphaerula and
Austropeplea ollula have been found to be naturally and experimentally infected with the cercariae of
E. cinetorchis (
Ahn et al., 1989;
Chung et al., 2001). Several freshwater snail species, i.e.,
H. cantori,
Radix auricularia coreana,
Physa acuta,
Cipangopaludina chinensis malleata,
S. hemisphaerula, and
A. ollula were also reported as the second molluscan intermediate host for this trematode (
Lee et al., 1988a;
Ann et al., 1989;
Lee et al., 1990;
Chung et al., 2001). Other second intermediate hosts include a loach,
Misgurnus anguillicaudatus (
Seo et al., 1984), and tadpole of
Rana nigromaculata (
Chung et al., 2001). Studies on the involvement of freshwater bivalves in trematode life cycles were previously carried out by many investigators. The cercarial emergence of the bucephalid trematodes
Rhipidocotyle fennica and
R. campanula from a freshwater unionid clam
Anodonta piscinalis kept under natural conditions was reported (
Taskinen et al., 1994;
Taskinen, 1998). Some bivalves act as the second intermediate hosts for digenean trematodes. Metacercariae of echinostmatid trematodes have been observed in several species of bivalve hosts; those of
E. revolutum and
E. macrorchis were experimentally infected in
Corbicula fluminea from Schukykill River in southeastern Pennsylvania (
Fried et al., 1987), and from Taiwan (
Lo, 1995), respectively. The zebra mussel
Dreissena polymorpha collected from the St. Lawrence River between Massena, New York and Cornwall, Ontario acted as the second intermediate host for
Echinoparyphium sp. (
Conn and Conn, 1995). In addition, the metacercariae of gymnophallid trematode
Parvatrema timondavidi were naturally found from
Tapes philippinarum, one of the most common marine clam in Korea (
Yu et al., 1993;
Sohn et al., 1996). It was suggested that
Corbicula spp. may act as a source of human infection for
E. cinetorchis. However, neither epidemiological studies on the naturally-infected cercariae and metacercariae in
C. fluminea clams nor the life-cycle studies with laboratory-reared clams have been conducted to date.
Therefore, the present study was designed to determine the extent of natural infection of E. cinetorchis from C. fluminea collected at several local sites in Korea as well as the susceptibility of the clams in the laboratory to infection with miracidia and cercariae of E. cinetorchis.
Corbicula fluminea Müller 1884, the most common freshwater bivalve in Asian countries and North America (
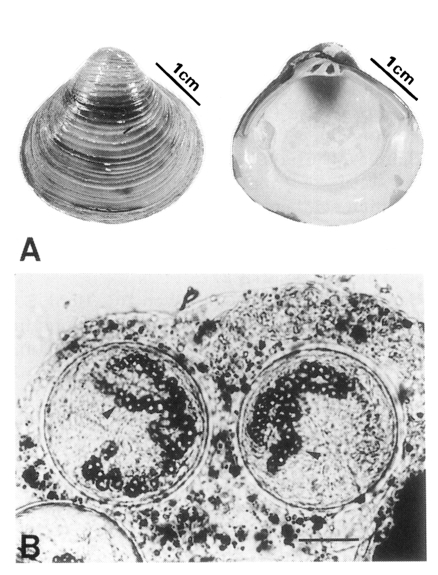
Morton, 1986), is easily found in freshwater systems, and edible as a food source in Korea. More than 1,500 clams collected in 5 local sites of Korea from August to September, 2000 (voucher numbers, 1UMC 104-108;
Fig. 1A) were first examined for the emergence of trematode cercariae and the presence of encysted metacercariae by the methods of Fried et al. (
1987). In field-collected
C. fluminea, no natural emergence of trematode cercariae was detected. However, 3 of 200 clams collected at Kangjin, Chollanam-do harbored degenerated echinostome-like metacercariae in which typical spines could not be identified except the excretory granules.
For the susceptibility experiments, eggs of
E. cinetorchis were collected from adult worms and incubated in conditioned water with a few drops of Fungizon solution (Gibco Life Technologies Inc., Grand Island, NY) at 26℃ under the illumination of 17,000 lux. About 67% of the eggs hatched 18 days after incubation. Each of 20 laboratory-reared clams was exposed to 20
E. cinetorchis miracidia for 20 hours in the laboratory, and the exposed clams were observed for shedding cercariae everyday. However, no cercaria was released up to 60 days after infection. A second experiment was conducted to determine if this corbiculid clams could serve as second intermediate hosts for this echinostome species. Each of 30 laboratory-reared clams, 12-17 mm in shell diameter, was exposed to 100 cercariae obtained from experimental infections of
E. cinetorchis miracidia in
S. hemisphaerula. More than 90% of the 30 clams employed were found to be infected with the metacercariae; however, average number of metacercariae in the 14-day old clams after cercarial exposure (5 per infected clam) was significantly decreased as compared with those of metacercariae in the 1-day and 7-day old clams (14 and 15 per infected clams, respectively) (
Table 1). The encysted metacercariae were mostly found in the gills and kidneys in this study (
Fig. 1B). It is considered that variations of metacercarial numbers in the clams taken more than 14 days postinfection should be observed in the further studies. The usual locations for echinostomatid metacercariae are within the metanephridial kidney or pericardial cavity of molluscan hosts (
Anderson and Fried, 1987). The cercariae crawl directly up the urinary ducts to encyst at or near the blind end (
Anderson and Fried, 1987). Lo (
1995) pointed out that the bivalves acquire their food by filtration of water and trapping plankton with the aid of mucus; thus, many cercariae must have been caught by the mucus and ingested. However, authors have not proved how the cercaria of
E. cinetorchis infected to the tissues of the
C. fluminea clam. The results obtained in the present study were generally in accord with those of the
E. revolutum studies on the
C. fluminea (
Fried et al., 1987).
Laboratory rats (Sprague-Dawley strain, 120 g/body weight) were used as the final host of this fluke. Fifty metacercariae from experimentally infected clams were fed per os to each rat. Rat feces were examined for eggs of E. cinetorchis daily, starting one week after exposure. Immediately after finding the eggs, rats were killed and examined for adult trematodes. Adult worms, characterized by 37-38 collar spines on the head crown, were recovered from the ileocecal regions of the intestine 4 weeks after being infected, with the average recovery rate of 2%. However, the metacercariae collected from the 1-day old clams after cercarial exposure were not infected to the rats.
In summary, this is the first report of C. fluminea serving as the potential second intermediate host of E. cinetorchis in Korea.
References
- 1. Ahn YK, Ryang YS, Chai JY, Sohn WM. Cercarial shedding of Echinostoma cinetorchis and experimental infection of the cercariae to several kinds of snails. Korean J Parasitol 1989;27:203-211. (in Korean).
- 2. Anderson JW, Fried B. Experimental infection of Physa heterostropha, Helisoma trivolvis and Biomphalaria glabrata (Gastropoda) with Echinostoma revolutum (Trematoda) cercaria. J Parasitol 1987;7:49-54.
- 3. Ando R, Ozaki Y. On four new species of trematodes of the family Echinostomatidae. Dobutsugaku Zasshi 1923;35:108-119. (in Japanese).
- 4. Chung PR, Jung Y, Park YK. Segmentina hemisphaerula: A new mollu-scan intermediate host for Echinostoma cinetorchis in Korea. J Parasitol 2001;87:1169-1171.
- 5. Conn DB, Conn DA. Experimental infection of zebra mussels Dreissena polymorpha (Mollusca: Bivalvia) by metacercariae of Echinoparyphium sp. (Platyhelminthes: Trematoda). J Parasitol 1995;81:304-305.
- 6. Fried B, Emili S, Ettinger W. Experimental infection of Corbicula fluminea (Bivalvia: Corbiculidae) with Echinostoma revolutum cercariae. J Parasitol 1987;73:655-656.
- 7. Lee SH, Chai JY, Hong ST, Sohn WM. Experimental life history of Echinostoma cinetorchis. Koeran J Parasitol 1990;28:39-44.
- 8. Lee SH, Jun HS, Sohn WM, Chai JY. Tegumental ultrastructure of juvenile and adult Echinostoma cinetorchis. Korean J Parasitol 1992;30:65-74. (in Korean).
- 9. Lee SH, Lee JK, Sohn WM, Hong ST, Hong SJ, Chai JY. Metacercariae of Echinostoma cinetorchis encysted in the fresh water snail, Hippeutis (Helicorbis) cantori, and their development in rats and mice. Korean J Parasitol 1988a;26:189-197.
- 10. Lee SK, Chung NS, Ko IH, Ko HI, Sohn WM. A case of natural human infection by Echinostoma cinetorchis. Korean J Parasitol 1988b;26:61-64. (in Korean).
- 11. Lo CT. Echinostoma macrorchis: life history, population dynamics of intra-molluscan stages, and the first and second intermediate hosts. J Parasitol 1995;81:569-576.
- 12. Morton B. Corbicula in Asia: an updated synthesis. Am Mai Bull 1986;2:113-124.
- 13. Ryang YS. Studies on Echinostoma spp. in the Chungju Reservoir and upper streams of the Namhan River. Korean J Parasitol 1990;28:221-233. (in Korean).
- 14. Ryang YS, Ahn YK, Kim WT, Shin KC, Lee KW, Kim TS. Two cases of human infection by Echinostoma cinetorchis. Korean J Parasitol 1986;24:71-76. (in Korean).
- 15. Seo BS, Cho SY, Chai JY. Studies on intestinal trematodes in Korea. I. A human case of Echinostoma cinetorchis infection with an epidemiological investigation. Seoul J Med 1980;21:21-29.
- 16. Seo BS, Park YH, Chai JY, Hong SJ, Lee SH. Studies on intestinal trematodes in Korea. XTV. Infection status of loaches with metacercariae of Echinostoma cinetorchis and their development in albino rats. Korean J Parasitol 1984;22:181-189. (in Korean).
- 17. Sohn WM, Chai JY, Lee SH. Infection status of Tapes philippinarum collected from southern coastal areas of Korea with Parvatrema spp. (Digenea: Gymnophallidae) metacercariae. Korean J Parasitol 1996;34:273-277.
- 18. Son WY, Huh S, Lee SU, Woo HC, Hong SJ. Intestinal trematode infections in the villagers in Koje-myon, Kochang-gun, Kyongsangnam-do, Korea. Korean J Parasitol 1994;32:149-155.
- 19. Taskinen J. Cercarial production of the trematode Rhipidocotyle fennica in clams kept in the field. J Parasitol 1998;84:345-349.
- 20. Taskinen J, Valtonen ET, Makela T. Quantity of sporocysts and seasonality of two Rhipidocotyle species (Digenea: Bucephalidea) in Anodonta piscinalis (Mollusca: Bivalvia). Int J Parasitol 1994;24:877-886.
- 21. Yu JR, Chai JY, Lee SH. Parvatrema timondavidi (Digenea: Gymnophallidae) transmitted by a clam, Tapes philippinarum, in Korea. Korean J Parasitol 1993;31:7-12.
Fig. 1The shells of Corbicula fluminea (A) and two metacercariae of Echinostoma cinetorchis in tissue of kidney of Corbicula fluminea (B). Note the excretory granules (arrow heads) in the cysts. Collar spines in the cysts are not focused (bar = 50 µm).

Table 1.Metacercarial infectivity in the laboratory-reared
Corbicula fluminea exposed to the cercariae of
Echinostoma cinetorchis shed from
Segmentina hemisphaerulaa)
Table 1.
|
Days after exposure |
No. of clams examined |
No. of clams infected (%) |
Total no. of metacercariae obtained |
Average no. ± SD of metacercariae per infected clam |
|
1 |
10 |
10 (100) |
135 |
14 ± 6.1 |
|
7 |
10 |
9 (90) |
134 |
15 ± 11.0 |
|
14 |
10 |
9 (90) |
49 |
5 ± 3.8b)
|
|
Total |
30 |
28 (93.3) |
318 |
11 ± 8.4 |
Citations
Citations to this article as recorded by

- Lower parasite pressure in invasive freshwater bivalves than in sympatric native Unionidae mussels in southern European lakes
Binglin Deng, Nicoletta Riccardi, Maria Urbańska, Timo J. Marjomäki, Wojciech Andrzejewski, Jouni Taskinen
Biological Invasions.2025;[Epub] CrossRef - The Nuclear Ribosomal Transcription Units of Two Echinostomes and Their Taxonomic Implications for the Family Echinostomatidae
Yu Cao, Ye Li, Zhong-Yan Gao, Bo-Tao Jiang
Biology.2025; 14(8): 1101. CrossRef - Infection of Corbicula clams by trematode cercariae in Myanmar
Alexander V. Kropotin, Yulia V. Bespalaya, Alexander V. Kondakov, Irina S. Khrebtova, Ilya V. Vikhrev, Ivan N. Bolotov
Ecologica Montenegrina.2023; 62: 1. CrossRef - Corbicula fluminea (Asian clam)
Uma Sabapathy Allen
CABI Compendium.2022;[Epub] CrossRef - Corbicula fluminalis
Fabiana Freitas
CABI Compendium.2022;[Epub] CrossRef - Parasites of Aquatic Exotic Invertebrates: Identification of Potential Risks Posed to the Great Lakes
Sergey E. Mastitsky, Alexander Y. Karatayev, Lyubov E. Burlakova
Human and Ecological Risk Assessment: An International Journal.2014; 20(3): 743. CrossRef - Korean molluscs as auxiliary hosts for parasites: A study of implications for pathogen transmission in a changing climate
Gab-Man Park
The Korean Journal of Malacology.2012; 28(1): 13. CrossRef - ENCYSTMENT AND METACERCARIAE DEVELOPMENT OF ECHINOSTOMA CINETORCHIS CERCARIAE IN AN IN VITRO CULTURE SYSTEM
Yun-Kyu Park, Myung-Ki Hwang, Pyung-Rim Chung
Journal of Parasitology.2006; 92(5): 1010. CrossRef - Host Specificity of Pisidium coreanum (Bivalvia: Sphaeriidae) to Larval Infection with a Human Intestinal Fluke Echinostoma cinetorchis (Trematoda: Echinostomatidae) in Korea
Y. K. Park, C. T. Soh, G. M. Park, M. K. Hwang, P. R. Chung
Journal of Parasitology.2006; 92(5): 1118. CrossRef