Abstract
Although some reports have been published on the protective effect of antibodies to Toxoplasma gondii surface membrane proteins, few address the inhibitory activity of antibodies to dense granular proteins (GRA proteins). Therefore, we performed a series of experiments to evaluate the inhibitory effects of monoclonal antibodies (mAbs) to GRA proteins (GRA2, 28 kDa; GRA6, 32 kDa) and surface membrane protein (SAG1, 30 kDa) on the invasion of T. gondii tachyzoites. Passive immunization of mice with one of three mAbs following challenge with a lethal dose of tachyzoites significantly increased survival compared with results for mice treated with control ascites. The survival times of mice challenged with tachyzoites pretreated with anti-GRA6 or anti-SAG1 mAb were significantly increased. Mice that received tachyzoites pretreated with both mAb and complement had longer survival times than those that received tachyzoites pretreated with mAb alone. Invasion of tachyzoites into fibroblasts and macrophages was significantly inhibited in the anti-GRA2, anti-GRA6 or anti-SAG1 mAb pretreated group. Pretreatment with mAb and complement inhibited invasion of tachyzoites in both fibroblasts and macrophages. These results suggest that specific antibodies to dense-granule molecules may be useful for controlling infection with T. gondii.
-
Key words: Toxoplasma gondii, monoclonal antibody, dense-granule molecules, complement, major surface protein, host cell invasion
INTRODUCTION
Toxoplasma gondii has an extremely broad host range, which includes birds, mammals, and humans, and it is remarkable for its ability to infect virtually any nucleated cell. Host cell invasion by tachyzoites plays a crucial role in maintaining infection. Most studies of tachyzoite antigens have focused on surface antigens expressed at the proliferative tachyzoite stage, particularly SAG1 (P30). SAG1 is the most abundant and immunogenic of these surface proteins, and it is fundamentally important for the success of invasion (
Grimwood and Smith, 1996).
Excretory/secretory antigens (ESA) expressed at both the tachyzoite and encysted bradyzoite stages are also important for host cell entry (
Cesbron and Capron, 1993). It has been observed that ESA is highly immunogenic, and induces a protective effect through antibody-dependent or cell-mediated immunity (
Zenner et al., 1999;
Prigione et al., 2000). The major components of ESA are dense-granule (GRA) molecules that are stored within
Toxoplasma dense core granules and secreted into the parasitophorous vacuole after parasite invasion (
Cesbron-Delauw, 1994;
Coppens et al., 1999). GRA proteins may be important protective antigens, since they are secreted in abundance and are major components of both the vacuole surrounding tachyzoites and the cyst wall surrounding the more slow-growing bradyzoites (
Cesbron-Delauw, 1994). Currently, many investigators are studying the protective immunity induced by the GRA proteins of
T. gondii. However, the role of antibodies against GRA proteins remains unclear.
Infection with
T. gondii results in the development of both humoral and cell-mediated immune responses. Cell-mediated immunity is effective during infection with
T. gondii, however it is uncertain whether B cells or antibodies are important components of acquired resistance to
T. gondii. Specific antibody shows down parasite growth when added to tissue culture, and rapidly destroys tachyzoites in the presence of complement in vitro (
Johnson et al., 1983;
Eisenhauer et al., 1988). Recently, it was reported that neutralizing SAG1 protein with a monoclonal antibody (mAb) caused a significant drop in the invasion of human fibroblasts and murine enterocysts. Thus, SAG1 has an important, functional role in the infection of host cells by
T. gondii (
Mineo et al., 1993;
Grimwood and Smith, 1996). However, it is not yet known whether any of these antibodies normally play a protective role against
T. gondii. Apart from mAbs to surface membrane proteins, there are only limited reports on the protective role of antibodies to
T. gondii ESA. Therefore, using in vivo and in vitro models, we performed a series of experiments to elucidate the protective activity of mAbs in combination with complement to the GRA proteins of
T. gondii, and to compare the these results with those of mAb to SAG1.
MATERIALS AND METHODS
Mice and parasites
Female BALB/c mice were obtained from the Korean Research Institute of Bioscience and Biotechnology (Daejeon, Korea). All mice used were 10-12 weeks old and documented to be specific-pathogen-free animals. The mice were used to prepare the peritoneal macrophages and to measure survival after lethal challenge with Toxoplasma tachyzoites.
The RH strain of T. gondii was used to infect mice and cell lines. The strain was maintained by peritoneal passages in BALB/c mice, and tachyzoites were purified by Percoll gradient centrifugation.
Preparation of ascites and complement
Ascites containing mAbs to
T. gondii were kindly provided by Dr. H. W. Nam, College of Medicine, Catholic University. The ascites contained mAb to GRA2 (28 kDa), GRA6 (32 kDa), or SGA1 (30 kDa) of
T. gondii, which were listed as Tg556, Tg737 and Tg563, respectively (
Sohn and Nam, 1999). Their presence was confirmed by Western blot and immunofluorescence. Ascites from BALB/c mice injected intraperitoneally with SP2/0 myeloma cells served as the ascitic control. Mouse serum complement was purchased from Sigma (Catalog # S3269; St. Louis, MO, USA).
Ascites were purified by chromatography using HiTrap affinity columns (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's instructions. Briefly, columns were equilibrated with start buffer (20 mM Naphosphate, pH 7.0), and then the filtered ascites were applied. The columns were washed with 5 column volumes of start buffer, and eluted with elution buffer (0.1 M glycine-HCl, pH 2.7). The purified fractions were neutralized in 0.1 M Tris-HCl (pH 9.0), and dialyzed against PBS.
Passive mAb immunization of mice
Mice were immunized intraperitoneally with 0.2 ml (1 mg/ml) of either purified mAb or ascitic control twice a week, total four times. Seven days after the last immunization, mice were infected intraperitoneally with 1×104 RH strain tachyzoites. The day of infection was referred to as day 0, and the survival periods were recorded daily until all mice were dead.
Pretreatment of Toxoplasma tachyzoites with either mAb or complement
Toxoplasma tachyzoites were suspended at a concentration of 4×107/ml in RPMI 1640 containing 10% fetal bovine serum (FBS) and antibiotics. Aliquots of 100 µl were dispensed into individual sterile tubes containing 100 µl of the purified mAb (1 mg/ml) with or without complement (1:100 or 1:1000 dilution), and incubated at 37℃ for 30 min on a platform rocker at 10 rpm. After incubation, the supernatants were collected and adjusted to 800 µ1 with medium containing 10% FBS and antibiotics.
Pretreated tachyzoite challenge of mice
Mice were challenged by intraperitoneal injection of 1×104 RH strain tachyzoites, which were pretreated with either purified mAb or complement (1:100 dilution). The day of infection was referred to as day 0, and the survival periods were recorded daily until all mice were dead.
Preparation of macrophage monolayers
Mice were injected intraperitoneally with 1 ml of 3% thioglycollate. After 3 days, peritoneal cells were harvested from the mice by repeated lavage of the peritoneal cavity with Hanks' balanced salt solution (HBSS). After washing, the cell pellets were resuspended in RPMI 1640 containing 10% heat-inactivated FBS and antibiotics, and cultured on 13-mm coverslips (Nunc, Denmark) for 2 hr at 37℃ in 5% CO2. Nonadherent cells were removed by rinsing with warm HBSS, and then were reincubated for 3-4 days to obtain the macrophage monolayers.
Pretreated tachyzoite challenge of macrophage monolayers
Cells on coverslips were infected with 1×106 pretreated tachyzoites, and incubated for 2 hr at 5% CO2 and 37℃. After 2 hr, the cells were rinsed with HBSS to remove noningested parasites and added complete culture medium to the cells. The coverslips were then incubated for another 18 hr at 5% CO2 and 37℃, and then fixed in methanol, and stained with Giemsa to determine the number of infected cells per 100 macrophages. Invasion of tachyzoites in peritoneal macrophages was expressed as percentage of macrophages infected with T. gondii.
Pretreated tachyzoite challenge of fibroblasts
Intracellular growth of
T. gondii tachyzoites in fibroblasts was determined by measuring the incorporation of [
3H]-uracil into acid-precipitable material, as previously described (
Chamberland et al., 1991). Briefly, normal human skin fibroblast cells (ATCC # CRL-2114) were incubated in 96-well flat-bottomed plates using MEM containing 10% FBS and antibiotics, and cultured at 37℃ and 5% CO
2. The medium was changed every day, and the cells were cultured for 2-3 days to obtain fibroblast monolayers. A 100-µl aliquot of 1×10
5 pretreated tachyzoites was added to each well and incubated for 24 hr at 37℃ and 5% CO
2. Plates were then re-incubated for 12 hr following the addition to each well of 50 µl of 1 µCi/ml of [5,6-
3H]-uracil (30-50 Ci/mmol, NEN Life Science Products, USA). The supernatant from each well was then discarded, and cells were dissolved in 50 µl of 1% SDS containing 100 µg of unlabelled uracil per ml (Sigma) and then precipitated with 150 µl of cold 10% trichloroacetic acid (TCA). The precipitates were collected in glass fiber, washed twice with 5% TCA, rinsed with 95% ethanol, dried, and counted in a liquid scintillation spectrophotometer. The results were expressed as counts per minute (CPM) of triplicateculture of each group.
The results are expressed as the mean ± SD of at least two independent experiments. Data from these experiments were analyzed with ANOVA (SPSS software) and the chi-square test. Differences were considered significant when p values were p < 0.05.
RESULTS
Passive immunization with anti-GRA2 or anti-GRA6 mAb significantly increased survival
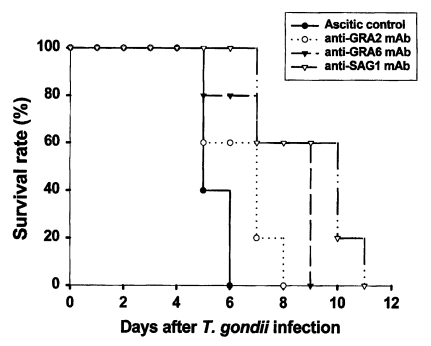
Ascitic control group survived 5.4 ± 0.5 days after lethal tachyzoite challenge. As shown in
Fig. 1, mice immunized with anti-GRA2, anti-GRA6, or anti-SAG1 mAb survived significantly longer compared to the ascitic control group (
p = 0.011,
p = 0.024 and
p = 0.007, respectively). Anti-GRA6 mAb immunized mice survived longer than anti-GRA2 immunized mice (8.8 ± 1.9 days vs. 7.4 ± 1.3 days,
p = 0.025). However, anti-SAG1 immunized mice had the longest survival (10.0 ± 1.9 days), a significantly longer survival than that of anti-GRA6 mAb immunized mice (
p = 0.033).
Toxoplasma tachyzoites were pretreated with mAb alone or with complement following the protocol, and were then injected into mice. As shown in
Table 1, the mean survival of the ascitic control group was 6.2 ± 1.3 days. This was not significantly different from the survival of mice that received either complement or anti-GRA2 mAb pretreated tachyzoites. However, combined pretreatment with anti-GRA2 mAb and complement produced significantly longer survival times. Mice that received anti-SAG1 mAb pretreated tachyzoites survived significantly longer than those that received ascitic control, complement or anti-GRA2 mAb pretreated tachyzoites (0.003 <
p < 0.009). Mice that received anti-SAG1/complement pretreated tachyzoites survived considerably longer than the other groups (0.001 <
p < 0.019), except for the anti-GRA6/complement pretreated group (
p = 0.142).
Anti-GRA6 mAb pretreatment inhibited parasite invasion in fibroblasts
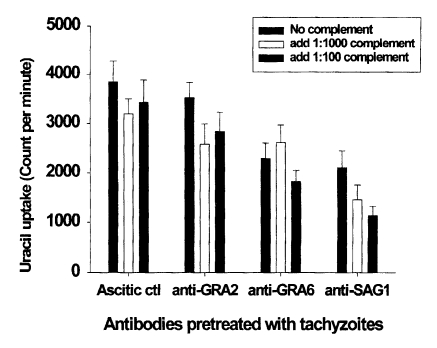
Invasion of mAb- or complement-pretreated tachyzoites in fibroblasts was assayed by [
3H]-uracil uptake assays. The mean CPM of [
3H]-uracil uptake in the ascitic control group was 3,850. There were no significant CPM differences in the ascitic control group, even after complement pretreatment. As shown in
Fig. 2, tachyzoites pretreated with anti-GRA2 mAb were invaded similar number of parasites to that of the ascitic control group. However, the invasion of anti-GRA6 or anti-SAG1 mAb pretreated tachyzoites was significantly inhibited compared to the ascitic control (
p < 0.01). The invasion of tachyzoites was inhibited by complement pretreatment in the anti-GRA2 and anti-SAG1 mAb groups than those of mAb alone pretreated group (18-25% in anti-GRA2/complement,
p < 0.05; 31-46% in anti-SAG1/complement,
p < 0.05), whereas complement pretreatment did not affect the anti-GRA6 mAb group (
p > 0.05). Inhibitory activity was not dependent on the concentration of complement.
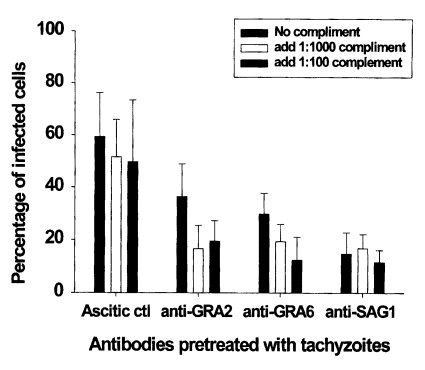
The percentage of infected macrophages in ascitic control group was 59.4 ± 16.9%. There were slightly decreased the mean percentage of infected macrophages after pretreatment with complement in the ascitic control group (
p > 0.05). As shown in
Fig. 3 and
4, pretreatment of tachyzoites with anti-GRA2, anti-GRA6 or anti-SAG1 mAb significantly inhibited parasite invasion in macrophages compared to the ascitic control group (59.4 ± 16.9 vs 36.4 ± 12.5 in anti-GRA2 mAb,
p < 0.05; 59.4 ± 16.9 vs 29.7 ± 8.1 in anti-GRA6 mAb,
p < 0.01; 59.4 ± 16.9 vs 14.7 ± 8.1 in anti-SAG1 mAb,
p < 0.01). Tachyzoite pretreatment with complement in the presence of anti-GRA2 or anti-GRA6 mAb inhibited significantly T. gondii invasion compared to those of mAb pretreated group (36.4 ± 12.5 vs 16.6 ± 8.9-19.5 ± 7.7 in anti-GRA2 mAbs,
p < 0.05; 29.7 ± 8.1 vs 19.3 ± 6.6-12.4 ± 8.7 in anti-GRA6 mAb,
p < 0.05), whereas significant inhibition was not seen in anti-SAG1 mAb/complement pretreated tachyzoites (14.7 ± 8.1 vs 16.8 ± 5.3-11.5 ± 4.6,
p > 0.05). Inhibitory activity was not dependent on the concentration of complement in groups pretreated with mAbs to
T. gondii.
DISCUSSION
GRA proteins are the major soluble components in ESA preparations obtained by serum-stimulated secretion from extracellular parasites. GRA2 belongs to a family of at least nine proteins associated with dense granules that are specialized secretory organelles found in all Apicomplexan parasites (
Coppens et al., 1999). GRA6 is expressed and secreted by both the tachyzoite and bradyzoite stages of the parasite and is therefore seen during both the acute and chronic phases of infection (
Lecordier et al., 2000). We did experiment to evaluate whether mAbs to GRA proteins are able to directly inhibit the invasion of parasites. Our results indicate that anti-GRA2 and anti-GRA6 mAb conferred partial protection against a highly virulent strain of
T. gondii. However, they revealed different inhibitory effects on the invasion of tachyzoites pretreated with one of these mAbs. The different effects of mAbs to GRA proteins may result from different isotypes or different antigen binding epitopes of the two mAbs.
Although cell-mediated immunity appears to be the major component of the host's defense mechanism against toxoplasmosis, the humoral immune response is necessary for the control and clearance of residual parasites (
Sayles et al., 2000). For example, treatment with high titers of
Toxoplasma antibody and activated macrophages has been shown to provide significant protection against congenital infection (
Eisenhauer et al., 1988). More recently, B cells have also been implicated in the regulation of the switch of T cells from the initial Th1-like response to a Th2-like response (
Langhorne et al., 1998). In this study, we compared the protective effect of passive immunization among mAbs to GRA2, GRA6, or SAG1 of
T. gondii. The period of survival days was significantly increased in passively transferred mAb mice compared to ascitic control group, although anti-SAG1 mAb immunized mice survived longer than mice immunized with mAb to GRA protein. From these results, it appears that mAbs to GRA proteins, as well as SAG1, are capable of providing protection in the absence of an already developed cell-mediated immunity (
Sharma et al., 1984;
Langhorne et al., 1998). However, these results do not address whether mAbs to GRA proteins generated in the normal course of infection are required for the protection of chronically infected mice.
Many factors are involved in host cell invasion or intracellular multiplication. It has been suggested that lamin may be an attachment factor (
Furtado et al., 1992), and that SAG1 may have a role in host cell invasion (
Mineo et al., 1993;
Grimwood and Smith, 1996). Recently, ESA has been implicated as an important attachment factor in cell invasion (
Lee et al., 2001;
Son and Nam, 2001;
Zenner et al., 1999). In this study, we evaluated the inhibitory effect of cell invasion by anti-GRA2, anti-GRA6, and anti-SAG1 mAbs using two different cell types: nonphagocytic fibroblasts and phagocytic macrophages. Anti-GRA6 and anti-SAG1 mAb pretreated tachyzoites exhibited inhibition of parasite invasion in both macrophages and fibroblasts compared to the ascitic control group. However anti-GRA2 mAb pretreated tachyzoites were not significantly inhibited in fibroblasts. These data suggest that anti-GRA6 or anti-SAG1 mAb blocks the tachyzoite attachment ligand into host cells. And then, pretreated tachyzoites are not able to actively invade nonphagocytic cells, or pretreated tachyzoites are phagocytosed by macrophages, and finally parasite invasion is inhibited (
Mineo et al., 1993;
Sibley, 1995;
Vercammen et al., 1999). In contrast, Grimwood and Smith (
1996) reported that antibodies against the secretion of rhoptries, dense granules, and micronemes had no effect on the invasion shown in an in vitro neutralization assay.
Complement plays a role in cytolysis, opsonization of organisms and immune complexes for phagocytosis, inflammation, enhancement of humoral immune responses, and solubilization and clearance of immune complexes. The Sabin-Feldman dye test, used clinically to diagnose infection with
Toxoplasma, is an assay for serum antibodies that lyse tachyzoites in a complement-dependent manner (
Dando et al., 2001). In this experiment, the inhibitory effects of mAbs pretreated tachyzoites were generally enhanced in cell line or mouse model after addition of complement. These protective effects may be mediated by a complement-dependent effector mechanism (
Sayles et al., 2000).
Taken together, anti-GRA2, anti-GRA6, and anti-SAG1 mAb induced partial resistance to T. gondii by passive immunization. Tachyzoites pretreated with anti-GRA2, anti-GRA6 or anti-SAG1 mAb with or without complement inhibited parasite invasion in fibroblast or macrophage cell line as well as mouse model, although tachyzoites pretreated with anti-GRA2 mAb were not significantly inhibited in vitro or in vivo compared to ascitic controls. Further study is needed to address whether mAbs to GRA proteins generated in the normal course of infection are required for the protection of chronically infected mice.
Notes
-
This work was supported by Grant No. 1999-1-20200-002-2 from the Basic Research Program of the Korea Science & Engineering Foundation.
ACKNOWLEDGES
We would like to thank Professor H.W. Nam in College of Medicine, Catholic University of Korea, for kindly supplying monoclonal antibodies to T. gondii.
References
- 1. Cesbron MF, Capron A. Excreted-secreted antigens of Toxoplasma gondii: their origin and role in the host-parasite interaction. Res Immunol 1993;144:41-44.
- 2. Cesbron-Delauw MF. Dense-granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today 1994;10:293-296.
- 3. Chamberland S, Kirst HA, Current WL. Comparative activity of macrolides against Toxoplasma gondii demonstrating utility of in vitro microassay. Antimicrob Agents Chemother 1991;35:903-909.
- 4. Coppens I, Andries M, Liu JL, Cesbron-Delauw MF. Intracellular trafficking of dense granule proteins in Toxoplasma gondii and experimental evidences for a regulated exocytosis. Eur J Cell Biol 1999;78:463-474.
- 5. Dando C, Gabriel KE, Remington JS, Parmley SF. Simple and efficient methodfor measuring anti-Toxoplasma immunoglobulin antibodies in human sera using complement-mediated lysis of transgenic tachyzoites expressing β-galactosidase. J Clin Microbiol 2001;39:2122-2125.
- 6. Eisenhauer P, Mack DG, McLeod R. Prevention of peroral and congenital acquisition of Toxoplasma gondii by antibody and activated macrophages. Infect Immun 1988;56:83-87.
- 7. Furtado GC, Cao Y, Joiner KA. Laminin on Toxoplasma gondii mediates parasite binding to the β1 integrin receptor α6β1 on human foreskin fibroblasts and Chinese hamster ovary cells. Infect Immun 1992;60:4925-4931.
- 8. Grimwood J, Smith JE. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int J Parasitol 1996;26:169-173.
- 9. Johnson AM, McDonalds PJ, Neoh SH. Monoclonal antibodies to Toxoplasma cell membrane surface antigens protect mice from toxoplasmosis. J Protozool 1983;30:351-356.
- 10. Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA 1998;95:1730-1734.
- 11. Lecordier L, Fourmaux MP, Mercier C, Dehecq E, Masy E, Cesbron-Delauw MF. Enzyme-linked immunosorbent assays using the recombinant dense granule antigens GRA6 and GRA1 of Toxoplasma gondii for detection of immunoglobulin G antibodies. Clin Diagn Lab Immunol 2000;7:607-611.
- 12. Lee BY, Ahn MH, Kim HC, Min DY. Toxoplasma gondii: Ultrastructural localization of specific antigens and inhibition of intracellular multiplication by monoclonal antibldies. Korean J Parasitol 2001;39:67-75.
- 13. Mineo JR, McLeod R, Mack D, et al. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestinal after per oral infection. J Immunol 1993;150:3951-3964.
- 14. Prigione I, Facchetti P, Lecordier L, et al. T cell clones raised from chronically infected healthy humans by stimulation with Toxoplasma gondii excretory-secretory antigens cross-react with live tachyzoites: characterization of the fine antigenic specificity of the clones and implications for vaccine development. J Immunol 2000;164:3741-3748.
- 15. Sayles PC, Gibson GW, Johnson LL. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect Immun 2000;68:1026-1033.
- 16. Sibly LD. Invasion of vertebrate cells by Toxoplasma gondii. Trends Cell Biol 1995;5:129-132.
- 17. Sharma SD, Araujo FG, Remington JS. Toxoplasma antigen isolated by affinity chromatography with monoclonal antibody protects mice against lethal infection with Toxoplasma gondii. J Immunol 1984;133:2818-2820.
- 18. Sohn EM, Nam HW. Western blot analysis of stray cat sera against Toxoplasma gondii and the diagnostic availability of monoclonal antibodies in sandwich-ELISA. Korean J Parasitol 1999;37:249-256.
- 19. Son ES, Nam HW. Detection and characterization of excretory/secretory proteins from Toxoplasma gondii by monoclonal antibodies. Korean J Parasitol 2001;39:49-56.
- 20. Vercammen M, Scorza T, Bouhdid AE, et al. Opsonization of Toxoplasma gondii tachyzoites with nonspecific immunoglobulins promotes their phagocytosis by macrophages and inhibits their proliferation in nonphagocytic cells in tissue culture. Parasite Immunol 1999;21:555-563.
- 21. Zenner L, Estaquier J, Darcy F, Maes P, Capron A, Caesbron-Delauw MF. Protective immunity in the rat model of congenital toxoplasmosis and the potential vaccinal role of excreted-secreted antigens (ESA). Parasite Immunol 1999;21:261-272.
Fig. 1Survival rate of BALB/c mice passively immunized with monoclonal antibodies following lethal challenge with T. gondii tachyzoites. Mice were infected intraperitoneally with 1 × 104 tachyzoites of RH strain Toxoplasma gondii. Mice (n = 5) were observed daily until all were dead. Data are representative of one of two experiments.
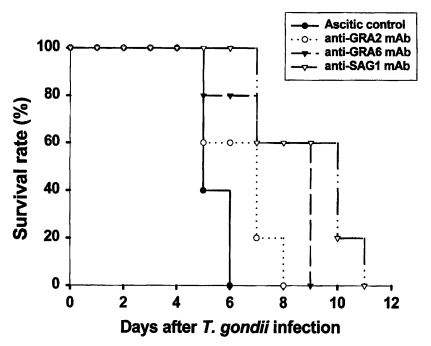
Fig. 2Effect of monoclonal antibody alone or in combination with complement on theinvasion of pretreated T. gondii tachyzoites in human fibroblast cells. Tachyzoites were pretreated with monoclonal antibodies and/or complement for 30 min before infection. Data are presented as the mean ± SD of two experiments.
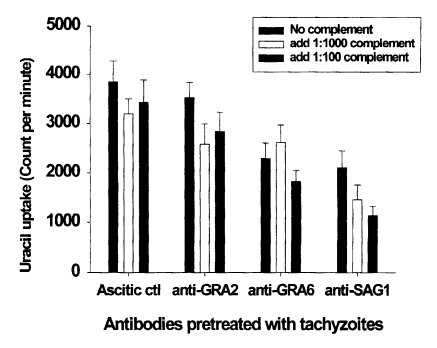
Fig. 3Effect of monoclonal antibody alone or in combination with complement on the invasion of pretreated T. gondii tachyzoites in peritoneal macrophages. Tachyzoites were pretreated with monoclonal antibodies and/or complement for 30 min before infection. The data are the mean ± SD of two experiments.
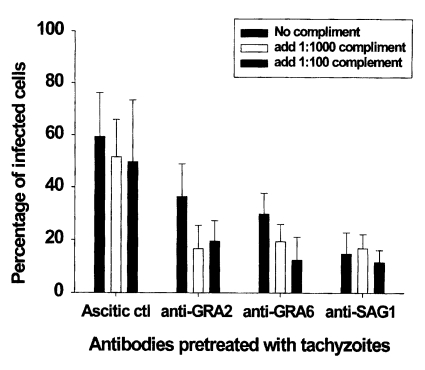
Fig. 4
T. gondii within murine peritoneal macrophages, fixed in methanol and stained with Giemsa solution (×200). The macrophages were exposed to tachyzoites for 18 hr.
Table 1.Survival days of BALB/c mice challenged intraperitoneally with lethal doses of tachyzoites pretreated with either mAb or complement
a)
Table 1.
|
Group |
Survival days (range) |
p-valueb)
|
|
Ascitic control |
6.2 ± 1.3 (5-7) |
— |
|
Complement |
6.4 ± 0.9 (5-7) |
0.374b)
|
|
Anti-GRA2 mAb |
7.2 ± 1.2 (5-8) |
0.099 |
|
Anti-GRA2/complement |
9.4 ± 1.3 (8-11) |
0.001 |
|
Anti-GRA6 mAb |
8.0 ± 1.2 (6-9) |
0.009 |
|
Anti-GRA6/complement |
11.2 ± 2.0 (9-13) |
0.001 |
|
Anti-SAG1 mAb |
8.8 ±1.8 (6-10) |
0.003 |
|
Anti-SAG1/complement |
12.2 ± 2.7 (8-14) |
0.001 |