Abstract
The last two decades witnessed significant advances in the efforts of immunoparasitologists to elucidate the nature and role of the host mucosal defence mechanisms against intestinal nematode parasites. Aided by recent advances in basic immunology and biotechnology with the concomitant development of well defined laboratory models of infection, immunoparasitologists have more precisely analyzed and defined the different immune effector mechanisms during the infection; resulting in great improvement in our current knowledge and understanding of protective immunity against gastrointestinal (GI) nematode parasites. Much of this current understanding comes from experimental studies in laboratory rodents, which have been used as models of livestock and human GI nematode infections. These rodent studies, which have concentrated on Heligmosomoides polygyrus, Nippostrongylus brasiliensis, Strongyloides ratti/S. venezuelensis, Trichinella spiralis and Trichuris muris infections in mice and rats, have helped in defining the types of T cell responses that regulate effector mechanisms and the effector mechanisms responsible for worm expulsion. In addition, these studies bear indications that traditionally accepted mechanisms of resistance such as eosinophilia and IgE responses may not play as important roles in protection as were previously conceived. In this review, we shall, from these rodent studies, attempt an overview of the mucosal and other effector responses against intestinal nematode parasites beginning with the indices of immune protection as a model of the protective immune responses that may occur in animals and man.
-
Key words: mucosal immunity, intestinal nematodes, antibody, cytokines, eosinophils, mast cells, goblet cells
INTRODUCTION
Gastrointestinal (GI) nematode parasites are important causes of disease in humans and domestic animals. In humans, species belonging to the genera
Ascaris,
Ancylostoma,
Necator,
Strongyloides and
Trichuris, all of which are known as soil-transmitted parasites, infect approximately one billion people worldwide and are believed to cause one million deaths annually, with prevalence in some endemic areas in developing countries approaching 100% especially in children (
Kightlinger et al., 1995;
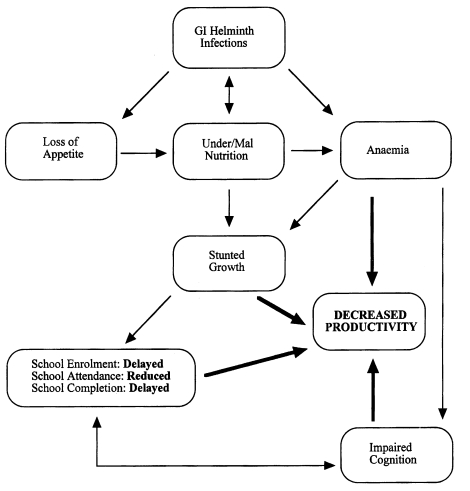
Finkelman et al., 1997). Since infections tend to be chronic, it is particularly damaging to severely infected children, causing anaemia, growth retardation, impaired cognitive function and lowered educational achievement (
Cooper and Bundy, 1988;
Nokes et al., 1992;
Guyatt, 2000). In addition, infections also cause impaired physical fitness, reduced productivity and low earning potential in adults as a result of high morbidity due to iron-deficiency anaemia and wasting (
Guyatt, 2000) (
Fig. 1). Parasitic gastroenteritis (PGE), caused by species of the genera
Bunostomum,
Cooperia,
Haemonchus,
Nematodirus,
Oesophagostomum,
Ostertagia,
Teladorsagia and
Trichostrongylus, is a major problem in farm animals worldwide where it can cause significant production losses and, in heavy infections, death of the host (
Balic et al., 2000;
Claerebout and Vercruysse, 2000). Control measures are expensive. For instance, based on 1994 sales figures in Britain alone, it was shown that control of PGE costs the livestock industry about one thousand million pound sterling (£1000 million) annually (
Newton and Munn, 1999). At present, the control of PGE in animals relies heavily on repeated use of anthelmintic drugs to cure existing infections, but increasing consumer concerns regarding drug residues in animal products and the environment and, the emerging development of resistance by the parasites against anthelmintic drugs in livestock, including the latest family of ivermectin drugs, all highlight the imperative need to seek and adopt more natural and alternative control strategies for the future (
Coles, 1998;
Newton and Munn, 1999;
Vercruysse and Dorny, 1999;
Balic et al., 2000). Similarly, control of GI nematode parasitism in humans relies on anthelmintic chemotherapy and, coupled with efficient primary health care and effective public sanitation, human GI nematode parasitism can be effectively eliminated (
Finkelman et al., 1997). However, these measures are also expensive and difficult to achieve and sustain in most under-developed and developing countries where GI nematode parasitism remains endemic and intractable. In both cases, immunological intervention through vaccination has been viewed as a feasible control alternative (
Emery et al., 1993). In fact, throughout much of the just ended century, researchers intensively studied systemic and mucosal host immune responses to the infections as well as various parasite antigens with the teleologic aim being the development of an effective vaccine as a more cost effective, practical and innovative therapeutic intervention. Accordingly, a great deal of information has been generated on various aspects of immune responses against GI nematode parasites. It is clear, mostly from rodent model studies, that significant protective immunity can be generated following natural and experimental infections. But this not withstanding effective vaccines are yet to be developed for the major GI nematode species. This can be attributed to the earlier difficulties in precise definition and understanding of the various underlying immune mechanisms involved in parasite rejection. Based on the rodent model studies the situation is different now and the next decade aught to see an accelerated advance towards vaccine development. However, although some similarities occur between effector responses in rodent models and some studies in livestock, care must be taken in extrapolating from one host-parasite system to another as differences between host species, nematode species and, in the localization of particular parasites within the host gut mucosa, have important consequences for the interaction between the parasite and the host's gut immune systems and on the type of effector mechanism that is expressed (
Nawa et al., 1994;
Claerebout and Vercruysse, 2000;
Balic et al., 2000).
INDICES OF MUCOSAL IMMUNITY AGAINST GI NEMATODE PARASITES
Immunity against gastrointestinal nematodes can be manifested as expulsion of adult parasites and reduction in worm length, decrease in female worm fecundity, failure of infective larvae to establish and arrested development of larvae.
Expulsion of adult worms
Rapid expulsion of adult worms following primary infections occurs in most rodent nematodes but the phenomenon is uncommon in large animals and man (
Behnke et al., 1992). The only exception is
Nematodirus species in which adult worms from primary infections of sheep with
N. battus were expelled in periods ranging from 18-21, 24-28, upto 72 days post infection (p.i.) depending on the dose of infective larvae given (
Balic et al., 2000). Most of the work on rapid expulsion of adult nematodes following primary infections have been in rodents using
T. spiralis,
N. brasiliensis,
Strongyloides species and
T. muris. Each of these parasites occupies a different niche in the gut and, as will be reviewed subsequently, evokes slightly different effector mechanisms; thus important differences exist in the ability of the rodent hosts to expel a primary infection with each of them (
Else and Finkelman, 1998).
T. spiralis,
N. brasiliensis,
S. ratti and
S. venezuelensis all have tissue migratory larval stages and their adults live in the small intestinal lumen from where they are expelled within 2-3 weeks after primary infection (
Ogilvie and Hockley, 1968;
Miller, 1984;
Wakelin and Lloyd, 1976;
Nawa et al., 1985;
Sato and Toma, 1990a,
b).
T. muris on the otherhand occupies a niche in the large intestine where it induces syncitium formation and lives partially or completely within the intestinal epithelium and the ability to expel the adult in a primary infection is genetically determined (
Wakelin, 1975,
Else and Wakelin, 1988). Thus, in some strains the worms are eliminated before they reach sexual maturity and produce eggs while in others a proportion fails to do so and allows the parasites to mature and establish a chronic infection (
Wakelin, 1987).
Observations on the changes in morphology of GI nematodes as an index of protective immunity have largely described reduced size (stunting) of adult nematodes although the loss of vulval flap in some adult female worms have been documented (
Balic et al., 2000). Most of the studies of immune stunting of adult nematodes in rodent models have been done with
T. spiralis although the evidence of stunting exists for
H. polygyrus and
S. ratti. Mice immunized by primary
T. spiralis infection followed by treatment prior to the production of newborn larvae and then challenged with infective larvae showed stunting of the adults as well as earlier expulsion with 95% reduction in the number of muscle larvae which encysted following the challenge (
James and Denham, 1975). A similar observation was made by DeVos et al. (
1992) in challenge
T. spiralis infection in mice. Although they recorded a dose-dependent response, with 28% reduction in the size of adult worms recovered from mice primed and challenged with 10 larvae each and 35% reduction in those from mice primed and challenged with 150 larvae each. In addition, vaccination of mice with various
T. spiralis antigen preparations results in protective immunity that also induces stunting of adult worms following challenge (
Grencis et al., 1986;
Goyal and Wakelin, 1993;
Boulos et al., 1993). Adult worms arising from challenge infections of mice that had previously experienced one or more infections of
H. polygyrus or from naive mice that had received immune serum prior to challenge were stunted and anaemic with female worms being more severely affected than males (
Ey, 1988). Similarly, adoptive transfer of immune mesenteric lymph node cells (MLNC) induced reduction in adult worm size in recepient rats challenged with
S. ratti (
Moqbel and Wakelin, 1981) and Uchikawa et al. (
1989) showed a dose dependent reduction in worm length following single and repeated inoculations of rats with
S. ratti. Stunting of adult worms as a result of acquired immunity has also been reported for the livestock parasites
Cooperia spp.,
H. contortus,
O. ostertagi, and
Trichostrongylus spp. (
Balic et al., 2000;
Claerebout and Vercruysse, 2000).
Immune-mediated reduction in female worm fecundity is a very important epidemiological factor and in sheep has been implicated as a major regulatory force for GI nematode populations (
Stear et al., 1997). It has been suggested that reduced female worm fecundity can also be as a result of density-dependent intraspecific parasite competition although data on the role of density dependence are conflicting (
Balic et al., 2000). Depending on the species of parasite and animal model, reduction in female worm fecundity as a result of developing or acquired immunity can be measured either by reduced faecal egg output, number of eggs in-utero or number of newborn larvae/female worm. In rodent models and ruminants, the first evidence of developing protective immunity to a primary infection and acquired protective immunity following challenge infection is usually a decreasing and no egg output in faeces respectively. Although faecal egg count is the only parasitological parameter of immunity that can be obtained sequentially and regularly in the same animal in the course of an infection, it does not strictly reflect the fecundity of the female worm population (
Claerebout and Vercruysse, 2000) as a lot of other factors may affect the faecal egg count. However, a very good correlation between faecal egg counts and the number of eggs in utero was found in
C. oncophora (
Claerebout and Vercruysse, 2000) and
O. circumcincta (
Stear et al., 1995) infections in calves and sheep respectively. In rodents infected with
Strongyloides or
N. brasiliensis faecal egg count as an index of immunity is feasible only in a primary infection as the immunity that develops is very strong and ablates any challenge infection prior to the enteric stage or before maturation and egg production if any larva reaches the gut. Several studies in our laboratory have shown that in such primary infections, development of immune-mediated effect on the female worms is usually manifested as decreasing number of eggs in faeces followed by adult worm expulsion. However, because the decrease may also be related to a gradual decrease in the number of female worms due to expulsion, determining the number of eggs in utero is a better index of decreased fecundity, as shown in
S. ratti infection in rats immunized either by adoptive transfer of MLNC (
Moqbel and Wakelin, 1981) or by repeated infections (
Uchikawa et al., 1989). Similarly, the number of in utero eggs/female was significantly less in euthymic than hypothymic (nude) mice on day 9 after infection with
S. venezuelensis (
Sato and Toma, 1990a). In BALB/c mice infected with either
S. ratti or
S. venezuelensis, the fecundity of both species was significantly decreased respectively from 6 and 5 in utero eggs/female worm on day 6 p.i. to 0.8 and 1 in utero eggs/female worm on day 10 p.i. (
Sato and Toma, 1990b). Conversely, female worms are significantly more fecund in IL-5 knockout than IL-5 sufficient mice infected with
S. ratti (
Ovington et al., 1998), although this result was based on dividing the total eggs+larvae by the total number of adult worms. Several workers have demonstrated reduced fecundity of female worms in
T. spiralis following infection- and/or vaccine antigen-induced immunity as measured by decrease in the number of newborn larvae/female worm (
Wakelin and Wilson, 1977;
Kennedy and Bruce, 1981;
Grencis et al., 1986;
DeVos et al., 1992;
Goyal and Wakelin, 1993). On the otherhand, in contrast to decreased fecundity in vaccinated mice, suppression of either vaccine- or infection-induced immunity by super infection with
Trypanosoma brucei either at the time of primary
T. spiralis infection or at the time of primary vaccination with muscle larva antigen followed by
T. spiralis challenge resulted in significantly increased female worm fecundity (
Onah and Wakelin, 1999,
2000). Reduction in female worm fecundity has also been described for
H. polygyrus (
Urban et al.,
1991b).
A major manifestation of acquired immunity to GI nematodes is failure of infective larvae to establish and mature to adults in the gut. This phenomenon has been described both in rodents and ruminants. In rodents, challenge infections with
Strongyloides or
N. brasiliensis are usually sterile with no egg output in the faeces nor recovery of adult worms. Studies in our laboratory as well as those of other workers have shown that in immune animals most infective larvae following challenge are destroyed at the tissue migratory stage usually with few larvae surviving the transition from the subcutaneous infection site to the lungs. Even fewer or none at all may migrate from the lungs to the intestine where they fail to thrive and are expelled prior to maturity and egg production (
Sato and Toma, 1990b;
Korenaga et al., 1991;
Dent et al., 1997b; Onah DN, Uchiyama F, Ishiwata K and Nawa Y, unpublished observation). Although resistance to larval establishment is most strongly expressed in secondary and subsequent larval challenges, the fact that in most experimental studies 40% or less of the infecting larval dose in a primary infection finally establishes as adults, indicates that resistance to larval establishment is not restricted to challenge infections. Resistance to larval establishment in primary
Strongyloides infections has been attributed to complement (
Brigandi et al., 1996), IL-5/eosinophils (
Ovington et al., 1998) and granulocytes (
Watanabe et al., 2000). Failure of larval establishment has also been demonstrated in many ruminant parasites where the resistance is generally stage-specific, requires homologous challenge for expression but can act against heterologous species present in the same tissue and is usually expressed as rapid expulsion of infective larvae from previously primed hosts (
Balic et al., 2000).
Arrested larval development at the L4 stage (hypobiosis of larvae) of GI nematodes in the host mucosa is a common phenomenon in ruminant hosts and is associated with increased resistance of the hosts to the parasites. However, it is also associated with a number of other factors such as seasonal changes, population density of the nematode in the host and strain of the nematode (
Balic et al., 2000). Its role is epidemiological as it provides the replacements for expelled or aged adult worm populations and its wide spread occurrence in ruminants suggests that it is a successful survival strategy for the parasites. The phenomenon has not been reported in rodent models.
ANTIGEN PROCESSING AND PRESENTATION AT THE GI MUCOSAL SURFACE
Before reviewing the various protective mucosal immune responses against parasitic GI nematodes, it may be helpful to briefly outline how immune responses to orally acquired antigens may occur, although whether they may also apply to nematodes is not clear. Despite significant advances in the understanding of antigen uptake, processing and presentation in general immune system, our knowledge of these events in the GI tract or other mucosal surfaces is limited (
Mayer, 1990). Moreover, the GI epithelium and its mucus are thought to constitute a prominent barrier to antigen uptake in the gut (
Russell and Walker, 1990), and unless disrupted, antigen uptake may be minimal (
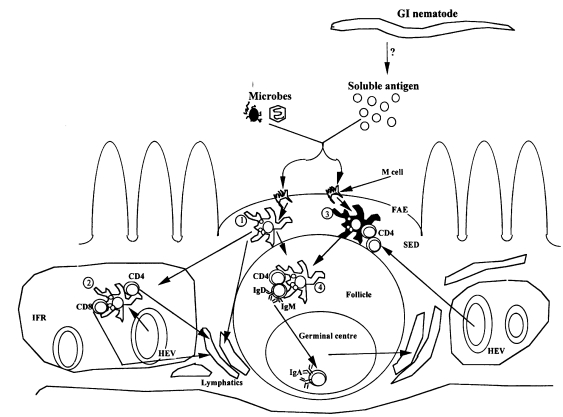
Befus, 1995). However, while antigen uptake in the GI tract is associated with specialized microfold (M) cells (
Fig. 2) in the follicle-associated epithelium overlying the Peyer's patches (PP) and other gut associated lymphoid tissues (GALT)(
Mayer, 1990;
Kelsall and Strober, 1999), antigen presenting cells (APC) such as B cells, dendritic cells, epithelial cells, fibroblasts and macrophages are involved in antigen processing and presentation (
Mayer, 1990). Following ingestion, soluble antigens and microorganisms are rapidly taken up and transported by M cells from the intestinal lumen to APC in the subepithelial dome (SED) of the PP where initial cognate interactions between APC and T cells, or T cells and B cells occur (
Owen and Jones, 1974;
Kelsall and Strober, 1999) (
Fig. 2). Detailed review of the sequence of these events can be found in many excellent reviews on the subject (
Mayer, 1990;
Owen, 1994;
Befus, 1995;
Kelsall and Strober, 1999;
Claerebout and Vercruysse, 2000).
There is a dearth of studies on the uptake, processing and presentation of helminth antigens in the GI tract or elsewhere, thus there is currently no solid in vivo proof of the capacity of any of the above cells to be involved in nematode antigen presentation in the gut (
Befus, 1995,
Miller, 1996a). No relationship between M cells and any intestinal helminth has been noted to date but it is thought that perhaps, they may provide some physical or chemical signals which helminths utilize either for site selection or as other microenvironmental clue for survival (
Befus, 1995). However, although there is limited information on the nature of the antigens of the abomasal nematode parasite of ruminants
Ostertagia (
Claerebout and Vercruysse, 2000) and it is not known which of its antigens elicit protective immune responses (
Hilderson et al., 1995), there is an evidence that soon after the infection of calves with
O. ostertagi, worm antigens were presented to the host cells in the abomasal lymph nodes (
Gasbarre, 1997). But in general, it remains to be determined which nematode antigens are relevant in the antigen handling mechanisms, the pathway(s) of their uptake, types of APC utilized and the nature of processing and presentation. In fact, whether or not the remarkable predominance of IgE, mast cell and eosinophil responses in GI nematode infections can be directly attributed to the pathways of uptake, processing and presentation of worm antigens, or to other characteristics of the host-parasite relationship remains to be established (
Befus, 1995).
EFFECTOR MECHANISMS IN THE GI MUCOSA AGAINST PARASITIC NEMATODES
There is diverse information concerning the generation of immune and inflammatory responses in the gut mucosa (
Lin and Befus, 1999;
Kelsall and Strober, 1999). However, investigations of effector mechanisms in intestinal nematode infections have focused on more limited areas such as the types of antigen-specific cells which can transfer immunity, mast and goblet cell responses and the role of selected chemical mediators, including mucus. In general, effector mechanisms against GI nematodes appear to involve antigen-specific T cell responses which induce antibody response and inflammatory changes, with the release of a plethora of chemical mediators ultimately leading to the expulsion of the worms (
Befus, 1995). This section will review some of the information available in these areas.
Several studies with different nematode species have demonstrated that protective immunity against GI nematodes is T cell-dependent. The foundation for this in immunoparasitology was laid by the works of DiNetta et al. (
1972) and Larsh et al. (
1972) using
T. spiralis infections in mice. Independently these authors reported that treatment of mice with anti-thymocyte serum resulted in delayed expulsion of adult
T. spiralis worms. These were to be confirmed and extended by the use of congenitally athymic 'nude' mice and rats infected with
T. spiralis (
Walls et al., 1973;
Ruitenberg and Elgersma, 1976;
Ruitenberg et al., 1977;
Vos et al., 1983). These studies in athymic animals have been corroborated by work with other nematode species such as
N. brasiliensis (
Mitchell, 1978;
Mckay et al., 1995),
Nematospiroides dubius (
Heligmosomoides polygyrus) (
Prowse et al., 1978),
S. ratti (
Abe and Nawa, 1988) and
S. venezuelensis (
Sato and Toma, 1990a).
Adoptive transfer experiments and/or in vivo depletion of CD4
+ T cells by cytotoxic anti-CD4 monoclonal antibody (mAb) treatments have demonstrated that CD4
+ helper T cells play a central role in the T cell-dependent protective immunity against GI nematode parasites (
Finkelman et al., 1997;
Else and Finkelman, 1998). Using accurate cell sorting and adoptive transfer of immune cells it was demonstrated that CD4
+ T cells were the T cell subset involved in the conferement of immunity against challenge infection with
T. spiralis in mice (
Grencis et al., 1985). Also, thoracic duct CD8
-OX22
- (presumably CD4
+) lymphocytes collected from rats 3 days after infection with
T. spiralis conferred immunity against the adult worms in naive rats (
Wang et al., 1990). Depletion of CD4
+ but not CD8
+ cells by mAb treatment at the time of
N. brasiliensis infection in mice blocked spontaneous elimination of adult worms as well as polyclonal IgE responses (
Katona et al., 1988;
Abe et al., 1994) and, was shown to be effective only against the intestinal phase of the infection (
Khan et al., 1995). Primary
H. polygyrus infection in mice results in chronic infection characterized by faecal egg production for several months. Evidence that protective immunity against
H. polygyrus in mice is CD4
+ T cell-dependent came from the fact that during a primary infection, mice treated with rat anti-mouse CD4
+ mAb had increased faecal egg production which was primarily due to an increase in female worm fecundity (
Urban et al., 1991a). It was further shown that if a primary infection was cleared by anthelmintic drug treatment a protective response against challenge developed which reduced adult worm establishment following challenge by up to 80% and their fecundity by more than 90%. This secondary protective response was completely abrogated by anti-CD4 but not anti-CD8 mAb treatment (
Urban et al., 1991a). Also following mAb treatment, the expulsion of
T. muris was inhibited in anti-CD4 treated mice but no evidence of suppressed immunity was observed in the anti-CD8 treated mice (
Koyama et al., 1995).
The discovery in mice that CD4
+ T cells can be segregated into two distinct T helper subsets, Th1 and Th2, based on their cytokine secretion profiles (Mosmann et al., 1986) provided the basis for subsequent understanding of the underlying T cell regulatory mechanisms controlling resistance to GI nematode infections. Th1 cells produce gamma-interferon (IFN-γ), interleukin 2 (IL-2) and alpha-lymphotoxin (LT-α), whereas Th2 cells secrete IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13, among others. Products of Th1 cells negatively regulate the Th2 cells and vice versa (
Mosmann and Coffman, 1989). Which T cell subset gains predominance over the other in an immune response following parasite infection depends on factors such as the type of APC, co-stimulatory molecules, the nature and dose of parasite antigen and, perhaps more importantly, the immediate cytokine environment the T cell experiences at the time of antigen presentation (
Grencis, 1996;
Constant and Bottomly, 1997). The predominant immune responses that are typical of nematode infections are all controlled by Th2 cytokines (
Finkelman et al., 1991), but of all the Th2 cytokines those that have been associated with resistance to various nematode parasites include IL-4, IL-5, IL-9, and IL-13.
The most compelling evidence for the importance of IL-4 in resistance to GI nematode infections came from studies with
H. polygyrus and
T. muris infections in mice (reviewed by
Finkelman et al., 1997;
Else and Finkelman, 1998). Anti-IL-4 or anti-IL-4 receptor (IL-4R) mAb treatment was shown to block the immune-mediated expulsion of
H. polygyrus challenge infection in mice (
Urban et al., 1991b). Similarly, BALB/k mice are normally resistant to
T. muris infection but when treated with anti-IL-4R mAb (which also blocks IL-13 receptor), host protective immunity against primary
T. muris infection is abrogated, resulting in the establishment of a chronic infection in which Th1 responses predominate while Th2 responses are down-regulated (
Else et al., 1994). Furthermore, treatment of mice with an IL-4 complex (IL-4C), obtained by mixing IL-4 and neutralizing anti-IL-4 mAb, which prolongs the in vivo half life of IL-4 by protecting it from degradation and excretion, was shown to be effective in enhancing worm expulsion and in curing even established chronic infections of
H. polygyrus (
Urban et al., 1995) and
T. muris (
Else et al., 1994). Further evidence for the significance of IL-4 in protective immunity against
H. polygyrus and
T. muris was obtained in experiments using IL-4 gene knockout (KO) mice (
Finkelman et al., 1997;
Bancroft et al., 1998;
Artis et al., 1999). The role of IL-4 in protective immunity has also been demonstrated in normal and immune deficient mice infected with
N. brasiliensis. IL-4 treatment terminated
N. brasiliensis infections in severe combined immunodeficiency (SCID) mice and in anti-CD4 mAb-treated BALB/c mice (
Urban et al., 1995). However, both anti-IL-4 mAb-treated mice and mice lacking the IL-4 gene expel
N. brasiliensis normally (
Madden et al., 1991;
Lawrence et al., 1996), suggesting that IL-4 is sufficient but not critical for expulsion of
N. brasiliensis.
The role of IL-5 in protective immunity to GI nematode parasites is not very clear, largely because it has been difficult to demonstrate anti-parasite effects of eosinophils in vivo, the production of which during parasitic infections depends on IL-5, although in vitro killing by eosinophils is easily demonstrated (
Butterworth et al., 1974;
Korenaga and Tada, 1994). Thus, the involvement of IL-5/eosinophilia in resistance to intestinal helminthiasis remains an unresolved controversial issue. Several studies involving IL-5 transgenic mice or mice treated with neutralising mAb against IL-5 prior to infection with various species of helminths indicate that IL-5 may enhance resistance to some but not all parasites and that some parasites may even fair better in IL-5 transgenic animals (reviewed by
Onah et al., 2001). For instance, following a primary
T. spiralis infection higher numbers of muscle larvae were established in IL-5 transgenic mice than their non-transgenic counterparts whereas in transgenic mice infected with
N. brasiliensis, few larvae survived to reach the lung and even fewer finally made it to the intestines where they failed to thrive and produce eggs (
Dent et al., 1997b).
Although limited, there is an evidence that IL-9 which in humans and mice has been shown to potentiate IL-4-induced Th2-type antibody production (
Dugas et al., 1993;
Petit-Frere et al., 1993), also plays a role in resistance against GI nematode parasites. First suggestive evidence for the role of IL-9 in protection came from studies with
T. muris infections in BALB/k and B10.BR mice which differ in an absolute fashion in their ability to resist the infection. Whereas the former mice strain expels
T. muris relatively rapidly, the latter are unable to expel the parasite before the infection reaches patency. When MLNC obtained from
T. muris-infected BALB/k and B10.BR mice were stimulated in vitro with Con A and analyzed for Th1 and Th2 cytokine production, MLNC from BALB/k mice were shown to produce elevated levels of IL-9 with little amount of IFN-γ whilst MLNC from B10.BR mice produced large amounts of IFN-γ in the relative absence of IL-9 (
Else et al., 1992a). When BALB/k mice were rendered susceptible to
T. muris by hydrocortisone treatment their MLNC produced significantly higher amounts of IFN-γ and low levels of IL-9 than MLNC from untreated infected mice whereas, in B10.BR mice immunized with
T. muris E/S antigen there was accelerated expulsion of
T. muris with elevated production of IL-9 and no IFN-γ by MLNC (
Else et al., 1992b). Studies with mice in which IL-9 was elevated in vivo prior to
T. muris infection and with IL-9 transgenic mice which constitutively overexpress IL-9 showed that the animals displayed an extremely rapid, but immune-mediated, expulsion of the parasite accompanied by pronounced intestinal mastocytosis and enhanced production of IgG
1 and IgE while the neutralization of IL-9 by treating normally resistant C57BL/6 mice with neutralizing anti-IL-9 coupled to ovalbumin rendered them susceptible (
Faulkner et al., 1998;
Richard et al., 2000). The importance of IL-9 in immune-mediated protection against
H. polygyrus (
Behnke et al., 1993,
Wahid et al., 1994) and
T. spiralis (
Behnke et al., 1993;
Faulkner et al., 1997;
Else and Finkelman; 1998) has also been demonstrated.
As mentioned above, although IL-4 plays a central role in Th2-dependent protective immunity against GI nematodes, a couple of studies showed that both IL-4 KO mice and mice treated with anti-IL-4 mAb expel
N. brasiliensis normally (
Madden et al., 1991;
Lawrence et al., 1996), suggesting an independence of the process on IL-4 and that another Th2 mediator may be required for worm expulsion. However, a number of recent studies reveal that both IL-4, and a related cytokine IL-13, play protective roles in
N. brasiliensis,
T. muris and
T. spiralis infections in mice, but show parasite-specific differences in their relative importance or redundancy (
Finkelman et al., 1999) (
Table 1). Studies that compared the kinetics of expulsion of
N. brasiliensis in wild type, IL-4 KO and IL-13 KO mice revealed a distinct and critical role for IL-13 in worm expulsion since only IL-13 KO mice failed to clear the infection (
McKenzie et al., 1998). IL-13 shares some, but not all, biological activities with IL-4 (
Finkelman et al., 1999), and as mentioned earlier, anti-IL4R mAb treatment blocks both IL-4 and IL-13 which would suggest that the IL-4 independent worm expulsion is not mediated by IL-13. It is therefore, rather paradoxical that whilst infected mice injected with recombinant IL-4 or IL-13 can expel the worm, as does IL-4 KO mice, IL-13 KO mice cannot. This apparent paradox is now explained by the observation that IL-4 receptor alpha chain-deficient (IL-4Rα KO) and signal transducer and activator of transcription molecule 6-deficient (STAT6 KO) mice, as well as mice treated with anti-IL-4R mAb all fail to expel a primary
N. brasiliensis infection (
Urban et al., 1998). Unlike IL-4, IL-4Rα chain is required for
N. brasiliensis expulsion; furthermore, Th2 development during the infection (as indexed by cytokine production, expression of the transcription factor GATA-3 and the cell surface receptor CD30) was more severely impaired in IL-4Rα KO than in IL-4 KO mice (
Barner et al., 1998). These authors also demonstrated that the injection of recombinant IL-13 induced worm expulsion in otherwise incompetent, B and T lymphocyte deficient RAG2 KO mice which suggests that IL-13 regulation of Th2 responses to nematode infection requires IL-4Rα. There is an evidence that IL-4 and IL-13 share receptor components (
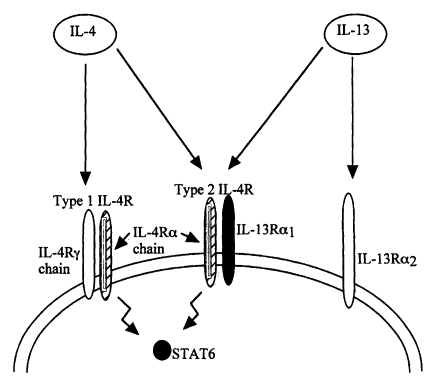
Fig. 3) and that IL-13 binds to a receptor which contains the IL-4Rα chain (
Zurawski et al., 1993,
1995;
Obiri et al., 1995;
Smerz-Bertling et al., 1995). In addition, only IL-4 and IL-13 are currently known to induce STAT6 activation via the IL-4Rα chain, so taken together the above studies show that IL-13 mediates resistance to
N. brasiliensis in the absence of IL-4 (
Else and Finkelman, 1998). Indeed, when immunocompetent mice infected with
N. brasiliensis were treated with a soluble IL-13Rα 2-human IgGFc fusion protein (sIL-13Rα 2-Fc), worm expulsion was dramatically inhibited with fecund adults still present on day 16 p.i., and similarly treated IL-4 KO mice harboured even higher numbers of more fecund parasites. These together suggest that both IL-4 and IL-13 contribute to
N. brasiliensis expulsion through an IL-4Rα-dependent and STAT6-dependent mechanism with IL-13 being quantitatively the more important cytokine (
Else and Finkelman, 1998;
Finkelman et al., 1999).
Recent studies also revealed a critical role for IL-13 in protection against
T. muris infection (
Bancroft et al, 1998) and that the process is tumour necrosis factor alpha (TNF-α)-dependent since it is impaired by blocking TNF-α and enhanced by administration of recombinant TNF-α (
Artis et al., 1999). In addition, IL-13-mediated
T. muris expulsion is independent of B7 costimulation but IFN-γ sensitive. Thus, in mice infected with
T. muris, blocking B7 ligand interaction by treatment with murine CTLA4-Ig fusion protein inhibited worm expulsion and enhanced IFN-γ production but did not inhibit IL-13 production (
Urban et al., 2000a). When both B7 and IFN-γ were blocked, protective immunity was restored suggesting that the B7-independent protective immune response to
T. muris is mediated by IL-13. This was confirmed by subsequent experiments in which additional administration of A25 to further block IL-13 in infected mice in which both B7 and IFN-γ had been blocked resulted in dramatic increase in worm burdens similar to but above those seen in
T. muris-infected mice treated with CTLA4-Ig alone (
Urban et al., 2000a). Furthermore, these authors demonstrated that IL-4/IFN-γ double knockout mice showed enhanced protection against
T. muris infection which was abrogated by IL-13 blockade following A25 administration. While endogenous production of IFN-γ also suppresses
T. spiralis expulsion, the production of IL-4 or IL-13 is essential and sufficient to induce spontaneous expulsion of
T. spiralis in addition to the expression of IL-4Rα and the associated signaling molecule STAT6, and mastocytosis (
Urban et al., 2000b).
Intestinal nematode infections are typically accompanied by elevated IgE and IgG
1 antibody isotypes, which over the years have been favoured as effector molecules in resistance based on the ability to transfer immunity with immune serum. However, passive transfer experiments have provided equivocal results, thus it was observed more than twenty years ago that although immunity can be transferred with immune serum it has proved difficult in an infection to correlate protection with circulating antibody levels (
Wakelin, 1978). This statement remains as valid today as it was then. Immunity to adult stage of
T. spiralis in mice, as assessed by an acceleration of worm expulsion, was markedly transferred to naive recipient mice but only when the immune serum was transferred along with immune mesenteric lymph node cells taken from the donor mice at day 8 p.i. (
Wakelin and Lloyd, 1976). Also in rats, complete destruction of
T. spiralis newborn larvae in vivo after passive transfer, as measured by muscle larvae burden, was only evident after exposure to both immune serum and immune cells, and not to either alone (
Wang and Bell, 1988). Similarly, rapid expulsion of infective muscle larvae could be transferred to naive adult rats with immune serum collected on day 28 p.i. and thoracic duct lymphocytes (TDL) collected on days 3-5 p.i., but neither serum nor cells could transfer rapid expulsion when given alone, even in large volumes (
Ahmad et al., 1990). In the day 28 p.i. serum, the most important Ig isotype was shown to be homocytotropic IgE which when purified could induce rapid expulsion after passive transfer with TDL in as little as 183 µg/rat whereas similarly purified monoclonal IgG of any isotype transferred in amounts of up to 35 mg/rat could not transfer rapid expulsion to rats previously transfused with TDL (
Ahmad et al., 1991). In contrast, there is a evidence that immune IgG monoclonal antibodies alone were effective in the transfer of rapid expulsion to neonatal rats (
Appleton et al., 1988) and in addition, when primed by prior infection with
H. polygyrus instead of immune cell transfer, both IgE and IgG mononclonal antibody isotypes were equally effective in inducing rapid expulsion of
T. spiralis in adult rats as well (
Bell et al., 1992). Related to this is the fact that high levels of immunity against
T. spiralis in mice can be achieved by passive transfer of only immune serum obtained from vaccinated, or from vaccinated and infected mice donors (
Robinson et al., 1995;
Boulos et al., 1998).
Studies with
T. muris also typify the inconsistency in demonstrating an essential role for antibodies in resistance to GI nematodes. Transfer of immune serum on days 0 and 3 p.i. accelerated worm expulsion but delaying serum transfer until days 7 and 8 p.i. failed to enhance expulsion, although immune MLNC accelerated expulsion whether transferred early or late in infection (
Wakelin, 1975). Also, the adoptive transfer of highly purified immune CD4
+ T cells to SCID mice protected them from primary
T. muris infection in the complete absence of an antibody response since they lack functional B or T cell function (
Else and Grencis, 1996). Furthermore, whilst immune serum from resistant (B10.BR × B10.G) F1 hybrid mice containing high levels of IgG
1 antibody specific for
T. muris E/S and other IgG antibodies which recognised two high molecular weight E/S antigens was effective in transferring protection to the non-responsive B10.BR mouse strain (
Else et al., 1990), passive transfer of immunity could not be achieved with IgG and IgM mAb although two IgA mAb effectively transferred immunity (
Roach et al., 1991). The importance of antibodies in protection against
T. muris infection is also questioned by studies with IL-9 transgenic mice which expel
T. muris very rapidly in the absence of any detectable parasite-specific IgG antibodies in the serum, though it was reasoned that this may have been because the majority of larvae traversed through the gut so rapidly that insufficient antigen was available for B cell activation and antibody production (
Faulkner et al., 1998).
Injection of immune serum alone induced highly specific protective immunity in mice infected with
N. dubius (
H. polygyrus) (
Brindley and Dobson, 1983) but in contrast, considerably greater protection was transferred to recipient mice only when they received both immune serum and immune MLNC (
Behnke and Parish, 1981;
Williams and Behnke, 1983). A non-essential role for antibody in protective immune responses is also seen in
N. brasiliensis infection where mice treated with anti-IgM produce little antibody but retain the ability to expel
N. brasiliensis (
Jacobson et al., 1977). In the other hand,
N. brasiliesnsis-infected STAT6 KO mice generate strong parasite-specific antibody responses yet they are unable to expel the parasites (
Urban et al., 1998). Studies of adoptive transfer of immunity in
Strongyloides infections also lacked consistency. Resistance to
S. ratti infection was transferred with either pooled immune serum or immune MLNC but not with immune spleen cells to mice (
Dawkins and Grove, 1981) and with either pooled immune serum or immune serum fraction containing predominantly IgG
1 to infected recipient rats (
Murrell, 1981). In contrast, serum transfer from immune mice to nude mice failed to confer protection against
S. venezuelensis whereas protective immunity was transferred to nude mice that received either normal or immune spleen cells from syngeneic heterozygous thymus-bearing littermates (
Sato and Toma, 1990a). In general therefore, there seems to be very little consistent and convincing data to indicate that the antibody represents a principal effector mechanism in resistance to lumen-dwelling gastrointestinal nematodes, although relatively little attention has been paid to local as opposed to systemic antibody production; thus antibodies secreted at gut mucosal surfaces may yet prove to be important in protective immunity against gut dwelling helminths (
Else and Finkelman, 1998).
Significant blood eosinophilia and increase in the number of eosinophils in the GI mucosa (
Rothwell, 1989) as well as a marked increase in the levels of IL-5, the Th2 cell cytokine responsible for the generation of eosinophils (
Korenaga and Tada, 1994), are prominent features of GI nematode infections. Despite this, the involvement of eosinophils/IL-5 in protective immunity remains controversial as a protective role for them during the infection has not been consistently demonstrated (
Table 2). There are evidences that eosinophils kill a variety of parasites in vitro (
Butterworth et al., 1975;
Kazura and Grove, 1978;
Butterworth, 1984;
Gransmuller et al., 1987). These studies projected eosinophils as effectors of anti-parasite immunity. However, while the in vitro studies seemed quite convincing, data from in vivo studies have been less clear and convincing. The first suggestive evidence of an eosinophil-mediated protection against a variety of parasites in vivo came from studies of the effects of anti-eosinophil serum administration. Thus, treatment of mice with a monospecific rabbit anti-eosinophil serum (AES) prior to primary infection with the vessle-dwelling helminth
Schistosoma mansoni had no effect on the number of schistosomula recovered from the lungs compared to untreated controls (
Mahmoud et al., 1975). However, a similar AES treatment prior to challenge abrogated the strong secondary immunity, as seen in untreated immune controls, with highly significant numbers of both schistosomula and adult worms recovered from their lungs and portal system respectively (
Mahmoud et al., 1975). In addition, a partial immunity conferred on naive recipient mice by adoptive immune serum transfer was also ablated by the AES treatment (
Mahmoud et al., 1975). Similarly, AES treatment in mice infected with
T. spiralis had no effect on the spontaneous expulsion of adult worms from the small intestine, but the numbers of larvae in the mucsles were almost doubled by the treatment (
Grove et al., 1977). On the otherhand, AES treatment resulted in a striking decrease in the number of intestinal eosinophils and significantly increased the susceptibility of guinea-pigs to both primary and secondary infections with the ruminant parasite
T. colubriformis (
Gleich et al., 1979).
In vivo studies involving the ablation of blood and tissue eosinophlia by anti-IL-5 or anti-IL-5R mAb treatment or by targeted disruption of the IL-5 or IL-5Rα gene (IL-5 or IL-5Rα KO), as well as studies with IL-5 transgenic mice, which constitutively over express IL-5 and over produce blood and tissue eosinophilia, have presented similar conflicting data as above regarding the role of eosinophils as anti-parasite effector cells. Anti-IL-5 or anti-IL-5R mAb treatment resulted in increase in the number of lung larvae recovered from mice following secondary
S. venezuelensis infection (
Korenaga et al., 1991). Similarly, anti-IL-5 mAb treatment reduced the resistance of immune mice challenged with
S. stercoralis (
Rotman et al., 1997) and enhanced the survival and numbers of tissue larvae recovered from mice primarily infected with the rat lung worm
Angiostrongylus cantonensis (
Sasaki et al., 1993) or the filarial parasite
Onchocerca lienalis (
Folkard et al., 1996). In contrast, following primary or secondary infection, anti-IL-5 mAb treatment had no effect on the number of recovered larvae in mice infected with
T. spiralis (
Herndon and Kayes, 1992),
Toxocara canis (
Parsons et al., 1993) and
S. stercoralis (
Rotman et al., 1996). Similarly, mAb treatment had no effect on the number of adult worm establishment in mice infected with
S. venezuelensis (
Korenaga et al., 1991),
S. mansoni (
Sher et al., 1990),
S. japonicum (
Cheevers et al., 1991),
H. polygyrus (
Urban et al., 1991b),
N. brasiliensis (
Coffman et al., 1989;
Khan et al., 1995) and
T. muris (
Betts and Else, 1999). Significantly greater numbers of intracranial larvae were recovered from IL-5Rα KO mice than from their IL-5Rα sufficient controls following primary infection with
A. cantonensis (
Yoshida et al., 1996;
Sugaya et al., 1997) but the number of tissue larvae recovered did not differ between IL-5 KO and their wild type controls following primary
T. canis infection (
Ovington and Behm, 1997;
Takamoto et al., 1997). However, IL-5 KO mice had higher numbers of adult parasites following primary infection with
H. polygyrus and
S. ratti (
Ovington and Behm, 1997) and after primary or secondary
T. spiralis infection (
Matthaei et al., 1997). Consistent with the mAb treatment and IL-5Rα KO results above, IL-5 transgenic mice are protected against
A. cantonensis infection (
Yoshida et al., 1996;
Sugaya et al., 1997). Similarly, resistance to both the tissue migratory larval and intestinal adult stages of
N. brasiliensis is enhanced in IL-5 transgenic mice but in contrast, IL-5 transgenic mice tend to harbour more larval or adult parasites than their non-transgenic littermates following primary or secondary infection with
S. mansoni (
Freeman et al., 1995;
Dent et al., 1997a) and
T. spiralis (Hokibara et al., 1995;
Dent et al., 1997b) while they fared neither better nor worse than non-transgenic controls following primary or secondary infection with
T. canis (
Sugane et al., 1996;
Dent et al., 1997b). From the foregoing it can be concluded that although eosinophils would appear not to be necessary for immunity to the gut stages of nematodes, they are very effective against the tissue migratory larval stages of at least some but not all helminth parasites (
Table 2).
Several studies demonstrated that GI nematode infection is almost invariably accompanied by mucosal mast cell (MMC) hyperplasia (
Rothwell, 1989;
Miller, 1996b), which may be concomitant with the immune-mediated elimination of the adult parasite following a primary infection (
Miller, 1996b). Thus, intestinal mast cells have long been considered as possible effector cells at the mucosal level against GI nematodes (
Askenase, 1980;
Bienenstock and Befus, 1980;
Miller, 1984). However, the period of mastocytosis varies depending on the parasite species and experimental animal host, and does not always coincide with the time of worm expulsion (
Lee and Wakelin, 1982;
Nawa et al., 1994;
Ishiwata et al., 1999). In addition, there are instances of ability to expel worms in the absence of mucosal mastocytosis or, conversely, absence of worm expulsion in the presence of marked MMC hyperplasia (
Crowle and Reed, 1981;
Nawa et al., 1994;
Ishiwata et al., 1999). For instance, mast cell-deficient
W/Wv mice, with mutation affecting the tyrosine kinase activity of the stem cell factor (SCF) receptor
c-kit, are still able to expel
N. brasiliensis (
Crowle and Reed, 1981) and, suppression of mucosal mastocytosis by anti-IL-3 and anti-IL-4 mAb treatment during primary
N. brasiliensis infection in mice did not prevent worm expulsion (
Madden et al., 1991). Similarly, treatment of a resistant strain of mice with anti-IL-3 mAb or with anti-
c-kit antibodies did not prevent the expulsion of
T. muris (
Betts and Else, 1999). On the otherhand, STAT6 KO mice develop a strong intestinal mastocytosis during
N. brasiliensis infection but are unable to expel the parasites (
Urban et al., 1998), just as primary
S. ratti and
S. venezuelensis infections persist in Mongolian gerbils and Syrian golden hamsters even when intense mastocytosis was observed (
Horii and Nawa, 1992;
Horii et al., 1993,
Khan et al., 1993a;
Shi et al., 1994a,
1995). In addition, faecal egg output in
N. brasiliensis-infected
Ws/Ws mast cell-deficient rats is reduced when compared with mast cell-sufficient littermates (
Arizono et al., 1993) and, suppression of MMC hyperplasia by anti-SCF antibodies treatment in rats infected with
N. brasiliensis also significantly reduces faecal egg output by the parasites (
Newlands et al., 1995) which together raise the interesting suggestion that mast cells may have some effects that favour the fecundity of
N. brasiliensis.
The above not withstanding, the most compelling evidence for the protective role of MMC against GI nematodes comes from studies of the expulsion kinetics of
Strongyloides spp. and
T. spiralis (
Nawa et al., 1994;
Miller, 1996b). Several workers had in the past associated MMC with the expulsion of
S. ratti (
Ishiwata et al., 1999) which was eventually substantiated by studies using mast cell-deficient
W/Wv mice as host as well as those that employed repetitive administration of IL-3 to infected animals. When
W/Wv mice were infected with either
S. ratti (
Nawa et al., 1985) or the related parasite
S. venezuelensis (
Khan et al., 1993b) adult worm expulsion was delayed, but both accelerated worm expulsion and mucosal mastocytosis were completely restored by bone marrow grafting (
Nawa et al., 1985). However, when bone marrow-derived cultured mast cells which are phenotypically similar to MMC were adoptively transferred to
W/Wv mice, intestinal mastocytosis but not worm expulsion was restored, thus casting a doubt over the role of MMC (
Abe and Nawa, 1987a). This doubt was subsequently cleared by the observation that MMC migrated intraepithelially in both
S. ratti-infected normal and bone marrow reconstituted
W/Wv mice but not in infected
W/Wv mice reconstituted with cultured mast cells (
Abe and Nawa, 1987b). Subsequently, it was clearly demonstrated that repetitive administration of recombinant IL-3 induced intestinal mastocytosis and enabled hypothymic nude mice to expel primary
S. ratti infection (
Abe and Nawa, 1988). Moreover, when normal C57BL/6 mice were also treated with IL-3 prior to oral infection with infective larvae of
S. ratti recovered from the head of infected rats, worm recovery from the intestine was significantly reduced as early as 6 h after which suggests that MMC are equally effective in preventing worm establishment as in the expulsion of established adults in the intestine (
Abe et al., 1993a). In addition,
KitW/KitWv, IL-3 KO mice, which are profoundly mast cell deficient, were unable to expel primary
S. venezuelensis infection when compared with thier
KitW/KitWv, IL-3 sufficient counterparts which expelled all worms by day 18 or 19 after the infection (
Lantz et al., 1998). Mastocytosis also represents a major component of the severe inflammatory response provoked in the small intestine of
T. spiralis-infected mice and there is an evidence that as in
Strongyloides, the expulsion of adult
T. spiralis is delayed in
W/Wv mice (
Alizadeh and Wakelin, 1984). Similarly, antibodies to SCF or
c-kit abrogated MMC response and inhibited the expulsion of a primary
T. spiralis infection (
Grencis et al., 1993;
Donaldson et al., 1996). In addition, when IL-9 transgenic mice which exhibit extremely fast expulsion kinetics of
T. spiralis are treated with anti-
c-kit mAb, worm expulsion is dramatically delayed accompanied by severe depression of intestinal mastocytosis (
Faulkner et al., 1997).
In situations where mast cells appear to play an important role in worm expulsion, the precise mechanism of the mediation was less clear. Results of concurrent infection with
S. ratti and
N. brasiliensis clearly indicated that whereas goblet cells are involved in the expulsion of the latter, but not goblet cells are involved in the expulsion of the former in mice and rats, which suggests that a yet to be defined effector molecule(s) for the expulsion of
Strongyloides could be derived from mast cells only (
Nawa and Korenaga, 1983;
Abe et al., 1992,
1993b). Ironically, the clue to the possible effector molecules in mast cell-mediated adult worm expulsion came from goblet cells in hamsters. Unlike in mice and rats, the expulsion of
S. venezuelensis in Syrian golden hamsters is associated with massive goblet cell hyperplasia and production of large quantities of mucin, and not with mastocytosis which although present do not migrate intraepithelially nor does the peak coincide with the time of expulsion (
Shi et al., 1994a,
1995). Goblet cell mucins in four different species of hamsters were shown to be highly sulphated but the degree of sulphation determined the rapidity of expulsion. Hamsters that have heavily sulphated goblet cell mucins expelled
S. venezuelensis in 2 weeks, whereas those with moderately sulphated mucins expelled in 50 days (
Shi et al., 1994b;
Ishiwata et al., 1999). These studies suggest that highly sulphated mucins may substitute for mast cell derived effector molecules in the expulsion of
Strongyloides in mice and rats. This possibility was strengthened by studies in rats where reserpine-induced sulphated intestinal goblet cell mucin inhibited the establishment of implanted adult
S. venezuelensis (
Ishikawa et al., 1995). High molecular weight proteoglycans in mast cell granules are highly sulphated and based on the above, it is tempting to propose that sulphated proteoglycans released from mast cell granules upon degranulation may be the effector molecules that prevent the establishment of adult
Strongyloides and thus mediate thier expulsion. Our recent studies support this proposal. The deletion of the gene for the γ subunit of Fc receptors results in loss of mast cell functions, including IgE-mediated degranulation and mediator release (
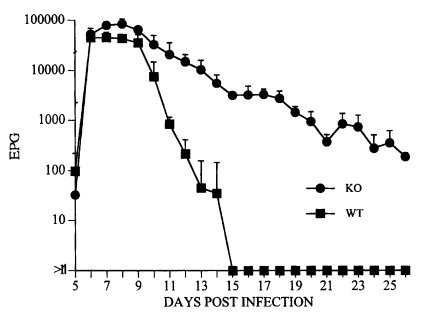
Takai et al., 1994). When we infected FcRγ KO and FcRγ sufficient wild type (FcRγ
+/+) mice with
S. venezuelensis, mastocytosis was similar in both, but whereas FcRγ
+/+ controls expelled nearly all the worms by day 13 post infection, the FcRγ KO mice retained the worms for over 25 days (
Onah et al., 2000) (
Fig. 4). Furthermore, recently it was shown that mast cells contribute to the expulsion of
S. venezuelensis from mice by preventing the invasion of the host intestinal mucosa by the adults since treatment with glycosaminoglycans of the type produced by mast cells, such as chondroitin sulphate-A (ChS-A), ChS-E, heparin and dextran sulphate, inhibited in vivo, the binding of
S. venezuelensis adhesion molecules to mucosal epithelium and thus the invasion of the gut mucosa by the adults (
Maruyama et al., 2000).
Goblet cell hyperplasia is a feature of many GI nematode infections and the protective function is considered to be mediated through its associated increased mucus production which excludes and traps worms in immune animals (
Miller and Nawa, 1979a,b;
Lee and Ogilvie, 1980,
1982;
Miller et al., 1981) and prevents thier establishment by hindering intimate contact with the gut mucosa (
Miller, 1987). Perhaps the strongest evidence of a protective role for goblet cells comes from
N. brasiliensis model studies in rats in which there is a chronological association between goblet cell hyperplasia and worm expulsion (
Nawa et al., 1994). When rats were concurrently infected with
N. brasiliensis and
S. ratti, the two parasites were expelled separately; the former in association with goblet cell hyperplasia and the latter with mastocytosis (
Nawa and Korenaga, 1983). Furthermore, adoptively transferred immune T cells induced both goblet cell hyperplasia and worm expulsion in rats infected with
N. brasiliensis (
Miller and Nawa, 1979a), indicating the involvement of T cells in the process. A major break-through in the elucidation of the role of goblet cells in mucosal defence against
N. brasiliensis came from simple lectin histochemical staining of jejunal sections which showed changes in the terminal sugars of the mucins produced and secreted by the goblet cells (
Koninkx et al., 1988;
Ishikawa et al., 1993;
1994;
Oinuma et al., 1995). Whereas lectins did not bind to goblet cell mucins in uninfected rats, terminal sugars of goblet cell mucins of
N. brasiliensis-infected rats were qualitatively altered and bound strongly to lectins with increase in the number of goblet cells as well as alteration in terminal sugars of goblet cell mucins consistently occurring in association with worm expulsion (
Ishikawa et al., 1993;
Oinuma et al., 1995). However, it was to emerge that the qualitative changes in goblet cell mucins rather than the quantitative change in goblet cells were more important in the final expulsion of adult
N. brasiliensis. When normal (obtained 7 days after primary infection) or immune damaged (obtained 13 days after primary infection) adult
N. brasiliensis were implanted intraduodenally into naive or immune euthymic and naive hypothymic (
rnu/rnu) rats, normal worms established in euthymic rats before being expelled between days 10-14 in association with goblet cell hyperplasia and alteration in terminal sugars of goblet cell mucins whereas the worms were retained in
rnu/rnu rats without significant change in goblet cell responses. In contrast, both strains of rat expelled implanted damaged worms by day 5 but while this occurred in association with goblet cell hyperplasia and alteration in terminal sugars of goblet cell mucins in euthymic rats only the alteration in terminal sugars of goblet cell mucins was observed in
rnu/rnu rats (
Ishikawa et al., 1993;
1994). Furthermore, dexamethasone treatment completely abolished goblet cell changes in both euthymic and hypothymic rats and suppressed the expulsion of implanted damaged worms. When both strains were primed by implantation of damaged worms to induce goblet cell changes, and challenged 3-5 days later by implantation of normal worms they completely prevented the establishment of the normal worms albeit less effectively in the hypothymic strain (
Ishikawa et al., 1994). Apart from
N. brasiliensis, goblet cell hyperplasia is also prominent during primary infections of mice with
T. spiralis (
Garside et al., 1992;
Ishikawa et al., 1997) and
T. muris (
Else and Finkelman, 1998) with a correlation between worm expulsion and peak mucin production observed for the latter parasite.
A substantial number of worms following expulsion from infected hosts are not irreversibly damaged by immune effectors but retain some viability and can reestablish when transferred to a naive host (
Kennedy and Bruce, 1981). Therefore, apart from immune responses, parasites may elicit in the hosts some physiological processes which assist in their eviction from the gut prior to thier irreversible damage by gut immune effectors. For instance, increase in water and electrolyte secretions is a common feature and occurs within days or minutes after primary and secondary infections respectively with nematode parasites (
Castro et al., 1979;
Castro, 1989). This can be interpreted as a host physiological mechanism that assist in parasite eviction (
Vallance and Collins, 1998) since secretory diarrhoea, a consequence of chloride (Cl
-) secretion, may flush the GI tract of its parasites and/or produce a hostile environment to the parasites (
Baird and O'Malley, 1993). Fluid and electrolyte transport is influenced by immunological reactions and, as stated above, is dramatically expressed following challenge. In
T. spiralis-sensitized rats, Cl
- secretion is mediated via an anti-
Trichinella reaction, triggered by IgE and mast cells, which release two preformed Cl
- secretagogues (5-hydroxytryptamine [5-HT] and histamine) and leads to local,
de novo synthesis of a third, prostaglandin E
2 (PGE
2) (
Castro et al., 1987). The amines work largely through enteric nerves to stimulate Cl- secretion transiently within the first 2 min after antigenic challenge, followed by a second phase of secretion mediated by PGE
2 that peaks at about 5 min and wanes over a period of about 20 min (
Castro et al., 1987;
Castro, 1989). Interestingly, the late but not early phase Cl
- secretion could be passively transferred to naive recipient rats with serum containing anti-
Trichinella IgE antibodies (
Harari et al., 1987,
Harari and Castro, 1989) whereas both phases could be passively achieved only when recipient rats were primed by
N. brasiliensis infection to induce MMC hyperplasia prior to immune serum transfer (
Harari and Castro, 1989).
In addition to the role of fluid and electrolyte secretion, there is a convincing evidence of the involvement of the intestinal motor system in host defence against GI nematode infections. It was demonstrated that increased propulsive forces occurred in extrinsically denervated gut segments of
T. spiralis-infected guinea pigs (
Alizadeh et al., 1987). Measurements of the correlate of gut motility (i.e. small bowel myoelectric activity) in conscious rats following challenge
T. spiralis infection suggest that the in vitro changes in the gut fluid propelling behaviour may also occur in vivo (
Palmer and Castro, 1986). The longitudinal muscles of the jejunum of rats infected with
T. spiralis developed increased tension by day 6 p.i. (
Vermillion and Collins, 1988) as well as hyperplasia and hypertrophy of jejunal smooth muscles (
Blennerhassett et al., 1992). The altered muscle contractility was partly attributed to suppression of Na-K ATPase activity in the mucsle (
Khan and Collins, 1993). Since it had been shown previously that the alteration in muscle contractility was attenuated in infected rats treated with corticosteroid (
Marzio et al., 1990) and in infected athymic rats (
Vermillion et al., 1991), it was inferred that the alteration reflected the host's inflammatory response to the infection, rather than a direct effect of the parasite and that the attenuation in athymic rats suggests a T cell dependence and a tangible interface between the immune and motor systems (
Vallance and Collins, 1998). The most convincing evidence for the role of the motor system came from works which compared the intestinal mucsle function in a mouse strain (NIH) which expels
T. spiralis infection quickly (strong responder) with that in a mouse strain (B10.BR) which expels poorly (poor responder). While intestinal mucsle function prior to infection was similar in both strains, the NIH mice developed significantly greater maximum tension (
Vallance et al., 1997) and chronic increase in muscle function (
Barbara et al., 1997) than B10.BR mice following
T. spiralis infection. As mentioned above, the change in muscle contractility appears to be modulated by T cells, especially CD4+ T cells, since CD4- but not CD8-deficient mice showed reduced muscle contractility following infection (
Vallance and Collins, 1998).
CONCLUSION
Experimental studies in rodent model infections have greatly advanced our understanding of the various mucosal immune responses acting against GI nematode parasites. As important as these studies may be it is still worth bearing in mind that experimental studies are stereotypical; being generally conducted in inbred, and more recently, gene-KO and transgenic, animals under controlled environments and defined immunizing and challenge protocols. Thus, both the resulting infections, aspects of the immune resposes measured and the overall conditions of the animals may differ significantly from the situation under natural infections. However, such controlled conditions are necessary in order to achieve repeatability of results which would in turn allow analysis of the precise roles of individual factors and cell populations in the overall protective responses of the host against a particular nematode parasite or group of nematode parasites (
Balic et al., 2000). We have based this review largely on such model system studies involving
T. spiralis,
T. muris,
S. ratti,
S. venezuelensis,
N. brasiliensis and
H. polygyrus whcih have provided most of our current understanding of mucosal immunity against GI nematodes. From these studies it is now evident that multiple effector mechanisms operate against GI nematodes and that the individual importance of each of the mechanism will vary depending on the host, species of parasites and the niche they occupy within the intestinal environment. This notwithstanding, two common features apparent from these studies are that protective immune responses are controlled by CD4+ Th2 lymphocyte responses and that resistance is impaired by CD4+ Th1 type responses. There is little doubt that anti-nematode immunity and the ability to expel nematodes from the gut depend on CD4+ T cells that can make Th2 cytokines and induce gut inflammatory responses, including mast or goblet cell responses and change in gut physiology all of which act in concert to create an environment hostile to worm survival and ultimately the dislogdement of adult worms from their intestinal niche within and expulsion from the host.
ACKNOWLEDGEMENTS
We thank Dr. Kenji Ishiwata for his help in preparing
figure 2. DN Onah is a Postdoctoral Fellow sponsored by the
Japan Society for the Promotion of Science (JSPS).
References
- 1. Abe T, Khan WI, Sugaya H, Ishida K, Yoshimura K. Prolongation of infection time and failure of restoring fecundity of mouse-nonadaptive Nippostrongylus brasiliensis by administration of cyclophosphamide or anti-CD4 antibody in mice. Jpn J Parasitol 1994;43:288-293.
- 2. Abe T, Nawa Y. Reconstitution of mucosal mast cells in W/Wv mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti infection. Parasite Immunol 1987a;9:31-38.
- 3. Abe T, Nawa Y. Localization of mucosal mast cells in W/Wv mice after reconstitution with bone marrow cells or cultured mast cells, and its relation to the protective capacity to Strongyloides infection. Parasite Immunol 1987b;9:477-485.
- 4. Abe T, Nawa Y. Worm expulsion and mucosal mast cell response induced by repetitive IL-3 administration in Strongyloides ratti-infected nude mice. Immunology 1988;63:181-185.
- 5. Abe T, Sugaya H, Ishida K, Khan WI, Tasdemir I, Yoshimura K. Intestinal protection against Strongyloides ratti and mastocytosis induced by administration of interleukin-3 in mice. Immunology 1993a;80:116-121.
- 6. Abe T, Sugaya H, Yoshimura K. Different susceptibility to IL-3-induced protective effects between Strongyloides ratti and Nippostrongylus brasiliensis in C57BL/6 mice. Parasite Immunol 1993b;15:643-645.
- 7. Abe T, Sugaya H, Yoshimura K, Nawa Y. Induction of expulsion of the Strongyloides ratti and retention of Nippostrongylus brasiliensis in athymic nude mice by repetitive administration of recombinant IL-3. Immunology 1992;76:10-14.
- 8. Ahmad A, Wang CH, Bell RG. A role for IgE in intestinal immunity. Expression of rapid expulsion of Trichinella spiralis in rats transfused with IgE and thoracic duct lymphocytes. J Immunol 1991;146:3563-3570.
- 9. Ahmad A, Wang CH, Korenaga M, Bell RG, Adams LS. Synergistic interaction between immune serum and thoracic duct cells in the adoptive transfer of rapid expulsion of Trichinella spiralis in adult rats. Exp Parasitol 1990;71:90-99.
- 10. Alizadeh H, Castro GA, Weems WA. Intrinsic jejunal propulsion in the guinea pig during parasitism with Trichinella spiralis. Gastroenterology 1987;93:784-790.
- 11. Alizadeh H, Wakelin D. The intestinal mast cell response to T. spiralis infection in mast cell deficient W/Wv mice. J Parasitol 1984;70:767-771.
- 12. Appleton JA, Schain LR, McGregor DD. Rapid expulsion of Trichinella spiralis in suckling rats: Mediation by monoclonal antibodies. Immunology 1988;65:487-492.
- 13. Arizono N, Kasugai T, Yamada M, et al. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood 1993;81:2572-2578.
- 14. Artis D, Humphreys NE, Bancroft AJ, Rothwell NJ, Potten CS, Grencis RK. Tumor necrosis factor α is a critical component of interleukin 13-mediated protective T helper cell type 2 responses during helminth infection. J Exp Med 1999;190:953-962.
- 15. Askenasae PW. Immunopathology of parasitic disease: Involvement of basophils and mast cells. Springer Sem Immunopathol 1980;2:417-442.
- 16. Baird AW, O'Malley KE. Epithelial ion transport - possible contribution to parasite expulsion. Parasitol Today 1993;9:141-143.
- 17. Balic A, Bowles VM, Meeusen ENT. The immunobiology of gastrointestinal nematodes in ruminants. Adv Parasitol 2000;45:181-241.
- 18. Bancroft AJ, McKenzie ANJ, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol 1998;160:3453-3461.
- 19. Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology 1997;113:1224-1232.
- 20. Barner M, Mohrs M, Brombacher F, Kopf M. Differences in IL-4Rα-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol 1998;8:669-672.
- 21. Befus AD. In Farthing MJG, Keusch GT, Wakelin D eds, Immune responses: protective immunity, adaptation and pathogenesis. Enteric Infection 2. 1995, 1st ed. London. Chapman and Hall Medical; pp 49-70.
- 22. Behnke JM, Barnard CJ, Wakelin D. Understanding chronic nematode infections: evolutionary considerations, current hypothesis and the way forward. Int J Parasitol 1992;22:860-907.
- 23. Behnke JM, Parish HA. Transfer of immunity to Nematospiroides dubius: co-operation between lymphoid cells and antibodies in mediating worm expulsion. Parasite Immunol 1981;3:249-259.
- 24. Behnke JM, Wahid FN, Grencis RK, Else KJ, Ben-Smith AW, Goyal PK. Immunological relationships during primary infection with Heligmosomoides polygyrus (Nematospiroides dubius): down regulation of specific cytokine secretion (IL-9 and IL-10) correlates with poor mastocytosis and chronic survival of adult worms. Parasite Immunol 1993;15:415-421.
- 25. Bell RG, Appleton JA, Negrao-Correa DA, Adams LS. Rapid expulsion of Trichinella spiralis in adult rats mediated by monoclonal antibodies of distinct IgG isotypes. Immunology 1992;75:520-527.
- 26. Betts CJ, Else KJ. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical to Trichuris muris. Parasite Immunol 1999;21:45-52.
- 27. Bienenstock J, Befus AD. Mucosal immunology. Immunology 1980;41:249-270.
- 28. Blennerhassett MG, Vignjevic P, Vermillion DL, et al. Inflammation causes hyperplasia and hypertrophy in smooth muscle of rat small intestine. Am J Physiol 1992;262:G1041-G1046.
- 29. Boulos LM, Abou Samra LM, Hegazy IH. Studies on T. spiralis and T. pseudospiralis larvae recovered from mice immunized with heterologous Trichinella antigen. J Egypt Soc Parasitol 1993;23:161-170.
- 30. Boulos LM, Ibrahim IR, Negm AY, El-Temsahi MM. Role of anti-Trichinella spiralis antibodies and interferon-gamma in the protection against the enteral phase of experimental trichinosis. J Egypt Soc Parasitol 1998;28:609-620.
- 31. Brigandi RA, Rotman HL, Yutanawiboonchai W, et al. Strongyloides stercoralis: Role of antibody and complement in immunity to the third stage larvae in BALB/cByJ mice. Exp Parasitol 1996;82:279-289.
- 32. Brindley PJ, Dobson C. Specificity of passive serum protection against Nippostrongylus brasiliensis and Nematospiroides dubius in mice. Aust J Exp Biol Med Sci 1983;61:37-45.
- 33. Butterworth AE. Cell-mediated damage to helminths. Adv Parasitol 1984;23:143-235.
- 34. Butterworth AE, Sturrock RF, Houba V, Mahmoud AAF, Sher A, Rees PH. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature 1975;256:727-729.
- 35. Butterworth AE, Sturrock RF, Houba V, Rees PH. Antibody-dependent cell mediated damage to schistosomula in vitro. Nature (Lond.) 1974;252:503-505.
- 36. Castro GA. Immunophysiology of enteric parasitism. Parasitol Today 1989;5:11-19.
- 37. Castro GA, Harari Y, Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol 1987;253:G540-G548.
- 38. Castro GA, Hessel JJ, Whalen G. Altered intestinal fluid movement in response to Trichinella spiralis in immunized rats. Parasite Immunol 1979;1:259-266.
- 39. Cheever AW, Xu Y, Sher A, Macedonia JG. Analysis of egg granuloma formation in Schistosoma japonicum-infected mice treated wtih antibodies to interleukin-5 and gamma interferon. Infect Immun 1991;59:4071-4074.
- 40. Claerebout E, Vercruysse J. The immune response and the evaluation of acquired immunity against gastrointestinal nematodes in cattle: a review. Parasitology 2000;120:S25-S42.
- 41. Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 1989;245:308-310.
- 42. Coles GC. Drug-resistant parasites of sheep: an emerging problem in Britain. Parasitol Today 1998;14:86-88.
- 43. Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol 1997;15:297-322.
- 44. Cooper ES, Bundy DAP. Trichuris is not trivial. Parasitol Today 1988;4:301-306.
- 45. Crowle PK, Reed ND. Rejection of intestinal parasite Nippostrongylus brasiliensis by mast cell deficient W/Wv anaemic mice. Infect Immun 1981;33:54-58.
- 46. Dawkins HJS, Grove DI. Transfer by serum and cells of resistance to infection with Strongyloides ratti in mice. Immunology 1981;43:317-322.
- 47. Dent LA, Daly C, Geddes A, et al. Immune responses of IL5 transgenic mice to parasites and aeroallergens. Mem Inst Oswaldo Cruz 1997b;92(suppl 2):45-54.
- 48. Dent LA, Munro G, Piper KP, et al. Eosinophilic interleukin 5 (IL-5) transgenic mice: eosinophil activity and impaired clearance of Schistosoma mansoni. Parasite Immunol 1997a;19:291-300.
- 49. DeVos T, Daniel G, Dick TA. Trichinella spiralis: Dose dependence and kinetics of the mucosal immune response in mice. Exp Parasitol 1992;75:99-111.
- 50. DiNetta J, Katz F, Campbell WC. Effect of heterologous anti-lymphocyte serum on the spontaneous cure of Trichinella spiralis infections in mice. J Parasitol 1972;58:636-637.
- 51. Donaldson LE, Schmitt E, Huntley JF, Newlands GFJ, Grencis RK. A critical role for the stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol 1996;8:559-567.
- 52. Dugas B, Renauld JC, Petit-Frrere C, et al. Interleukin-9 potentiates the interleukin-4-induced immunoglobulin (IgG, IgM and IgE) production by normal human B lymphocytes. Eur J Immunol 1993;23:1687-1692.
- 53. Else KJ, Finkelman FD. Intestinal nematode parasites, cytokines and effector mechanisms. Int J Parasitol 1998;28:1145-1158.
- 54. Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine mediated regulation of chronic intestinal helminth infection. J Exp Med 1994;179:347-351.
- 55. Else KJ, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. I. Differential cytokine production during acute or chronic infection. Immunology 1991;72:508-513.
- 56. Else KJ, Grencis RK. Antibody independent effector mechanisms in resistance to the intestinal nematode parasite Trichuris muris. Infect Immun 1996;64:2950-2954.
- 57. Else KJ, Hultner L, Grencis RK. Cellular immune responses to the murine nematode parasite Trichuris muris. II. Differential induction of TH-cell subsets in resistant versus susceptible mice. Immunology 1992a;75:232-237.
- 58. Else KJ, Hultner L, Grencis RK. Modulation of cytokine production and response phenotypes in murine trichuriasis. Parasite Immunol 1992b;14:441-449.
- 59. Else KJ, Wakelin D. The effects of H-2 and non-H-2 genes on expulsion of the nematode Trichuris muris from inbred and congenic mice. Parasitology 1988;96:543-550.
- 60. Else KJ, Wakelin D, Wassom DL, Hauda KM. MHC-restricted antibody responses to Trichuris muris excretory/secretory (E/S) antigen. Parasite Immunol 1990;12:509-527.
- 61. Emery DL, McClure SJ, Wagland BM. Production of vaccines against gastrointestinal nematodes of livestock. Immunol Cell Biol 1993;71:463-472.
- 62. Ey PL. Heligmosomoides polygyrus: retarded development and stunting of larvae by antibodies specific for excretory/secretory antigens. Exp Parasitol 1988;65:232-243.
- 63. Faulkner H, Humphreys N, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 is involved in host protective immunity to intestinal nematode infection. Eur J Immunol 1997;27:2536-2540.
- 64. Faulkner H, Renauld JC, Van Snick J, Grencis RK. Interleukin-9 enhances resistance to the intestinal nematode Trichuris muris. Infect Immun 1998;66:3832-3840.
- 65. Finkelman FD, Pearce EJ, Urban JF Jr, Sher A. Regulation and biological function of helminth-induced cytokine responses. Immunol Today 1991;12:62-67.
- 66. Finkelman FD, Shea-Donohue T, Goldhill J, et al. Cytokine regulation of host defence against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol 1997;15:505-533.
- 67. Finkelman FD, Wynn TA, Donaldson DD, Urban JF Jr. The role of IL-13 in helminth-induced inflammation and protective immunity against nematode infections. Cur Opin Immunol 1999;11:420-426.
- 68. Folkard SG, Hogarth PJ, Taylor MJ, Bianco AE. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology 1996;112:323-329.
- 69. Freeman GL Jr, Tominaga A, Takatsu K, Secor WE, Colley DG. Elevated innate peripheral blood eosinophilia fails to augument irradiated cercarial vaccine-induced resistance to Schistosoma mansoni in IL-5 transgenic mice. J Parasitol 1995;81:1010-1011.
- 70. Garside P, Grencis RK, Mowatt AM. T lymphocyte dependent enteropathy in murine Trichinella spiralis infection. Parasite Immunol 1992;14:217-225.
- 71. Gasbarre LC. Effects of gastrointestinal nematode infection on the ruminant immune system. Vet Parasitol 1997;72:327-343.
- 72. Germann T, Gately MK, Schoenhaut DS, et al. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not Th2 cells. Eur J Immunol 1993;23:1762-1770.
- 73. Gleich GJ, Olson GM, Herlich H. The effect of antiserum to eosinophils on susceptibility and acquired immunity of the guinea-pig to Trichostrongylus colubriformis. Immunology 1979;37:873-880.
- 74. Goyal PK, Wakelin D. Vaccination against Trichinella spiralis in mice using antigens from different isolates. Parasitology 1993;107:311-317.
- 75. Gransmuller A, Anteunis A, Venturiello SM, Bruschi F, Binaghi RA. Antibody-dependent in vitro cytotoxicity of newborn Trichinella spiralis larvae: nature of cells involved. Parasite Immunol 1987;9:281-293.
- 76. Grencis RK. T cell and cytokine basis of host variability in response to intestinal nematode infections. Parasitology 1996;112:S31-S37.
- 77. Grencis RK, Crawford C, Pritchard DI, Behnke JM, Wakelin D. Immunization of mice with surface antigens from the muscle larvae of Trichinella spiralis. Parasite Immunol 1986;8:587-596.
- 78. Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol 1993;15:55-59.
- 79. Grencis RK, Riedlinger J, Wakelin D. L3T4-positive T lymphoblasts are responsible for transfer of immunity to Trichinella spiralis in mice. Immunology 1985;56:213-218.
- 80. Grove DI, Mahmoud AAF, Warren KS. Eosinophils and resistance to Trichinella spiralis. J Exp Med 1977;145:755-759.
- 81. Guyatt H. Do intestinal nematodes affect productivity in adulthood? Parasitol Today 2000;16:153-158.
- 82. Harari Y, Castro GA. Simulation of parasite-induced gut hypersensitivity: implications for vaccination. Immunology 1989;66:302-307.
- 83. Harari Y, Russell DA, Castro GA. Anaphylaxis-mediated epithelial Cl- secretion and parasite rejection in rat intestine. J Immunol 1987;138:1250-1255.
- 84. Herndon F, Kayes SG. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J. Immunol 1992;149:3642-3647.
- 85. Hilderson H, Vercruysse J, Claerebout E, De Graaf DC, Fransen J, Berghen P. Interactions between Ostertagia ostertagi and Cooperia oncophora in calves. Vet Parasitol 1995;56:107-119.
- 86. Horii Y, Khan AI, Nawa Y. Persistent infection of Strongyloides venezuelensis and normal expulsion of Nippostrongylus brasiliensis in Mongolian gerbils, Meriones unguiculatus, with reference to the cellular responses in the intestinal mucosa. Parasite Immunol 1993;15:175-179.
- 87. Horii Y, Nawa Y. Peculiar phenotype and localization of mast cells in the jejunum of Mongolian gerbils and their significance to mucosal defence against intestinal helminths. Jpn J Parasitol 1992;41:505-512.
- 88. Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci USA 1992;89:6065-6069.
- 89. Ishikawa N, Horii Y, Nawa Y. Immune-mediated alteration of the terminal sugars of goblet cell mucins in the small intestine of Nippostrongylus brasiliensis-infected rats. Immunology 1993;78:303-307.
- 90. Ishikawa N, Horii Y, Oinuma T, Suganuma T, Nawa Y. Goblet cell mucins as the selective barrier for the intestinal helminths: T-cell independent alteration of goblet cell mucins by immunologically 'damaged' Nippostrongylus brasiliensis worms and its significance on the challenge infection with homologous and heterologous parasites. Immunology 1994;81:480-486.
- 91. Ishikawa N, Shi B-B, Khan AI, Nawa Y. Reserpine-induced sulphomucin production by goblet cells in the jejunum of rats and its significance in the establishment of intestinal helminths. Parasite Immunol 1995;17:581-586.
- 92. Ishikawa N, Wakelin D, Mahida YR. Role of Th2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 1997;113:542-549.
- 93. Ishiwata K, Uchiyama F, Maruyama H, Kobayashi T, Kurokawa M, Nawa Y. In Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR eds, Glycoconjugates and host-parasite relationship in the mucosal defence against intestinal nematodes. Mucosal Immunology. 1999, 2nd. ed. San Diego. Academic Press; pp 925-933.
- 94. Jacobson RH, Reed ND, Manning DD. Expulsion of Nippostrongylus brasiliensis from mice lacking antibody production potential. Immunology 1977;32:867-874.
- 95. James ER, Denham DA. Immunity to Trichinella spiralis VI. The specificity of the immune response stimulated by the intestinal stage. J Helminthol 1975;49:43-47.
- 96. Katona IM, Urban JF Jr, Finkelman FD. The role of L3T4; and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol 1988;140:3206-3211.
- 97. Kazura JW, Grove DI. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature 1978;274:588-589.
- 98. Kelsall B, Strober W. In Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR eds, Gut-associated lymphoid tissue: antigen handling and T-lymphocyte responses. Mucosal Immunology. 1999, 2nd. ed. San Diego. Academic Press; pp 925-933.
- 99. Kennedy MW, Bruce RG. Reversibility of the effects of the host immune response on the intestinal phase of Trichinella spiralis in the mouse, following transplantation to a new host. Parasitology 1981;82:39-48.
- 100. Khan AI, Horii Y, Nawa Y. Defective mucosal immunity and normal systemic immunity of Mongolian gerbils, Meriones unguiculatus, to reinfection with Strongyloides venezuelensis. Parasite Immunol 1993a;15:565-571.
- 101. Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol 1993b;23:551-555.
- 102. Khan I, Collins SM. Altered expression of sodium pump isoforms in the inflamed intestine of Trichinella spiralis-infected rats. Am J Physiol 1993;264:G1160-G1168.
- 103. Khan WI, Abe T, Ishikawa N, Nawa Y, Yoshimura K. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol 1995;17:485-491.
- 104. Knightlinger LK, Seed JR, Knightlinger MB. The epidemiology of Ascaris lumbricoides, Trichuris trichuria, and hookworm in children in the Ranomafana rain forest, Madagascar. J Parasitol 1995;81:159-169.
- 105. Koninkx JF, Mirck MH, Hendriks HG, Mouwen JM, van Dijk JE. Nippostrongylus brasiliensis: histochemical changes in the composition of mucins in goblet cells during infection in rats. Exp Parasitol 1988;65:84-90.
- 106. Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology 1991;72:502-507.
- 107. Korenaga M, Tada I. The role of IL-5 in the immune responses to nematodes in rodents. Parasitol Today 1994;10:234-236.
- 108. Koyama K, Tamauchi H, Ito Y. The role of CD4+ and CD8+ T cells in protective immunity to the murine nematode parasite Trichuris muris. Parasite Immunol 1995;17:161-165.
- 109. Lantz CS, Boesiger J, Song CH, et al. Role of interleukin-3 in mast cell and basophil development and in immunity to parasites. Nature 1998;392:90-93.
- 110. Larsh JE Jr, Weatherly NF, Goulson HT, Chaffee EF. Studies on delayed (cellular) hypersensitivity in mice infected with Trichinella spiralis. VII. The effect of ATS injections on the numbers of adult worms recovered after challenge. J Parasitol 1972;58:1052-1060.
- 111. Lawrence RA, Gray CA, Osbourne J, Maizels R. Nippostrongylus brasiliensis. Cytokine responses and nematode expulsion in normal and IL-4-deficient mice. Exp Parasitol 1996;84:65-73.
- 112. Lee GB, Ogilvie BM eds, In Ogra PL, Bienenstock J eds, The mucus layer in intestinal nematode infections. Mucosal Immune System in Health and Disease. 1980. the 81st Ross Conference on Pediatric Research. Ross Laboratories; Colombus: 175-187.
- 113. Lee GB, Ogilvie BM. In Strober W, Hanson LA, Sell KW eds, The intestinal mucus layer in Trichinella spiralis-infected rats. Recent Advances in Mucosal Immunity. 1982, New York. Raven Press; pp 319-329.
- 114. Lee TD, Wakelin D. The use of host strain variation to assess the significance of mucosal mast cells in the spontaneous cure response of mice to the nematode Trichuris muris. Int Arch Allergy Appl Immunol 1982;67:302-305.
- 115. Lin T, Befus AD. In Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR eds, Mast cells and eosinophils in mucosal defences and pathogenesis. Mucosal Immunology. 1999, 2nd. ed. Academic Press. San Diego; pp 925-933.
- 116. Madden KB, Urban JF Jr, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth induced intestinal mastocytosis. J Immunol 1991;147:1387-1391.
- 117. Mahmoud AF, Warren KS, Peters PA. A role of the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med 1975;142:805-813.
- 118. Maruyama H, Yabu Y, Yoshida A, Nawa Y, Ohta N. A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J Immunol 2000;164:3749-3754.
- 119. Matthaei KI, Foster PS, Young IG. The role of IL-5 in vivo: Studies with IL-5 deficient mice. Mem Inst Oswaldo Cruz 1997;92(Suppl 2):63-68.
- 120. Mayer L. In Targan SR, Shanahan F eds, Antigen presentation in the intestine. Immunology and Immunopathology of the Liver and Gastrointestinal Tract. 1990, New York. Igaku-Shoin; pp 33-48.
- 121. Marzio L, Blennerhassett P, Chiverton S, et al. Altered smooth muscle function in worm-free gut regions of Trichinella-infected rats. Am J Physiol 1990;259:G306-G313.
- 122. Mckay DM, Benjamin M, Baca-Estrada M, D'Inca R, Croitoru K, Perdue MH. Role of T lymphocytes in secretory response to an enteric nematode parasite. Studies in athymic rats. Dig Dis Sci 1995;40:331-337.
- 123. McKenzie GJ, Bancroft A, Grencis RK, McKenzie NJ. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Cur Biol 1998;8:339-342.
- 124. Miller HRP. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Vet Immunol Immunopathol 1984;6:167-259.
- 125. Miller HRP. Prospects for the immunological control of ruminant gastrointestinal nematodes: natural immunity, can it be harnessed? Int J Parasitol 1996a;26:801-811.
- 126. Miller HRP. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol 1996b;54:331-336.
- 127. Miller HRP. Gastrointestinal mucus, a medium for survival and for elimination of parasitic nematodes and protozoa. Parasitology 1987;94:S77-S100.
- 128. Miller HRP, Huntley JF, Wallace GR. Immune exclusion and mucus trapping during the rapid expulsion of Nippostrongylus brasiliensis from primed rats. Immunology 1981;44:419-429.
- 129. Miller HRP, Nawa Y. Nippostrongylus brasiliensis. Intestinal goblet-cell response in adoptively immunized rats. Exp Parasitol 1979a;47:81-90.
- 130. Miller HRP, Nawa Y. Immune regulation of intestinal goblet cell differentiation: Specific induction of nonspecific protection against helminths? Nouv Rev Fr Hematol 1979a;21:31-45.
- 131. Mitchell GF. Metazoan and protozoan parasitic infections in nude mice. Contemp Top Immunobiol 1978;8:55-67.
- 132. Moqbel R, Wakelin D. Immunity to Strongyloides ratti in rats. 1. Adoptive transfer with mesenteric lymph node cells. Parasite Immunol 1981;3:181-189.
- 133. Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145-173.
- 134. Murrell KD. Protective role of immunoglobulin G in immunity to Strongyloides ratti. J Parasitol 1981;67:167-173.
- 135. Nawa Y, Ishikawa N, Tsuchiya K, et al. Selective effector mechanisms for the expulsion of intestinal helminths. Parasite Immunol 1994;16:333-338.
- 136. Nawa Y, Kiyota M, Korenaga M, Kotani M. Defective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol 1985;7:429-438.
- 137. Nawa Y, Korenaga M. Mast and goblet cell responses in the small intestine of rats concurrently infected with Nippostrongylus brasiliensis and Strongyloides ratti. J Parasitol 1983;69:1168-1170.
- 138. Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N. brasiliensis infection. Blood 1995;86:1968-1976.
- 139. Newton SE, Munn EA. The development of vaccines against gastrointestinal nematode parasites, particularly Haemonchus contortus. Parasitol Today 1999;15:116-122.
- 140. Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Robinson BA, Bundy DA. Moderate to heavy infections of Trichuris trichuria affect cognitive function in Jamaican school children. Parasitology 1992;104:539-547.
- 141. Obiri NI, Debinski W, Leonard WJ, Puri RK. Receptor for interleukin13. Interaction with interleukin 4 by a mechanism that does not involve the common γ chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem 1995;270:8797-8804.
- 142. Ogilvie BM, Hockley DJ. Effects of immunity on Nippostrongylus brasiliensis adult worms: reversible and irreversible changes in infectivity, reproduction and morphology. J Parasitol 1968;54:1073-1084.
- 143. Oinuma T, Abe T, Nawa Y, Kawano J, Suganuma T. Glycoconjugates in rat small intestinal mucosa during infection with the intestinal nematode Nippostrongylus brasiliensis. Adv Exp Med Biol 1995;371:975-978.
- 144. Onah DN, Ishiwata K, Nawa Y. In Chowdhury N, Tada I eds, Host responses to helminths with emphasis on eosinophils and mast cells. Helminthology. 2001, 2nd. ed. New Delhi. Oxford and IBH Publishing Co.; pp 243-257 (in press).
- 145. Onah DN, Uchiyama F, Nagakui Y, Ono M, Takai T, Nawa Y. Mucosal defense against gastrointestinal nematodes: Mucosal mast cell and serum mouse mast cell protease 1 responses during primary Strongyloides venezuelensis infection in FcRγ-knockout mice. Infect Immun 2000;68:4968-4971.
- 146. Onah DN, Wakelin D. Trypanosome-induced suppression of responses to Trichinella spiralis in vaccinated mice. Int J Parasitol 1999;29:1017-1026.
- 147. Onah DN, Wakelin D. Murine model study of the practical implication of trypanosome-induced immunosuppression in vaccine-based disease control programmes. Vet Immunol Immunopathol 2000;74:271-284.
- 148. Ovington KS, Behm CA. The enigmatic eosinophil: investigation of the biological role of eosinophils in parasitic helminth infection. Mem Inst Oswaldo Cruz 1997;92(Suppl II):93-104.
- 149. Ovington KS, McKie K, Matthaei KI, Young IG, Behm CA. Regulation of primary Strongyloides ratti infections in mice: a role for interleukin-5. Immunology 1998;95:488-493.
- 150. Owen RL. M cells - Entryways of opportunity for enteropathogens. J Exp Med 1994;180:7-9.
- 151. Owen RL, Jones AL. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 1974;66:189-203.
- 152. Palmer JM, Castro GA. Anamnestic stimulus-specific myoelectric responses associated with intestinal immunity in the rat. Am J Physiol 1986;250:G266-G273.
- 153. Parsons JC, Coffman RL, Grieve RB. Antibody to interleukin 5 prevents blood and tissue eosinophilia but not liver trapping in murine larval toxocariasis. Parasite Immunol 1993;15:501-508.
- 154. Petit-Frrere C, Dugas B, Braquet P, Mencia-Huerta JM. Interleukin-9 potentiates the interleukin-4 induced IgE and IgG1 release from murine B lymphocytes. Immunology 1993;79:146-151.
- 155. Prowse SJ, Mitchell GF, Ey PL, Jenkin CR. Nematospiroides dubius: susceptibility to infection and the development of resistance in hypothymic (nude) BALB/c mice. Aust J Exp Biol Med Sci 1978;56:561-570.
- 156. Richard M, Grencis RK, Humphreys NE, Renauld JC, Van Snick J. Anti-IL-9 vaccination prevents worm expulsion and blood eosinophilia in Trichuris muris-infected mice. Proc Natl Acad Sci USA 2000;97:767-772.
- 157. Roach TI, Else KJ, Wakelin D, McLaren DJ, Grencis RK. Trichuris muris: antigen recognition and transfer of immunity in mice by IgA monoclonal antibodies. Parasite Immunol 1991;13:1-12.
- 158. Robinson K, Bellaby T, Wakelin D. Immunity to Trichinella spiralis transferred by serum from vaccinated mice not protected by immunization. Parasite Immunol 1995;17:85-90.
- 159. Rothwell TLW. Immune expulsion of parasitic nematodes from the alimentary tract. Int J Parasitol 1989;19:139-168.
- 160. Rotman HL, Schnyder-Candrian S, Scott P, Nolan TJ, Schad GA, Abraham D. IL-12 eliminates the Th2-dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol 1997;19:29-39.
- 161. Rotman HL, Yutanawiboonchai W, Brigandi RA, et al. Strongyloides stercoralis: Eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice. Exp Parasitol 1996;82:267-278.
- 162. Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature 1976;264:258-260.
- 163. Ruitenberg EJ, Leenstra F, Elgersma A. Thymus dependence and independence of intestinal pathology in a Trichinella spiralis infection: a study in congenitally athymic (nude) mice. Bri J Exp Pathol 1977;58:311-314.
- 164. Russell GA, Walker WA. In Targan SR, Shanahan F eds, Role of the intestinal mucosal barrier and antigen uptake. Immunology and Immuno-pathology of the Liver and Gastrointestinal Tract. 1990, Igaku-Shoin. New York; pp 15-31.
- 165. Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol 1993;15:349-354.
- 166. Sato Y, Toma H. Effects of spleen cells and serum on transfer of immunity to Strongyloides venezuelensis infection in hypothymic (nude) mice. Int J Parasitol 1990a;20:63-67.
- 167. Sato Y, Toma H. Strongyloides venezuelensis infection in mice. Int J Parasitol 1990b;20:52-62.
- 168. Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interluekin 4 inhibition of such priming. Proc Natl Acad Sci USA 1993;90:10188-10192.
- 169. Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp med 1992;176:1091-1098.
- 170. Sher A, Coffman RL, Hieny S, Cheever AW. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mice. J Immunol 1990;145:3911-3916.
- 171. Shi B-B, Ishikawa N, Itoh H, et al. Goblet cell mucins of four genera of the subfamily Cricetinae with reference to the protective activity against Strongyloides venezuelensis. Parasite Immunol 1994b;16:553-559.
- 172. Shi B-B, Ishikawa N, Itoh H, et al. Goblet cell hyperplasia induced by Strongyloides venezuelensis infection in Syrian golden hamster, Mesocricetus auratus. Int J Parasitol 1995;25:399-402.
- 173. Shi B-B, Ishikawa N, Khan AI, Tsuchiya K, Horii Y, Nawa Y. Strongyloides venezuelensis infection in Syrian golden hamster, Mesocricetus auratus, with reference to the phenotype of intestinal mucosal mast cells. Parasite Immunol 1994a;16:545-551.
- 174. Smerz-Bertling C, Duschl A. Both interleukin 4 and interleukin 13 induce tyrosine phosphorylation of the 140 kDa subunit of the interleukin 4 receptor. J Biol Chem 1995;270:966-970.
- 175. Stear MJ, Bairden K, Duncan JL, et al. How hosts control worms [letter]. Nature 1997;389:27.
- 176. Stear MJ, Bishop SC, Doligalska M, et al. Regulation of egg production, worm burden, worm length and worm fecundity by host responses in sheep infected with Ostertagia circumcincta. Parasite Immunol 1995;17:643-652.
- 177. Sugane K, Kusama Y, Takamoto M, Tominaga A, Takatsu K. Eosinophilia, IL-5 level and recovery of larvae in IL-5 transgenic mice infected with Toxocara canis. J Helminthol 1996;70:153-158.
- 178. Sugaya H, Aoki M, Yoshida T, Takatsu K, Yoshimura K. Eosinophilia and intracranial worm recovery in interleukin-5 transgenic and interleukin-5 receptor α chain-knockout mice infected with Angiostrongylus cantonensis. Parasitol Res 1997;83:583-590.
- 179. Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR γ chain deletion results in pleitropic effector cell defects. Cell 1994;76:519-529.
- 180. Takamoto M, Ovington KS, Behm CA, Sugane K, Young IG, Matthaei KI. Eosinophilia, parasite burden and lung damage in Toxocara canis infection in C57BL/6 mice genetically deficient in IL-5. Immunology 1997;90:511-517.
- 181. Uchikawa R, Nojima H, Sato A. The effects of single and repeated inoculations of various larval doses on Strongyloides ratti burden and distribution in rats. J Parasitol 1989;75:577-584.
- 182. Urban JF Jr, Fang H, Liu Q, et al. IL-13-mediated worm expulsion is B7 independent and IFN-γ sensitive. J Immunol 2000a;164:4250-4256.
- 183. Urban JF Jr, Katona IM, Finkelman FD. Heligmosomoides polygyrus: CD4; but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol 1991a;73:500-511.
- 184. Urban JF Jr, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci USA 1991b;88:5513-5517.
- 185. Urban JF Jr, Maliszewski CR, Madden KB, Katona IM, Finkelman FD. Interleukin-4 treatment can cure established gastrointestinal nematode infections in immunocompetent and immunodeficient mice. J Immunol 1995;154:4675-4684.
- 186. Urban JF Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 1998;8:255-264.
- 187. Urban JF Jr, Schopf L, Morris SC, et al. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol 2000b;164:2046-2052.
- 188. Vallance BA, Blennerhassett PA, Collins SM. Increased intestinal muscle contratility and worm expulsion in nematode-infected mice. Am J Physiol 1997;272:G321-G327.
- 189. Vallance BA, Collins SM. The effect of nematode infection upon intestinal smooth muscle function. Parasite Immunol 1998;20:249-253.
- 190. Vercruysse J, Dorny P. Integrated control of nematode infections in cattle: a reality? A need? A future? Int J Parasitol 1999;29:165-175.
- 191. Vermillion DL, Collins SM. Increased responsiveness of jejunal longitudinal muscle in Trichinella-infected rats. Am J Physiol 1988;254:G124-G129.
- 192. Vermillion DL, Ernst PB, Collins SM. T lymphocyte modulation of intestinal muscle function in Trichinella-infected rat. Gastroenterology 1991;101:31-38.
- 193. Vos JG, Ruitenberg EJ, Baste NV, Buys J, Elgersma A, Kruizinga W. The athymic nude rat. IV. Immunocytochemical study to detect T-cells, and immunological and histopathological reactions against Trichinella spiralis. Parasite Immunol 1983;5:195-215.
- 194. Wahid FN, Behnke JM, Grencis RK, Else KJ, Ben-Smith AW. Immunological relationships during primary infection with Heligmosomoides polygyrus: Th2 cytokines and primary response phenotype. Parasitology 1994;108:461-471.
- 195. Wakelin D. Immune expulsion of Trichuris muris from mice during a primary infection: analysis of the components involved. Parasitology 1975;70:397-405.
- 196. Wakelin D. Immunity to intestinal parasites. Nature 1978;273:617-620.
- 197. Wakelin D. Parasite survival and variability in host immune responsiveness. Mammal Rev 1987;17:135-141.
- 198. Wakelin D, Lloyd ML. Immunity to primary and challenge infections of Trichinella spiralis in mice: a re-examination of conventional parameters. Parasitology 1976;72:173-182.
- 199. Wakelin D, Wilson MM. Transfer of immunity to Trichinella spiralis in the mouse with mesenteric lymph node cells: time of appearance of effective cells in donors and expression of immunity in recipients. Parasitology 1977;74:215-224.
- 200. Walls RG, Carter RL, Leuchars E, Davies AJS. The immunopathology of trichiniasis in T-cell deficient mice. Clin Exp Immunol 1973;13:231-242.
- 201. Wang CH, Bell RG. Antibody-mediated in vivo cytotoxicity to Trichinella spiralis newborn larvae in immune rats. Parasite Immunol 1988;10:293-308.
- 202. Wang CH, Korenaga M, Greenwood A, Bell RG. T helper subset function in the gut of rats: Differential stimulation of eosinophils, mucosal mast cells and antibody forming cells by OX8- OX22-+ and OX8- OX22-. Immunology 1990;71:166-175.
- 203. Watanabe K, Noda K, Hamano S, et al. The crucial role of granulocytes in the early host defense against Strongyloides ratti in mice. Parasitol Res 2000;86:188-193.
- 204. Wescott RB, Todd AC. Adaptation of Nippostrongylus brasiliensis to the mouse. J Parasitol 1966;52:233-236.
- 205. Williams DJ, Behnke JM. Host protective antibodies and serum immunoglobulin isotypes in mice chronically infected or repeatedly immunized with the nematode parasite Nematospiroides dubius. Immunology 1983;48:37-47.
- 206. Yoshida T, Ikuta K, Sugaya H, et al. Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R alpha-deficient mice. Immunity 1996;4:483-494.
- 207. Zurawski SM, Chomarat P, Djossou O, et al. The primary binding subunit of the human interleukin-4 receptor is also a component of the interleukin-13 receptor. J Biol Chem 1995;270:13869-13878.
- 208. Zurawski SM, Vega F Jr, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J 1993;12:2663-2670.
Fig. 1A schematic representation of the interaction between GI nematode infections and under/malnutrition in the genesis of childhood anaemia and the effects on growth, cognitive ability and the ultimate decreased productivity in adulthood (
Adapted and modified with permission from Guyatt, 2000).
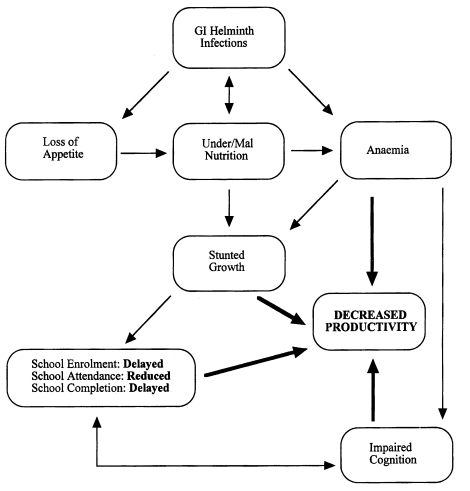
Fig. 2Antigen processing and presentation in the gut mucosa. Possible roles of Peyer's patch (PP) dendritic cells (DC) in the processing of luminal antigens which gain access to the PP across M cells located in the follicle-associated epithelium (FAE). Immature DC in the subepithelial dome, SED (1) acquire antigens, such as microbes via phagocytosis, and soluble antigens via pinocytosis. As these DC differentiate during movement to the interfollicular region (IFR), acquired antigens are processed and peptides are expressed in association with MHC class I and II antigens. In addition, adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and costimulatory molecules, such as B7-1 (CD80), B7-2 (CD86), and CD40, are upregulated, and the differentiation antigens M342 and NLDC-145 are expressed at high levels. In the IFR (2) they stimulate resident CD4
+ and CD8
+ T cells that have gained entry into the PP across high endothelial venules (HEV) located in the IFR, or these DC move into draining lymphatics, where they traffic to the mesenteric lymph nodes (MLN). A second possibility is that less differentiated DC in the SED process and present antigens to CD4
+ T cells at this site (3) or after migration into the follicle (4), resulting in the induction of T cells with a phenotype that is unique to the PP, such as one producing transforming growth factor-β (TGF-β) and/or IL-10. In the follicule, such T cells would be ideally positioned to provide help for switching to IgA, a process that is then completed in the germinal centre. Following IgA switch and affinity maturation, B cells rapidly migrate from the PP to the MLN via efferent lymphatics, and finally to the lamina propria where they undergo terminal differentiation into plasma cells. It is however, not clear whether these possibilities are also applicable to the processing and presentation of nematode antigens in the gut mucosa (
Adapted with permission from Kelsall and Strober, 1999).
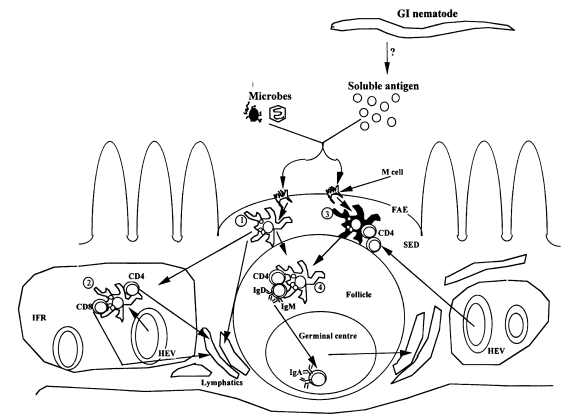
Fig. 3Schematic representation of IL-4 and IL-13 receptors. Anti-IL-4 mAb treatment blocks both IL-4 and IL-13 because both cytokines share (bind to) the type 2 IL-4R and activate STAT6 through this receptor. Thus, in the absence of IL-4, IL-13 is able to mediate worm expulsion via the IL-4Rα chain (
Adapted and modified with permission from Finkelman et al., 1999).
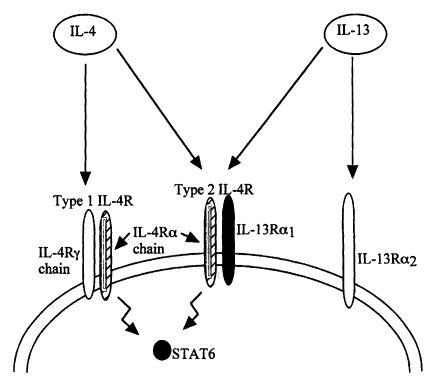
Fig. 4Daily faecal egg out (EPG) from wild type and FcRγ KO C57BL/6 mice infected subcutaneously with 3000 infective third stage larvae of
S. venezuelensis. The
figure 1 on the x-axis represents zero EPG.

Table 1.Summary of the outcome of various forms of cytokine manipulation in mice infected with GI nematode parasites
Table 1.
|
Effects on expulsion of |
|
Cytokine manipulation |
H.p |
N.b |
S.r/Sv |
T. m |
T. s |
|
IL-3 |
N.D |
N.D |
Enhanced |
N.D |
N.D |
|
IL-4C |
Enhanced |
N.D |
N.D |
Enhanced |
N.D |
|
Anti-IL-4 mAb |
Blocked |
None |
N.D |
N.D |
N.D |
|
Anti-IL-4R mAb |
Blocked |
N.D |
N.D |
Blocked |
N.D |
|
IL-4 KO |
Blocked |
None |
N.D |
Impaired |
None |
|
IL-4 KO/anti-TNF-α
|
N.D |
N.D |
N.D |
Blocked |
N.D |
|
IL-4 KO/IFN-γ KO |
N.D |
N.D |
N.D |
Enhanced |
None |
|
IL-4 KO/IL-13 Agt. |
N.D |
Blocked |
N.D |
Blocked |
Blocked |
|
IL-4 KO/IFN-γ KO/IL-13 Agt. |
N.D |
N.D |
N.D |
Blocked |
Blocked |
|
IL-13 KO |
N.D |
Delayed |
N.D |
Blocked |
None |
|
IL-13 Agt |
N.D |
Delayed |
N.D |
Blocked |
None |
Table 2.Summary of the protective role of eosinophils in helminth infections as determined by antibody treatment or manipulation of IL-5 and IL-5R
Table 2.
|
Parasite |
Stage assayed |
Infection |
Treatment |
Protection |
|
S. mansoni
|
Larvae |
1ory & 2ndry |
AES |
No |
|
Adult |
2ndry |
〃 |
Yes |
|
T. spiralis
|
Lavae |
1ory |
〃 |
Yes |
|
Adult |
1ory |
〃 |
No |
|
T. colubriformis
|
Adult |
1ory & 2ndry |
〃 |
Yes |
|
A. cantonensis
|
Larvae |
1ory & 2ndry |
IL-5 or IL-5R mAb |
Yes |
|
S. venezuelensis
|
Larvae |
2ndry |
〃 |
Yes |
|
Adult |
1ory |
〃 |
No |
|
O. lienalis
|
Larvae |
1ory |
〃 |
Yes |
|
T. spiralis
|
Larvae |
1ory & 2ndry |
〃 |
No |
|
T. canis
|
Larvae |
1ory & 2ndry |
〃 |
No |
|
S. mansoni
|
Adult |
1ory |
〃 |
No |
|
S. japonicum
|
Adult |
1ory |
〃 |
No |
|
H. polygyrus
|
Adult |
1ory |
〃 |
No |
|
N. brasiliensis
|
Adult |
1ory |
〃 |
No |
|
T. muris
|
Adult |
1ory |
〃 |
No |
|
A. cantonensis
|
Larvae |
1ory |
IL-5 or IL-5Ra KO |
Yes |
|
T. canis
|
Larvae |
1ory |
〃 |
No |
|
H. polygyrus
|
Adult |
1ory |
〃 |
Yes |
|
S. ratti
|
Adult |
1ory |
〃 |
Yes |
|
T. spiralis
|
Adult |
1ory & 2ndry |
〃 |
Yes |
|
A. cantonensis
|
Larvae |
1ory |
IL-5 Transgenic |
Yes |
|
N. brasiliensis
|
Adult |
1ory |
〃 |
Yes |
|
Adult |
2ndry |
〃 |
Comparable |
|
S. mansoni
|
Larvae |
1ory & 2ndry |
〃 |
No |
|
T. spiralis
|
Larvae |
1ory & 2ndry |
〃 |
No |
|
T. canis
|
Larvae |
1ory & 2ndry |
〃 |
Comparable |