Abstract
We investigated the seasonality of Anopheles mosquitoes, including its species composition, density, parity, and population densities of mosquitoes infected with the parasite in Ganghwa-do (Island), a vivax malaria endemic area in the Republic of Korea. Mosquitoes were collected periodically with a dry-ice-tent trap and a blacklight trap during the mosquito season (April-October) in 2008. Anopheles sinensis (94.9%) was the most abundant species collected, followed by Anopheles belenrae (3.8%), Anopheles pullus (1.2%), and Anopheles lesteri (0.1%). Hibernating Anopheles mosquitoes were also collected from December 2007 to March 2008. An. pullus (72.1%) was the most frequently collected, followed by An. sinensis (18.4%) and An. belenrae (9.5%). The composition of Anopheles species differed between the mosquito season and hibernation seasons. The parous rate fluctuated from 0% to 92.9%, and the highest rate was recorded on 10 September 2008. Sporozoite infections were detected by PCR in the head and thorax of female Anopheles mosquitoes. The annual sporozoite rate of mosquitoes was 0.11% (2 of 1,845 mosquitoes). The 2 mosquitoes that tested positive for sporozoites were An. sinensis. Malarial infections in anopheline mosquitoes from a population pool were also tried irrespective of the mosquito species. Nine of 2,331 pools of Anopheles mosquitoes were positive. From our study, it can be concluded that An. sinensis, which was the predominant vector species and confirmed as sporozoite-infected, plays an important role in malaria transmission in Ganghwa-do.
-
Key words: Anopheles mosquito, Plasmodium vivax, sporozoite rate, parous rate, PCR, Ganghwa-do
INTRODUCTION
Malaria is a vector-borne infectious disease caused by several species of protozoan parasites. During a blood meal, a malaria-infected female
Anopheles mosquito inoculates sporozoites into the human host. Only vivax malaria is endemic to the Republic of Korea (ROK). The outbreak area is confined to the northern part of Gyeonggi-do (province), Gangwon-do (province), and Incheon city (including Ganghwa-do), near the demilitarized zone (DMZ; the border between ROK and the Democratic People's Republic of Korea, DPRK). Ganghwa-do (Island) has the highest reported incidence of malaria among these areas [
1].
Eight species of
Anopheles mosquitoes are found in ROK;
Anopheles sinensis Wiedemann
*,
An. pullus M. Yamada
*,
An. lesteri Baisas and Hu
*,
An. kleini Rueda
*,
An. belenrae Rueda,
An. sineroides Yamada,
An. koreicus Yamada and Watanabe, and
An. lindesayi japonicus Yamada [
2-
4]. The 4
Anopheles species with an astrix (
*) have been confirmed as vectors of vivax malaria in KOK [
4-
6]. Among these species,
An. sinensis is the most widespread, whereas the others are variably distributed in each area [
7]. Factors that affect the
Anopheles species' ability to transmit malaria include its innate susceptibility to
Plasmodium, its host choice, and the longevity. There are also variations in these parameters within a same mosquito species [
8].
Malaria transmission ultimately depends upon the sporozoite positive rate of vector mosquitoes. Therefore, determining the presence of sporozoites in
Anopheles mosquitoes caught in the wild is an important factor in epidemiological studies in malaria endemic areas [
9]. The standard method for detecting sporozoites in mosquito salivary glands is based on light microscopy. However, the salivary gland dissection is impractical when large numbers of mosquitoes are to be processed. In recent years, the PCR method has been used to amplify specific DNA sequences of
Plasmodium for detection of parasites in mosquitoes [
10,
11].
Despite efforts of previous studies on malaria in ROK, our knowledge of the vector systems and their contribution to malaria transmission remains incomplete. More information on the vector systems will help the study of malaria epidemiology and benefit the vector control programs. In the present study, in view of the seasonal incidence of malaria in ROK, we investigated a survey on the seasonal composition, density, longevity, and parasite infection rate of Anopheles species, to determine the sporozoite rates and to confirm the main vector in Ganghwa-do.
MATERIALS AND METHODS
Study sites and mosquito collection
The study was conducted in Ganghwa-do, where the incidence of malaria was 1.2 cases/1,000 persons per year during the study period of 2008 [
1]. Ganghwa-do has a total population of 65,510 and is consisted with an area of 411.189 km
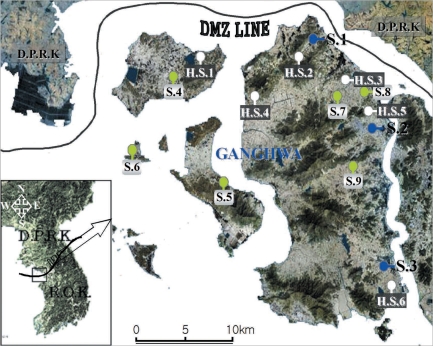
2. It is located at the west end of the central Korean peninsula (37°-31'-37°45'N, 125°33'-126°2'W), and the main Ganghwa-do Island shares a border with Yeonbaek-gun and Gaepung-gun of DPRK. Mosquitoes were collected from April to October 2008 at 9 study sites.
Mosquitoes were periodically collected at 3 study sites, i.e., Gyosan-ri (Yangsa-myeon, S.1), Namsan-ri (Ganghwa-eup, S.2), and Chogi-ri (Gilsang-myeon, S.3), using a dry-ice-tent trap and a blacklight trap (Nozawa type; Shinyoung Korea Co., Seoul, Korea). The traps were placed around the villages, and collections began at 19 : 00 and ended at 06 : 00 the next morning. The captured mosquitoes were transported to the laboratory within 2 hr and stored at -18℃ until processing. The female anopheline mosquitoes were determined parity, identified, and individually detected parasites.
Mosquitoes were collected at another 6 study sites, i.e., Daeryong-ri (Gyodong-myeon, S.4), Seokmo-ri (Samsan-myeon, S.5), Seogeom-ri (Samsan-myeon, S.6), Sungroe-ri (Songhae-myeon, S.7), Daesan-ri (Ganghwa-eup, S.8), and Geumwol-ri (Seonwon-myeon, S.9), using a blacklight trap. Mosquito collection at these sites has been conducted by the Korea Centers for Disease Control and Prevention (KCDC) during a research for National Vector Control and Surveillance. The traps were set every night throughout the study period (30 weeks). At each of the 6 study sites, the dried bodies of the captured mosquitoes were transported weekly to the laboratory. The female anopheline mosquitoes were counted and detected parasites in pools because of the large numbers collected.
Parous rate, probability of daily survival, and P11
At each of the collection times, the anopheline mosquitoes collected at the 3 study sites (S.1-S.3) were dissected to determine their parity according to the method of World Health Organization (WHO) [
12]. Mosquitoes stored at -18℃ were dissected within 5 days to avoid freeze-drying. The probability of daily survival was calculated as the cube root of the proportion of parous females in the population sample, because the gonotrophic cycle takes 3 days in Korea [
13]. P
11 was calculated as the 11th root of the proportion of parous females in the population sample, because
Plasmodium vivax requires 10.7 days to mature and to migrate to the salivary gland at 25℃ [
14].
Anopheline mosquitoes were identified to the species level using a PCR method with multiplex primers. The genomic DNA was extracted from single legs of individual mosquitoes [
2,
4], according to the manufacturer's instructions of DNAzol (Invitrogen, USA).
At each of the 3 study sites (S.1-S.3), DNA was extracted from individual mosquitoes after the wings, legs, and abdomens were removed. The head and thorax were placed in individual 1.5 ml microcentrifuge tubes (Axygen, USA) containing 100 µl of DNAzol.
At each of the 6 study sites (S.4-S.9), DNA was extracted from pooled mosquitoes.
Anopheles mosquitoes were grouped into pools of up to 10-20 in number, irrespective of the species. To each pool was added 500 µl of DNAzol in 1.5 ml tubes. The tissues, prepared as described above, were ground with a pellet pestle (Kimble/Kontes, USA). To overcome PCR inhibitors in the mosquito extracts [
15,
16], we added 500 µl of sterile water and incubated the samples at 37℃ overnight [
17]. DNA extraction was performed according to the manufacturer's instructions of DNAzol. The eluted DNA was stored at 4℃ until required.
Nested PCR was used to detect the presence of malarial parasites in the mosquitoes with
P. vivax species-specific small subunit (SSU) rRNA primers [
18]. The reaction mixture included the Maxime PCR premix kit (iNtRON Biotechnology, Seoul, Korea), to which was added the template, 1 µl of each primer (20 pmol/µl), and distilled water to a total volume of 20 µl. The reactions were subjected to 30 cycles (nest-1) of the following conditions: predenaturation at 94℃ for 5 min, denaturation at 94℃ for 1 min, annealing at 58℃ for 2 min, and extension at 72℃ for 2 min, with a final extension at 72℃ for 5 min. Nest-2 was the same as nest-1, except that 38 cycles were performed and the annealing temperature was 50℃. The DNA of
P. vivax from clinical patients was used as the positive control and sterile water was used as the negative control.
Hibernating mosquitoes were collected from December 2007 to March 2008 at 6 study sites, i.e., Gogu-ri (Gyodong-myeon, H.S.1), Gyosan-ri (Yangsa-myeon, H.S.2), Sangdo-ri (Songhae-myeon, H.S.3), Changhu-ri (Hajeom-myeon, H.S.4), Daesan-ri (Ganghwa-eup, H.S.5), Chogi-ri (Gilsang-myeon, H.S.6), using a sunlit tent trap, which covered the dried heavy grasses on the bank around a rice field (
Fig. 1). When the temperature inside the tent was increased by sunlight and a portable heater, the hibernating mosquitoes flew out from grasses in a few minutes. The mosquitoes, collected using a mouth aspirator, were placed in screen-topped pint cartons, provided with a 5% sugar solution on cotton wool, and transported to the laboratory. The mosquitoes were stored at -18℃ until they were processed for DNA extraction.
RESULTS
Mosquito species and seasonal prevalence
Mosquitoes collected periodically from April to October at 3 study sites (S.1-S.3) in Ganghwa-do showed seasonal prevalence of the
Anopheles species (
Table 1). The density of anopheline mosquitoes was high in July, August, and early September.
Anopheles sinensis (94.9%) was the most abundant species, followed by
An. belenrae (3.8%),
An. pullus (1.2%), and
An. lesteri (0.1%). The nocturnal activities of the mosquitoes were confirmed between April and October (
Table 2).
Details of the malarial infection rates of the mosquitoes are shown in
Tables 1 and
2. We analyzed 1,845 individual
Anopheles mosquitoes collected at sites S.1-S.3. In total, 2
Anopheles mosquitoes were found to be infected. Therefore, the annual sporozoite rate was 0.11% in Ganghwa-do. The infected mosquitoes were collected on July 8 in Gyosan-ri (S.1) and on September 10 in Namsan-ri (S.2). The
Anopheles mosquitoes positive for the malarial parasite were
An. sinensis.
At sites S.4-S.9, a total of 29,346 Anopheles mosquitoes were captured. Nine of the 2,331 pools of mosquitoes examined were positive for malarial parasites. Of the 9 pools of malaria-positive mosquitoes, 1 was collected in the period 4-10 July, 1 in the period 25-31 July, 4 in the period 4-8 August, 2 in the period 22-28 August, and 1 in the period 26 September to 2 October. The total 11 samples that were positive on PCR were confirmed by DNA sequencing. The sequencing results confirmed that the amplified fragments were identical to the SSU rDNA fragment of P. vivax.
Parous rate, probability of daily survival, and P11
The parous rate was fluctuated from 0.0% to 92.9%, and the highest rate was recorded on 10 September. The probability of daily survival and P
11 are shown in
Table 3. The expectancy of an infective life through 11 days was 0.766 on 10 September, which means that 76.6% of the female mosquitoes may have lived long enough to transmit malaria.
Of the 662 hibernating
Anopheles mosquitoes collected,
An. pullus (72.1%) was the most frequently collected, followed by
An. sinensis (18.4%) and
An. belenrae (9.5%) (
Table 4). These results differ from the distribution of
Anopheles mosquitoes during the mosquito season (April-October).
DISCUSSION
Knowledge of mosquito bionomics, including their seasonal distributions and population densities, is a prerequisite for a comprehensive understanding of mosquito-borne diseases. However, few studies of the malaria vector have been undertaken in the Ganghwa-do region. Eight species of
Anopheles mosquitoes are found in ROK. Among them, 2 new species of
Anopheles mosquitoes have been recently identified (
An. kleini and
An. belenrae) [
3], while
An. yatsushiroensis was synonymized with
An. pullus, and
An. anthropophagus Baisas and Hu, considered to be a primary malaria vector in China, was synonymized with
An. lesteri [
4]. Therefore, before 2005, there was much confusion concerning the vector status of these species, which can be identified by PCR techniques, but not based on morphology.
In our study, mosquito collection was conducted during the mosquito season (April-October) and the hibernation season (December-March) to investigate the seasonal distributions of malaria vectors. The density of
Anopheles mosquitoes was high in July, August, and early September.
An. sinensis (94.9%) was the most abundant
Anopheles species collected.
An. belenrae was consistently collected as a small proportion of the mosquitoes and
An. pullus was collected for a limited period in June, September, and October, at a relatively low temperatures.
An. kleini is particularly known for its distribution near the DMZ [
6,
7], but was not indentified in Ganghwa-do. Although the 4 species described above are known to be collectable with a light trap and dry-ice trap [
6,
7], further studies are required to confirm the collection of species by other methods. In the hibernation season,
An. pullus (72.1%) was the most frequently collected species, followed by
An. sinensis (18.4%) and
An. belenrae (9.5%). We confirmed that the composition of
Anopheles species showed marked difference between seasons. These data suggest that
An. sinensis is sensitive to low temperatures compared with the other species, and that
An. pullus is resistant to low temperatures.
Any mosquito that lives for less than the duration of the sporogonic cycle will be unable to transmit infection through sporozoite production. Therefore, mosquito longevity is an important factor in malarial transmission and can be estimated from the parous rate of the mosquito. The parous rates of Anopheles mosquitoes in Ganghwa-do fluctuated from 12.5% to 92.9% between June and October. It is noteworthy that there was a high parous rate time with no reduction in the population and thereafter the population of mosquitoes decreased dynamically. This finding indicates that older populations of mosquitoes tend to accumulate with time and that relatively few new adults emerge during this period. This also shows that the environmental conditions for mosquito survivorship deteriorated suddenly at this time.
PCR was used to identify sporozoite-infected mosquitoes because false positive results have been recorded with ELISA [
19,
20]. Vythilingam et al. [
21] reported that 20 pools were positive for
P. vivax according to PCR, whereas only 7 were positive on ELISA. Two pools were positive for
P. falciparum by both ELISA and PCR [
21]. Most malaria prevalence areas were difficult to determine because the sporozoite rate was exceedingly low. Therefore, the highly sensitive and specific PCR is considered the best method for determining the exact sporozoite rate.
In areas of Africa with high malarial transmission, the sporozoite rate generally ranges from 1% to 20% when evaluated by dissection of salivary glands [
22]. The sporozoite rate in Bangladesh was determined to be 2.8% using PCR [
23]. In ROK, it was 0.09% in Paju-shi in 2000 [
24] and 4% in northern Gyeonggi-do (province) in 2005 [
6]. However, both these studies detected the malarial parasite by ELISA. In our study, 2 malaria-positive
Anopheles mosquitoes were detected among 1,845 mosquitoes by PCR, indicating an annual sporozoite rate in Ganghwa-do of 0.11%. The infected mosquitoes were
An. sinensis. Because the malarial parasite DNA was extracted from the head and thorax and not from the whole mosquito, we confirmed that all the positive
Anopheles mosquitoes had infective sporozoites.
At the 6 study sites S.4-S.9, we examined malarial parasite infections in population pools of mosquitoes. We also determined the relationships between parasite infection rates in the mosquitoes and the incidence of malaria in patients. Malaria-positive mosquitoes were detected at 4 study sites. These sites had higher incidences of malaria than those of the other areas in Ganghwa-do [
1]. Mosquito collection has also been performed annually at these study sites by KCDC. Therefore, a program of continuous monitoring should confirm the annual variations in parasite infection rates in the mosquito.
Lee et al. [
6] reported that the infection rate of
Anopheles species was 9.2% for
An. pullus, 3.2% for
An. sinensis, and 1.8% for
An. kleini in northern Gyeonggi-do (province) in 2005, using ELISA [
6]. However, in our study, we found the malarial parasite only in
An. sinensis, which may be attributable to the low abundance of the other species in Ganghwa-do. Although the difference in the innate susceptibility of the different
Anopheles species is not well known, our results indicate that the infection rate in
An. pullus was 2.9-fold higher than that in
An. sinensis in the field. In Korea, the malarial life cycle is suspended in the mosquito's hibernation season and then resumes in the mosquito season. Therefore, the high density of
An. pullus in the hibernation season may promote the resumption of the malarial life cycle. To confirm these propositions, further studies are required to investigate the vectorial capacity of
Anopheles species, including their host preferences and innate susceptibility to
P. vivax infection.
Because the high density and sporozoite infection of An. sinensis were confirmed, we consider that An. sinensis is the main vector of P. vivax in Ganghwa-do. Our results constitute the first report of the sporozoite rate calculated in individual Anopheles mosquitoes in ROK using PCR. Our study should also extend our understanding of the prevalence of malaria, and benefit vector control in Ganghwa-do.
ACKNOWLEDGEMENTS
We thank the staff of the Public Health Centers of Ganghwa-do for their efforts in mosquito collection. We also appreciate the efforts of Yeon Ja Go, Gyeong Ae Kim, Gyu Cheol Lee, Ui Hwa Hwang, and Seong Hui Gwon at the Incheon Research Institute for Public Health and the Environment. This work was funded by the National Vector Control and Surveillance, the Construction of a Regional Diagnostic System, and the Incheon Research Institute for Public Health and the Environment.
References
- 1. Disease web statistics system. K-CDC. [http://stat.cdc.go.kr/index.aspx]
- 2. Li C, Lee JS, Groebner JL, Kim HC, Klein TA, Oj'Guinn ML, Wilkerson RC. A newly recognized species in the Anopheles hyrcanus group and molecular identification of related species from the Republic of (South) Korea (Diptera: Culicidae). Zootaxa 2005;939:1-8.
- 3. Rueda LM. Two new species of Anopheles (Anopheles) hyrcanus group (Diptera: Culicidae) from the Republic of (South) Korea. Zootaxa 2005;941:1-26.
- 4. Wilkerson RC, Li C, Rueda LM, Kim HC, Klein TA, Song GH, Strickman D. Molecular confirmation of Anopheles (Anopheles) lesteri from the Republic of (South) Korea and its genetic identity with An. (Ano.) anthropophagus from China (Diptera: Culicidae). Zootaxa 2003;378:1-14.
- 5. Shin EH, Kim DS, Lee HW, Lee JS, Lee WJ. Vector competence of Anopheles lesteri Baisas and Hu (Diptera: Culicidae) to Plasmodium vivax in Korea. Korean J Parasitol 2002;40:41-44.
- 6. Lee WJ, Klein TA, Kim HC, Choi YM, Yoon SH, Chang KS, Chong ST, Lee IY, Jones JW, Jacobs JS, Sattabongkot J, Park JS. Anopheles kleini, Anopheles pullus, and Anopheles sinensis: potential vectors of Plasmodium vivax in the Republic of Korea. J Med Entomol 2007;44:1086-1090.
- 7. Kim HC, Klein TA, Lee WJ, Collier BW, Chong ST, Sames WJ, Lee IY, Lee YJ, Lee DK. Mosquito species distribution and larval breeding habitats with taxonomic identification of anopheline mosquitoes in Korea. Entomol Res 2007;37:29-35.
- 8. MacDonald G. The analysis of the sporozoite rate. Trop Dis Bull 1952;49:569-586.
- 9. Mahapatra N, Marai NS, Ranjit MR, Parida SK, Hansdah DP, Hazra RK, Kar SK. Detection of Plasmodium falciparum infection in anopheles mosquitoes from Keonjhar district, Orissa, India. J Vector Borne Dis 2006;43:191-194.
- 10. Moreno M, Cano J, Nzambo S, Bobuakasi L, Buatiche JN, Ondo M, Micha F, Benito A. Malaria panel asay versus PCR: detection of naturally infected Anopheles melas in a coastal village of Equatorial Guinea. Malar J 2004;3:20.
- 11. Tassanakajon A, Boonsaeng V, Wilairatana P, Panyim S. Polymerase chain reaction detection of Plasmodium falciparum in mosquitoes. Trans R Soc Trop Med Hyg 1993;87:273-275.
- 12. World Health Organization. Entomological laboratory techniques for malaria control. Part 1: learner's guide. 1975, Geneva, Switzerland. WHO.
- 13. Ree HI, Hwang UW, Lee IY, Kim TE. Daily survival and human blood index of Anopheles sinensis, the vector species of malaria in Korea. J Am Mosq Control Assoc 2001;17:67-72.
- 14. World Health Organization. Manual on Practical Entomology in Malaria. Part I: vector bionomics and organization of anti-malaria activities. 1975, Geneva, Switzerland. WHO, Division of Malaria and Other Parasitic Diseases.
- 15. Harada M, Ishikawa H, Matsuoka H, Ishii A, Suguri S. Estimation of the sporozoite rate of malaria vectors using the polymerase chain reaction and a mathematical model. Acta Med Okayama 2000;54:165-171.
- 16. Ranford-Cartwright LC, Balfe P, Carter R, Walliker D. Genetic hybrids of Plasmodium falciparum identified by amplification of genomic DNA from single oocysts. Mol Biochem Parasitol 1991;49:239-243.
- 17. Li F, Niu C, Ye B. Nested polymerase chain reaction in detection of Plasmodium vivax sporozoites in mosquitoes. Chin Med J 2001;114:654-657.
- 18. Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol 1993;58:283-292.
- 19. Wirtz RA, Burkot TR, Andre RG, Rosenberg R, Collins WE, Roberts DR. Identification of Plasmodium vivax sporozoites in mosquitoes using an enzyme-linked immunosorbent assay. Am J Trop Med Hyg 1985;34:1048-1054.
- 20. Ryan JR, Day K, Emmerich E, Garcia L, Yi L, Coleman RE, Sattabongkot J, Dunton RF, Chan AS, Wirtz RA. Dipsticks for rapid detection of Plasmodium in vectoring Anopheles mosquitoes. Med Vet Entomol 2001;15:225-230.
- 21. Vythilingam I, Nitiavathy K, Yi P, Bakotee B, Hugo B, Singh B, Wirtz RA, Palmer K. A highly sensitive, nested polymerase chain reaction based method using simple DNA extraction to detect malaria sporozoites in mosquitos. Southeast Asian J Trop Med Public Health 1999;30:631-635.
- 22. Wirtz RA, Burkot TR. Detection of malarial parasites in mosquitoes. Advances in Disease Vector Research. 1991, New York, USA. Springer-Verlag.
- 23. Tangin A, Komichi Y, Wagatsuma Y, Rashidul H, Wataya Y, Kim HS. Detection of malaria parasites in mosquitoes from the malaria-endemic area of Chakaria, Bangladesh. Biol Pharm Bull 2008;31:703-708.
- 24. Lee HW, Shin EH, Cho SH, Lee HI, Kim CL, Lee WG, Moon SU, Lee JS, Lee WJ, Kim TS. Detection of vivax malaria sporozoites naturally infected in anopheline mosquitoes from endemic areas of northern parts of Gyeonggi-do (Province) in Korea. Korean J Parasitol 2002;40:75-81.
Fig. 1Map of Ganghwa-do (Republic of Korea) showing the locations of the 15 study sites.
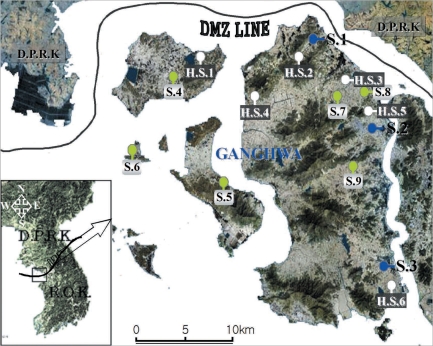
Table 1.The number of Anopheles mosquitoes collected in Gangwha-do (2008) which were analyzed by PCR to detect Plasmodium vivax infection
Table 1.
|
Collection date |
Anopheles sinensis
|
An. belenrae
|
An. pullus
|
An. lesteri
|
Total
|
|
No. of Mosq. |
No. of positive |
No. of Mosq. |
No. of positive |
No. of Mosq. |
No. of positive |
No. of Mosq. |
No. of positive |
No. of Mosq. |
No. of positive |
|
22 May |
0 |
- |
0 |
- |
1 |
0 |
0 |
- |
1 |
0 |
|
4 Jun |
0 |
- |
1 |
0 |
1 |
0 |
0 |
- |
2 |
0 |
|
12 Jun |
11 |
0 |
0 |
- |
1 |
0 |
0 |
- |
12 |
0 |
|
26 Jun |
52 |
0 |
4 |
0 |
0 |
- |
0 |
- |
56 |
0 |
|
8 Jul |
91 |
1 |
3 |
0 |
0 |
- |
0 |
- |
94 |
1 |
|
4 Aug |
206 |
0 |
7 |
0 |
0 |
- |
0 |
- |
213 |
0 |
|
19 Aug |
419 |
0 |
32 |
0 |
0 |
- |
0 |
- |
451 |
0 |
|
25 Aug |
378 |
0 |
9 |
0 |
0 |
- |
0 |
- |
387 |
0 |
|
3 Sep |
211 |
0 |
12 |
0 |
0 |
- |
0 |
- |
223 |
0 |
|
10 Sep |
283 |
1 |
15 |
0 |
5 |
0 |
0 |
- |
303 |
1 |
|
17 Sep |
32 |
0 |
3 |
0 |
1 |
0 |
0 |
- |
36 |
0 |
|
23 Sep |
20 |
0 |
1 |
0 |
2 |
0 |
0 |
- |
23 |
0 |
|
30 Sep |
3 |
0 |
1 |
0 |
8 |
0 |
0 |
- |
12 |
0 |
|
8 Oct |
5 |
0 |
0 |
- |
2 |
0 |
1 |
0 |
8 |
0 |
|
15 Oct |
3 |
0 |
1 |
0 |
0 |
- |
0 |
- |
4 |
0 |
|
21 Oct |
11 |
0 |
6 |
0 |
1 |
0 |
0 |
- |
18 |
0 |
|
28 Oct |
1 |
0 |
1 |
0 |
0 |
- |
0 |
- |
2 |
0 |
|
Total |
1,726 |
2 |
96 |
0 |
22 |
0 |
1 |
0 |
1,845 |
2 |
Table 2.Detection of Plasmodium vivax by PCR in pooled anopheline mosquitoes collected in Ganghwa-do, Incheon city, during the mosquito season of 2008
Table 2.
|
Collection date |
Daeryong (S.4)
|
Seokmo (S.5)
|
Seogeom (S.6)
|
Sungroe (S.7)
|
Daesan (S.8)
|
Geumwol (S.9)
|
|
No. of Anopheles
|
No. of pooling |
Malaria positive No. |
No. of Anopheles
|
No. of pooling |
Malaria positive No. |
No. of Anopheles
|
No. of pooling |
Malaria positive No. |
No. of Anopheles
|
No. of pooling |
Malaria positive No. |
No. of Anopheles
|
No. of pooling |
Malaria positive No. |
No. of Anopheles
|
No. of pooling |
Malaria positive No. |
|
11 Apr-1 Maya
|
0 |
- |
- |
0 |
- |
- |
3 |
2 |
0 |
3 |
3 |
0 |
4 |
3 |
0 |
0 |
- |
- |
|
2-22 Maya
|
0 |
- |
- |
2 |
1 |
0 |
10 |
2 |
0 |
4 |
3 |
0 |
16 |
3 |
0 |
0 |
- |
- |
|
23-29 May |
0 |
- |
- |
0 |
- |
- |
1 |
1 |
0 |
0 |
- |
- |
20 |
2 |
0 |
2 |
1 |
0 |
|
30 May-4 Jun |
0 |
- |
- |
8 |
1 |
0 |
0 |
- |
- |
10 |
1 |
0 |
7 |
1 |
0 |
6 |
1 |
0 |
|
5-12 Jun |
5 |
1 |
0 |
5 |
1 |
0 |
60 |
6 |
0 |
- |
- |
- |
- |
- |
- |
9 |
1 |
0 |
|
13-19 Jun |
4 |
1 |
0 |
12 |
2 |
0 |
- |
- |
- |
10 |
1 |
0 |
14 |
2 |
0 |
4 |
1 |
0 |
|
20-26 Jun |
1 |
1 |
0 |
132 |
14 |
0 |
132 |
14 |
0 |
32 |
4 |
0 |
120 |
12 |
0 |
44 |
5 |
0 |
|
27 Jun-3 Jul |
19 |
2 |
0 |
310 |
31 |
0 |
247 |
25 |
0 |
50 |
5 |
0 |
200 |
20 |
0 |
36 |
4 |
0 |
|
4-10 Jul |
74 |
8 |
0 |
600 |
60 |
0 |
947 |
95 |
0 |
800 |
80 |
0 |
57 |
6 |
1 |
110 |
11 |
0 |
|
11-17 Jul |
98 |
10 |
0 |
680 |
68 |
0 |
470 |
47 |
0 |
592 |
60 |
0 |
560 |
56 |
0 |
98 |
10 |
0 |
|
18-24 Jul |
160 |
16 |
0 |
276 |
30 |
0 |
97 |
10 |
0 |
35 |
4 |
0 |
40 |
4 |
0 |
61 |
6 |
0 |
|
25-31 Jul |
226 |
23 |
0 |
1,038 |
104 |
1 |
84 |
9 |
0 |
134 |
14 |
0 |
298 |
30 |
0 |
30 |
3 |
0 |
|
1-7 Aug |
90 |
9 |
0 |
1,300 |
130 |
0 |
- |
|
|
157 |
16 |
0 |
46 |
5 |
0 |
4 |
1 |
0 |
|
8-14 Aug |
338 |
34 |
0 |
1,720 |
86b
|
2 |
347 |
35 |
2 |
39 |
4 |
0 |
190 |
19 |
0 |
17 |
2 |
0 |
|
15-21 Aug |
4 |
1 |
0 |
2,460 |
123b
|
0 |
335 |
34 |
0 |
45 |
5 |
0 |
820 |
82 |
0 |
51 |
5 |
0 |
|
22-28 Aug |
960 |
96 |
2 |
1,994 |
100b
|
0 |
1,580 |
79b
|
0 |
207 |
21 |
0 |
169 |
17 |
0 |
16 |
2 |
0 |
|
29 Aug-4 Sep |
134 |
14 |
0 |
1,880 |
94b
|
0 |
1,240 |
62b
|
0 |
57 |
6 |
0 |
82 |
18 |
0 |
15 |
2 |
0 |
|
5-11 Sep |
206 |
21 |
0 |
1,012 |
51b
|
0 |
1,468 |
74b
|
0 |
84 |
9 |
0 |
43 |
5 |
0 |
23 |
3 |
0 |
|
12-18 Sep |
8 |
1 |
0 |
86 |
9 |
0 |
84 |
9 |
0 |
30 |
3 |
0 |
30 |
3 |
0 |
13 |
2 |
0 |
|
19-25 Sep |
10 |
1 |
0 |
89 |
9 |
0 |
169 |
17 |
0 |
52 |
6 |
0 |
34 |
4 |
0 |
14 |
2 |
0 |
|
26 Sep-2 Oct |
1 |
1 |
0 |
12 |
2 |
0 |
126 |
13 |
1 |
4 |
1 |
0 |
34 |
4 |
0 |
13 |
2 |
0 |
|
3-9 Oct |
3 |
1 |
0 |
19 |
2 |
0 |
113 |
12 |
0 |
4 |
1 |
0 |
20 |
2 |
0 |
11 |
1 |
0 |
|
10-16 Oct |
0 |
- |
- |
- |
- |
- |
- |
- |
- |
8 |
1 |
0 |
7 |
1 |
0 |
6 |
1 |
0 |
|
17-23 Oct |
0 |
- |
- |
4 |
1 |
0 |
76 |
8 |
0 |
1 |
1 |
0 |
7 |
1 |
0 |
2 |
1 |
0 |
|
24-30 Oct |
0 |
- |
- |
0 |
- |
- |
2 |
1 |
0 |
0 |
- |
- |
13 |
2 |
0 |
1 |
1 |
0 |
|
Total |
2,341 |
241 |
2 |
13,639 |
919 |
3 |
7,591 |
555 |
3 |
2,358 |
248 |
0 |
2,831 |
300 |
1 |
586 |
68 |
0 |
Table 3.The parous rate, probability of daily survival, and P11 of Anopheles species collected at 3 study sites (S.1-S.3) in Ganghwa-do (2008)
Table 3.
|
Collection date |
No. of dissected |
No. of parous |
Parous rate (%) |
Probability of daily survivala
|
P11 (%)b
|
|
22 May |
1 |
0 |
0.0 |
|
|
|
4 Jun |
2 |
0 |
0.0 |
|
|
|
12 Jun |
12 |
4 |
33.3 |
0.691 |
1.7 |
|
26 Jun |
49 |
22 |
44.9 |
0.766 |
5.3 |
|
8 Jul |
83 |
34 |
41.0 |
0.743 |
3.8 |
|
4 Aug |
196 |
122 |
62.2 |
0.854 |
17.6 |
|
19 Aug |
435 |
224 |
51.5 |
0.802 |
8.8 |
|
25 Aug |
333 |
176 |
52.9 |
0.809 |
9.7 |
|
3 Sep |
206 |
151 |
73.3 |
0.902 |
32.2 |
|
10 Sep |
285 |
265 |
92.9 |
0.976 |
76.6 |
|
17 Sep |
31 |
20 |
64.5 |
0.864 |
20.0 |
|
23 Sep |
19 |
14 |
73.7 |
0.903 |
32.6 |
|
30 Sep |
12 |
5 |
41.7 |
0.747 |
4.0 |
|
8 Oct |
8 |
1 |
12.5 |
0.5 |
0.05 |
|
15 Oct |
3 |
0 |
0.0 |
|
|
|
21 Oct |
14 |
0 |
0.0 |
|
|
|
28 Oct |
2 |
0 |
0.0 |
|
|
|
Total |
1,691 |
1,038 |
61.4 |
0.850 |
|
Table 4.Number (percentage) of Anopheles species collected at hibernation sites in Ganghwa-do between December 2007 and March 2008
Table 4.
|
Collection site |
Collection date |
No. of collected mosquitoes |
Anopheles species (%)
|
|
Sinensis
|
Belenrae
|
Pullus
|
|
Gogu (H.S.1) |
27 Dec |
151 |
25 (16.4) |
9 (5.9) |
117 (77.0) |
|
Gyosan (H.S.2) |
13 Dec, 3 Mar |
85 |
9 (10.6) |
28 (32.9) |
48 (56.5) |
|
Songdo (H.S.3) |
5 Dec |
134 |
36 (26.9) |
16 (11.9) |
82 (61.2) |
|
Changhu (H.S.4) |
14 Dec, 14 Feb |
102 |
2 (2.0) |
1 (1.0) |
99 (97.1) |
|
Daesan (H.S.5) |
21-Dec |
82 |
8 (9.8) |
4 (4.9) |
70 (85.4) |
|
Chogi (H.S.6) |
26 Dec |
108 |
42 (38.9) |
5 (4.6) |
61 (56.5) |
|
Total |
|
662 |
122 (18.4) |
63 (9.5) |
477 (72.1) |