Abstract
Mucosal mast cell (MMC) responses and worm recovery rates in rats infected with Echinostoma hortense were investigated from day 3 to day 56 post-infection (p.i.). Experimental infected group showed apparently higher number of MMC in each part of the small intestine than that of the control group. The number of MMC in the duodenum increased gradually after the infection and reached a peak on day 35 p.i. Thereafter, the number of MMC continued to decrease at a slow pace. The kinetics of MMC responses in the upper and lower jejunum were similar to that of the duodenum, but the number of MMC in the jejunum was lower. The worm recovery rate decreased with respect to time of which it was markedly reduced on day 49 and 56 p.i. The duration in which a high number of MMC appeared was similar to that in which a low rate in worm recovery was recorded. These results indicate that intestinal mastocytosis may play an important role in the expulsion of E. hortense.
-
Key words: Echinostoma hortense, mucosal mast cell, worm recovery rate, rats
INTRODUCTION
Intestinal mastocytosis is observed in certain intestinal helminth infections. The increase of mast cell in the intestinal mucosa is known to play an important role in host defense against intestinal parasites (
Miller, 1984). At least three gastrointestinal nematode parasites have been identified:
Trichinella spiralis,
Strongyloides ratti, and
Strongyloides venezuelensis (
Ha et al., 1983;
Abe et al., 1992;
Grencis et al., 1993;
Khan et al., 1993;
Donaldson et al., 1996;
Lantz et al., 1998). The expulsion of these parasites is severely impaired in mast cell-deficient W/W
v mice and in mice deficient in the mast cell-stimulating cytokine, IL-3 (
Lantz et al., 1998). Intestinal mastocytosis is also observed in rats infected with tapeworms such as
Hymenolepis diminuta and
H. microstoma and intestinal trematode such as
Fibricola seoulensis (
Andreassen et al., 1978;
Novak and Nombrado, 1988;
Kho et al., 1990). However, the role of mucosal mast cell (MMC) has not been completely elucidated and there is lack of information on MMC responses against intestinal trematode infection.
Echinostoma hortense was first discovered by Asada (
1926). In Korea, the human cases have been increased gradually since the first case of human infection was reported in the early 1980s (
Seo et al., 1983). In experimentally rats infected with
E. hortense, the worms are found in the intestinal villi from day 1 to day 3 p.i. and in the lumen from day 7 to day 44 p.i.. Intestinal infection causes villous loss, villous atrophy, and crypt hyperplasia (
Lee et al., 1990).
E. hortense is primarily located in the duodenum and the jejunum of infected rats.
In the present study, we observed the kinetics of intestinal MMCs at various sites of the small intestine of rats infected with E. hortense and compared it to the worm expulsion.
MATERIALS AND METHODS
Experimental infection and worm recovery
Metacercariae of E. hortense were isolated from the muscles of raw loaches, Misgurnus anguillicaudatus, obtained from Sumgin-gang (River), Kurye, Chonnam, Korea, by using artificial gastric juice. Sprague-Dawley rats weighing approximately 150 gm were orally infected with 150 metacercariae through a polyethylene capillary tube. The rats were sacraficed in each week p.i. and the worms were collected from the small intestines of the rats.
Observation of MMC responses
MMC was examined in the small intestines of rats on day 3, 5 and each week for day 56 p.i.. For histological samples, the intestinal segments were cut approximately 1.5-2 cm each in length and were taken from three sites of the small intestine: the duodenum (10 cm posterior to the pylorus), and the upper and lower parts of the jejunum. The excised segments of the small intestine were fixed in Carnoy's solution for 2-4 hrs. The fixed tissues were embedded in paraffin and sectioned at about 5 µm thickness using microtome. The sectioned samples were stained with 1% alcian blue (pH 0.3) and counter-stained with 0.5% safranin (pH 1.0) (
Strobel et al., 1981). Stained MMCs were counted in the graticule (500 × 500 µm). The values of of MMC were recorded as the average number of MMC per graticule (0.25 mm
2).
RESULTS
Kinetics of MMC responses
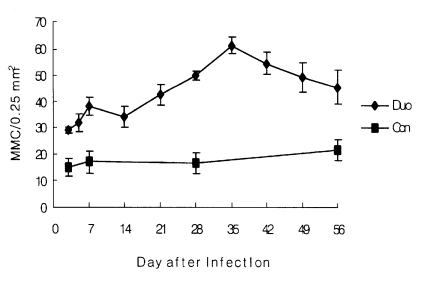
In the experimentally infected group, the number of MMC in the duodenum increased slightly from day 3 (29.0 ± 1.0) to day 5 p.i. (31.7 ± 3.4). At 14 day p.i., it decreased slightly but began to increase from day 21 p.i. It peaked on day 35 p.i. (61.3 ± 3.1); thereafter, it decreased slowly until day 56 p.i. (
Table 1,
Fig 1 and
2). The number of MMC in the upper and the lower parts of the jejunum was relatively lower than that in the duodenum. However, the kinetics of MMC responses were similar (
Table 1).
In contrast, no evident changes in the kinetics of MMC responses was shown in non-infected control group (
Table 1).
The worm recovery rate decreased slowly from day 7 to 35 p.i.. It continued to decrease apparently from day 42 to day 56 p.i., 20.8% on day 42, 12.9% on day 49 and 5.5% on day 56 p.i. (
Table 2).
DISCUSSION
This study shows that intestinal mastocytosis was induced in rats infected with
E. hortence and that this response may be associated with explusion of the worm. The highest number of MMC was observed in the duodenum where the most of the worms dwelled. Similar result about the mast cell response was shown in the small intestine of rats infected with
S. ratti (
Mimori et al, 1982) In contrast to our results, Kho et al. (
1990) reported that the increase in MMC number of rats was limited to the upper part of the small intestine in
F. seoulensis infection. In this experiment, the peak level of mastocytosis was observed at day 35 p.i.. the value decreased thereafter. The worm recovery rate was evidently decreased on day 49 and day 56 when the higher level of mastocytosis was persisted. In contrast to our results, the peak level of mastocytosis was observed after the worms were expelled in
T. spiralis infection or
S. ratti infection (
Woodbury et al., 1984;
Mimori et al., 1982). In
F. seoulensis infection, however, the peak level of mastocytosis was observed on the same time when the worm recovery rate began to decrease significantly. These different aspects between their and our results may be due to the different biological properties of the species of parasites.
There are many suggestions that MMC may play an important role in the host defense against intestinal parasites. Recent studies, however, showed that the effector cell in immune response against the parasites is not mast cell but goblet cell. It was reported, using concurrent infection with
S. ratti and
N. brasiliensis in rats, that mast cells were important in the expulsion of the former but not of the latter (
Nawa and Korenaga, 1983). In mice infected with
E. trivolvis, it was observed that the expulsion was associated mainly with goblet cell hyperplasia (
Weinstein and Fried, 1991;
Fujino et al., 1993). Fujino et. al. (
1996) re-ported that goblet cell hyperplasia was inhibited and the number of mast cells and eosinophils increased when the expulsion of
E. trivolvis was delayed by dexamethasone treatment. Considering the above these studies, main effector cells of immune responses in the intestinal paraisite infection may be different according to the species of intestinal helminths and kinds of hosts.
In conclusion, we suggest that MMC in rats are associated with the expulsion of E. hortense. In order to reach a more definite role of mast cells in protective immunity to E. hortense infection, we will need to further study on other effector cells such as goblet cells.
References
- 1. Abe T, Sugaya H, Yoshimura K, Nawa Y. Induction of the expulsion of Strongyloides ratti and retention of Nippostrongylus brasiliensis in athymic nude mice by repetitive administration of recombinant interleukin-3. Immunology 1992;76:10-14.
- 2. Andreassen J, Hindsbo O, Ruitenberg EJ. Hymenolepis diminuta infections in congenitally athymic(nude) mice: worm kinetics and intestinal histopathology. Immunology 1978;34:105-113.
- 3. Asada S. On a new echinostomatid trematode and its life history. Trans KJap Pathol Soc 1926;16:293-294. (in Japanese).
- 4. Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol 1996;8:559-567.
- 5. Fujino T, Fried B, Tada I. The expulsion of Echinostoma trivolvis: worm kinetics and intestinal cytopathology in conventional and congenitally athymic BALB/c mice. Parasitology 1993;106:297-304.
- 6. Fujino T, Ichkawa H, Fried B, Fukuda K. The expulsion of Echinostoma trivolvis: suppressive effects of dexamethasone on goblet cell hyperplasia and worm rejection in C3H/HeN mice. Parasite 1996;63:283-289.
- 7. Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol 1993;15:55-59.
- 8. Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun 1983;41:445-447.
- 9. Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol 1993;23:551-555.
- 10. Kho WG, Chai JY, Chun DH, Lee SH. Mucosal mast cell responses to experimental Fibricola seoulensis infection in rats. Seoul J Med 1990;31:191-199.
- 11. Lantz CS, Boesiger J, Song CH, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature 1998;392:90-93.
- 12. Lee SH, Noh TY, Sohn WM, Kho WG, Hong ST, Chai JY. Chronological observation of intestinal lesions of rats experimentally infected with Echinostoma hortense. Korean J Parasitol 1990;28:45-52.
- 13. Miller HRP. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Veterinary Immunology and Immunopathology 1984;6:167-259.
- 14. Mimori T, Nawa Y, Koremaga M, Tada I. Strongyloides ratti: Mast cell and goblet cell responses in the small intestine of infected rats. Exp Parasit 1982;54:366-370.
- 15. Nawa Y, Korenaga M. Mast and goblet cell responses in the small intestine of rats concurrently infected with Nippostrongylus brasiliensis and Strongyloides ratti. Journal of Parasitology 1983;69:1168-1170.
- 16. Novak M, Nombrado S. Mast cell responses to Hymenolepis microstoma infection in mice. J Parasitol 1988;74:81-88.
- 17. Seo BS, Hong ST, Chai JY, Lee SH. Studies on intestinal trematodes in Korea VIII. A human case of Echinostoma hortense infection. Korean J Parasitol 1983;21:219-223.
- 18. Strobel S, Miller HRP, Ferguson A. Human intestinal mucosal mast cells: Evaluation of fixation and staining techniques. J Clin Pathol 1981;34:851-858.
- 19. Weinstein MS, Fried B. The expulsion of Echinostoma trivolvis and retention of Echnostoma caproni in the ICR mouse: pathological effects. Int J Parasitol 1991;21:255-257.
- 20. Woodbury RG, Miller HRP, Huntley JF, Newlands GFJ, Palliser AC, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature 1984;312:450-452.
Fig. 1Chronological changes of MMC numbers (per 0.25 mm2) in the duodenum in control group (-■-) and experimental group(-◆-) infected with Echinostoma hortense.

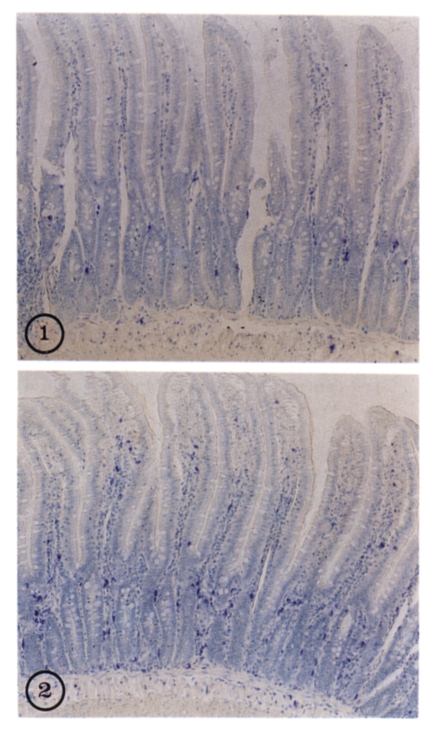
Fig. 2Mucosal mastocytosis in the duodenum of a rat infected with Echinostoma hortense (2) on day 35 p.i. compared with non-infected control (1) (× 100). Bule spots represent mast cells stained with alcian blue and safranin.

Table 1.Mucosal mast cell (MMC) numbers in the small intestine of rats infected with Echinostoma hortense
Table 1.
|
No. of MMCs per 0.25 mm2 in the mucosa of the small intestine
|
|
group |
duodenum |
jejunum
|
|
upper |
lower |
|
Control |
|
|
|
|
day 3 |
15.1 ± 3.9a)
|
15.2 ± 4.1 |
13.4 ± 2.6 |
|
day 7 |
17.2 ± 3.4 |
16.9 ± 6.5 |
15.0 ± 3.7 |
|
day 28 |
16.9 ± 4.2 |
20.4 ± 1.2 |
19.6 ± 3.2 |
|
day 56 |
22.0 ± 3.9 |
21.5 ± 4.3 |
22.7 ± 3.5 |
|
Infected |
|
|
|
|
day 3 |
29.0 ± 1.0 |
24.8 ± 4.9 |
25.0 ± 2.3 |
|
day 5 |
31.7 ± 3.4 |
25.3 ± 3.5 |
27.4 ± 5.0 |
|
day 7 |
38.1 ± 3.2 |
35.6 ± 3.2 |
37.2 ± 4.0 |
|
day 14 |
34.2 ± 4.1 |
37.8 ± 3.8 |
31.4 ± 2.6 |
|
day 21 |
42.5 ± 3.9 |
39.0 ± 5.0 |
40.6 ± 2.7 |
|
day 28 |
49.9 ± 1.6 |
35.0 ± 3.9 |
39.6 ± 6.2 |
|
day 35 |
61.3 ± 3.1 |
50.2 ± 6.2 |
42.0 ± 2.7 |
|
day 42 |
54.5 ± 4.2 |
44.5 ± 4.8 |
37.7 ± 2.1 |
|
day 49 |
49.5 ± 5.6 |
33.5 ± 1.5 |
36.0 ± 4.1 |
|
day 56 |
45.6 ± 6.2 |
38.0 ± 1.9 |
39.0 ± 2.7 |
Table 2.Chronological changes in the worm recovery rate in the rats infected with Echinostoma hortense
Table 2.
|
Duration (day) |
No. of rats examined |
No. of recovered worms
|
|
mean ± SD |
% |
|
7 |
5 |
43.0 ± 3.5 |
28.7 |
|
14 |
5 |
42.6 ± 4.2 |
28.4 |
|
21 |
5 |
36.0 ± 4.6 |
24.0 |
|
28 |
5 |
37.2 ± 5.6 |
24.8 |
|
35 |
5 |
34.6 ± 3.5 |
23.1 |
|
42 |
5 |
31.2 ± 4.6 |
20.8 |
|
49 |
5 |
19.4 ± 2.4 |
12.9 |
|
56 |
5 |
8.2 ± 1.5 |
5.5 |
Citations
Citations to this article as recorded by

- Neglected food-borne trematodiases: echinostomiasis and gastrodiscoidiasis
Rafael Toledo, María Álvarez-Izquierdo, J. Guillermo Esteban, Carla Muñoz-Antoli
Parasitology.2022; 149(10): 1319. CrossRef - Increased Intestinal Epithelial Cell Turnover and Intestinal Motility in Gymnophalloides seoi-Infected C57BL/6 Mice
Sang Hyub Lee, Bong-Kwang Jung, Jae-Hwan Park, Eun-Hee Shin, Jong-Yil Chai
The Korean Journal of Parasitology.2014; 52(3): 273. CrossRef - History of echinostomes (Trematoda)
Rafael Toledo, Valentin Radev, Ivan Kanev, Scott Gardner, Bernard Fried
Acta Parasitologica.2014;[Epub] CrossRef - Mucosal Immune Responses of Mice Experimentally Infected with Pygidiopsis summa (Trematoda: Heterophyidae)
Jong-Yil Chai, Young-Jin Park, Jae-Hwan Park, Bong-Kwang Jung, Eun-Hee Shin
The Korean Journal of Parasitology.2014; 52(1): 27. CrossRef - Foodborne Intestinal Flukes in Southeast Asia
Jong-Yil Chai, Eun-Hee Shin, Soon-Hyung Lee, Han-Jong Rim
The Korean Journal of Parasitology.2009; 47(Suppl): S69. CrossRef - Haplorchis taichui: Worm recovery rate and immune responses in infected rats (Rattus norvegicus)
Supap Saenphet, Chalobol Wongsawad, Kanokporn Saenphet, Amnat Rojanapaibul, Pramote Vanittanakom, Jong-Yil Chai
Experimental Parasitology.2008; 120(2): 175. CrossRef - Differential immune profiles following experimental Echinostoma hortense infection in BALB/c and C3H/HeN mice
Yoon Kyung Cho, Yong Suk Ryang, In Sik Kim, Seung Kyu Park, Jee Aee Im, Kyu Jae Lee
Parasitology Research.2007; 100(5): 1053. CrossRef - Somatostatin inhibits intestinal mucosal mast cell degranulation in normal conditions and during mast cell hyperplasia
Y Saavedra, P Vergara
Regulatory Peptides.2003; 111(1-3): 67. CrossRef - Food-borne intestinal trematode infections in the Republic of Korea
Jong-Yil Chai, Soon-Hyung Lee
Parasitology International.2002; 51(2): 129. CrossRef