Abstract
Changes in the expression level of splenocyte IFN-γ mRNA of Sprague-Dawley (SD) rats infected with Paragonimus westermani were analyzed by competitive reverse transcription-polymerase chain reaction (RT-PCR) followed by southern blot. The template RNA was extracted from the splenocytes of rats infected with 20 metacercariae of P. westermani. The products of competitive RT-PCR were subjected to southern blot and enhanced chemiluminescence (ECL), and analyzed with a densitometer. In comparison with that of uninfected control rat splenocytes (value of 1), the levels of mRNA expression of IFN-γ had changed to 0.747 at 1 week post infection (PI), 0.00175 at 2 week PI, 0.0217 at 3 week PI, 0.194 at 4 week PI and then to 0.537 at 5 week PI. The level at 7 week PI had returned to 1.25, comparable with that of uninfected rats. These results show that, when infected with P. westermani, the levels of IFN-γ mRNA of SD rat splenocytes were remarkably reduced by more than 500 times at 2 week PI and restored to normal level at 7 week PI.
-
Key words: Paragonimus westermani, IFN-γ, mRNA, competitive RT-PCR, rat
Paragonimus westermani is a parasite which lives in the lung of mammalian hosts, where active immune reaction is induced by various immune cells. The immune reaction is accompanied by the production of cytokine. The levels of different cytokines induced by the parasite infection varies, and depending on the relative prevalence of Th1 or Th2, either cellular or humoral immune is directed. Such a deliberate course of immune response has been elucidated with
Angiostrongylus cantonensis (
Sugaya et al., 1997) and
Echinococcus multilocularis (
Emery et al., 1997). Although the true meaning of this varying cytokine production is not yet known, cytokines are important for parasite immune defense as shown in leishimaniaisis (
Swihart et al., 1995). Although rats are an abnormal host for
P. westermani and the worm recovery rate is only 21%, rats are better than mice in mature worm recovery rate (
Fan et al, 1993). More information has also been documented about rat immune function than other experimental animals (except mice). For these reasons, researchers are encouraged to use rats in investigations of immune response to the parasite using cytokine mRNA synthesis. IFN-γ has been valuable in its use as an indicator to assess the immune response direction of the host, because it is controlled by certain cytokines (
Katsura, 1997) and can reflect an immune response. In this study, in order to understand the kinetics of cytokine production during
P. westermani infection, IFN-γ mRNA levels of rat splenocytes were analyzed by competitive RT-PCR (
Sung et al., 1996) and a southern blot with chemiluminescence.
Sprague-Dawley rats (10-week-old, 150-200 g, Samyook Animal center, Osan-shi, Korea) were each infected with 20 metacercariae of
P. westermani. A single cell suspension of splenocytes was prepared by discontinuous percoll (Sigma, St. Louis, USA) (70%/50%) (
Cho and Conrad 1997) from two rats sacrificed each week and preserved at -70℃. RNA was extracted from 1-×10
7 splenocytes with Trizol (Gibco-BRL, Grand Island, USA). Competitive reverse transcription-polymerase chain reaction (RT-PCR) for rat IFN-γ was done using the method of Sung et al. (
1996) and the kit of access RT-PCR system (Promega, Madison, USA). The mRNA level of each sample was adjusted based on β-actin RT-PCR products (23 PCR cycle). The density of the samples was analyzed with ethidium bromide staining and an Eagle Eye system (Stratagene, La Jolla, USA). In the process of conditioning, 26 cycles were selected for the competitive RT-PCR. These products were applied to 4% TBE agarose electrophoresis, and then a southern blot. The probes for hybridization were prepared by PCR with a sense primer of 24 mer (5'-CCCTCTCTGGG CTGTTACTGC-3') (
Siegling et al., 1994) and an anti-sense primer of 24 mer (5'-GAGTTCATTG ACAGCTTTGTGCTGG-3') (
Sung et al., 1996) and labeled with alkaline phosphatase with an AlkPhos Direct System (Amersham, Little Chalfont, England). The hybridized membrane was reacted with the substrate of enhanced chemiluminescence (ECL) (Amersham) and exposed to Hyper ECL film (Amersham). The film was developed in an M35A X-OMAT film autoprocessor (Kodak, Rochester, USA) and the band intensity was analyzed with the laser densitometer of an ultrascan XL (Pharmacia LKB, Uppsala, Sweden). The levels of IFN-γ in each sample were calculated in comparison with the intensity of competitor of each sample.
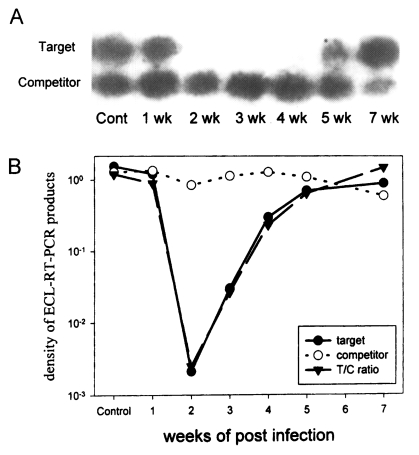
In comparison with that of rat IFN-γ from uninfected control rat (value of 1), the level of IFN-γ dropped to 0.747 on 1 week PI, to 0.00179 on 2 week PI, and 0.0217 on 3 week PI. It began to increase at 4 week PI, rising to 0.194 and at 5 week PI to 0.537. On 7 week PI, it had returned to 1.25, comparable to the normal rat (
Fig.1).
When the levels of cytokine production are measured with an ELISA capture assay, the tissue cells are usually activated with concanavalin-A or phytohemagglutinin-P because the cytokine levels from the non-activated cells are lower than measurable standards. However, the activation is accompanied with the proliferation of non-specific memory T cells, and IFN-γ produced by these memory cells causes confusion in the background. We have adopted a PCR method to compensate the restriction of cell activation in the analysis of cytokine production (
Saiki et al., 1985), because PCR is a sensitive method to measure the change of cytokine levels without any cell activation.
It is considered that the remarkable reduction in splenocyte IFN-γ mRNA level by more than 500 times at 2 week PI reflects a violent change in host immune response. This dramatic shift in splenocyte IFN-γ mRNA at 2 week PI is also shown in
Paragonimus iloktsuenensis and
P. miyazakii, in the number of spine tegumentum and the susceptibility to cyclosporin-A (
Hashiguchi and Okamura 1988,
Lee et al. 1989). However, the levels of IFN-γ from mice infected with
P. westermani measured by the capture ELISA assay increased by two times at 1 week PI (
Shin and Min, 1996). It is hypothesized that the discrepancy is a result of different species of experimental animal, rats and mice, and the analyzing methods, RT-PCR and capture ELISA.
These results shows that, when infected with P. westermani, the levels of IFN-γ mRNA of Sprague-Dawley rat splenocytes were reduced by more than 500 times at 2 week PI and restored to normal level at 7 week PI.
Notes
-
This study was supported by 1997 Basic Medical Science Research Fund, KMA
References
- 1. Cho SW, Conrad DH. A new multivalent B cell activation model anti-IgD bound to FcγRI: properties and comparison with CD40L-mediated activation. Int Immunol 1997;9:239-248.
- 2. Emery I, Liance M, Leclerc C. Secondary Echinococcus multilocularis infection in A/J mice: delayed metacestode development is associated with Th1 cytokine production. Parasite Immunol 1997;19:493-503.
- 3. Fan PC, Lu H, Lin HL. Experimental infection of Paragonimus westermani in mice and rats. Korean J Parasitol 1993;31:91-97.
- 4. Hashiguchi Y, Okamura Y. The effect of cyclosporin A on the course of Paragonimus miyazakii infection in rats. J Helminthol 1988;62:251-256.
- 5. Katsura Y. Cell-mediated and humoral immune responses in mice. III. Dynamic balance between delayed-type hypersensitivity and antibody response. Immunology 1977;32:227-235.
- 6. Lee SH, Kim SJ, Chai JY, et al. Tegumental ultrastructures of Paragonimus iloktsuenensis according to the developmental stages. Korean J Parasitol 1989;27:57-66. (in Korean).
- 7. Saiki RK, Scharf S, Fallona F, et al. Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 1985;230:1350-1354.
- 8. Shin MH, Min DY. Production of interferon γ and interleukin-4 by splenocytes in mice infected with Paragonimus westermani. Korean J Parasitol 1996;34:185-189.
- 9. Siegling A, Lehmann M, Platzer C, Emmrich F, Volk HD. A novel multi-specific competitor fragment for quantitative PCR analysis of cytokine gene expression in rats. J Immunol Methods 1994;177:23-28.
- 10. Sugaya H, Aoki M, Abe T, Ishida K, Yoshimura K. Cytokine responses in mice infected with Angiostrongylus cantonensis. Parasitol Res 1997;83:10-15.
- 11. Sung B, Wells J, Goldmuntz E, et al. A simplified, competitive RT-PCR method for measuring rat IFN-γ mRNA expression. J Immunol Methods 1996;195:139-149.
- 12. Swihart K, Fruth U, Messmer N, et al. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishimania major but mount a polarized T helper cell 1-type CD4+ T cell response. J Exp Med 1995;181:961-971.
Fig. 1Analysis of the levels of rat IFN-γ mRNA with competitive RT-PCR and enhanced chemiluminescence. Splenocytes were collected each week from 1 week PI to 7 week PI, excluding 6 week PI. A. Autoradiography of hybridized southern blot. B. Graph of the density ratio.
Citations
Citations to this article as recorded by

- Adjuvant effect of Korean mistletoe lectin on mucosal immunity induction following intranasal immunization with hemagglutinin antigen
Jin Hyuk Jung, Young Hoon Kim, Tae Jun Song, Hyosun An, Kyu Dae Kim, In Bo Kim, Taek Joon Yoon, Jong Bae Kim
Food Science and Biotechnology.2011; 20(3): 629. CrossRef