Abstract
Neospora caninum is an important cause of abortion in dairy cattle worldwide. Dog is the definitive host for N. caninum and can infect dairy cattle. The aim of this study is to determine the prevalence of Neospora oocysts in feces of dogs from dairy farms. A total of 174 fecal samples was collected from 89 farm dogs and 85 household dogs during 2006 and 2008. Fecal samples of dogs were microscopically examined for detecting Hammondia Neospora-like oocysts (HNLO) by Mini Parasep®SF fecal parasite concentrator. HNLO were microscopically detected in 4 fecal samples (2.2%). The fecal samples with HNLO were examined by N. caninum-specific PCR. Two of the samples were positive for N. caninum. The 2 positive fecal samples were selected for inoculation to calves. Two inoculated calves were seronegative by ELISA for 4 months post-infection. This is the first report of finding N. caninum DNA in feces of farm dogs in Mashhad area, Iran.
-
Key words: Neospora caninum, oocyst, dog, Iran
Neosporosis is a major cause of abortion in cattle [
1-
3]. There are 2 transmission routes for
Neospora caninum in cattle. The first route is vertical or transplacental transmission. In cattle, transplacental transmission from infected dams to offspring appears to be the major route of infection [
4]. Prenatally infected but health calves remain persistently infected and can pass infection to their own offspring. This leads to endogenous transplacental transmission of the infection through successive pregnancies and cattle generation [
3]. The second route is referred to as horizontal or postnatal transmission that occurs in cattle after ingesting sporulated
N. caninum oocysts [
4]. Dogs and coyotes are definitive hosts for
N. caninum [
5,
6]. Dogs can transiently shed oocysts upon ingestion of
N. caninum infected tissues of intermediated hosts [
3]. In some epidemiological studies of dairy herds, the presence of farm dogs either was a risk factor for seropositivity in cattle [
2]. In Iran, there are a few reports on the seroprevalence of
N. caninum infection in cattle and dogs [
7-
10]. Also,
N. caninum has been recognized as an important agent of abortion in dairy cattle in Iran [
11]. In these studies, correlation of seropositivity of dairy cattle to abortion was shown; however, the role of farm dogs in spreading
Neospora infection in dairy farms of Iran has not been investigated. It seems that the presence of farm dogs could be associated with abortion due to
N. caninum infection in dairy cattle. The aim of this study is to investigate the potential role of dogs as a source of
Neospora oocysts shedding in infection of dairy cattle in this area and to demonstrate cyclical oral transmission of
N. caninum between dogs and cattle.
The study was done in Mashhad area, capital city of the Razvai Khorasan province, situated in the northeast of Iran. The Razavi Khorasan province is located in northern temperature zone. The climate is semi-arid with cold winters and moderate summer. This area has an estimated 25,000 cattle on 110 dairy farms.
A total of 174 fecal samples were collected from 89 farm dogs and 85 household dogs during 2006 and 2008. Samples were kept at cold condition until laboratory examinations took place. Samples were examined by Mini Parasep®SF faecal parasite concentrator (Diasys Europe Ltd.). Briefly, the lid was unscrewed and 3.3 ml of 10% buffered formalin was added to the mixing tube, and a pea-sized (0.4 g) fecal sample was introduced by using the spoon on the end of the Parasep. The sample was mixed thoroughly with the Parasep spoon. The Parasep was immediately sealed by screwing the filter thrimble and conical tube. The mixture was vortexed and the Parasep was then inverted to allow the mixture tube filtered through the filter thimble. The Parasep was then centrifuged at 600 g for 1 min. The mixing chamber and filter thimble were unscrewed and discarded. All the liquid above the sediment was poured off and 1 ml water was added to the sediment. The sediment was re suspended with water by shaking. The sediment then was pipetted to a slide for microscopic examination. If the sample had oocysts, the oocysts of feces were measured with a calibrated ocular micrometer using bright-field microscopy. The oocysts with a diameter of 11.5 ± 1.5 µm and exhibiting morphology similar to non-sporulated
T. gondii-oocysts were considered to be positive for
Hammondia Neospora-like oocysts (HNLO) [
12-
14]. For sporulation of HNLO, the positive sample was reexamined from feces by a combined sedimentation and flotation procedure as described by Schares et al. [
15]. Then, the number of isolated oocysts was estimated by microscopic examination of 10 µl samples using a Neubaur hemocytometer. The fecal samples containing oocysts were mixed with 2% potassium dichromate in a Petri dish and incubated at room temperature for 3-5 days.
Oocysts of
N. caninum are morphologically indistinguishable from those of
Hammondia heydorni and
Toxoplasma gondii [
12,
15]. Thus, it was necessary to do molecular methods, such as PCR for differentiating oocysts of
N. caninum from those of
H. heydorni and
T. gondii. Therefore, NC5-PCR was used since a high diagnostic value of this method has been demonstrated in European interlaboratory evaluation [
16]. First, suspensions from sporulated oocysts were washed by repeated centrifugation in distilled water to remove potassium dichromate. Then, the oocysts were ruptured by 2-3 freeze-thaw cycles. DNA was subsequently isolated from purified oocysts with the DNeasykit according to the manufacturer's instructions (Cinagen, Tehran, Iran). After that, DNA amplification was performed as described by Müller et al. [
17] using the primer pair Np6
+/Np21
+. Briefly, 2 oligonucleotide PCR primers were used to detect
N. caninum for the PCR (Np6
+): 5'-CTCGCCAGTCAACCTACGTCTTCT-3', and the reverse (Np21
+): 5'-CCCAGTGCGTCCAATCCTGTAAC-3' were used for amplification reaction. The 50 µl reaction mixture contained; 2 µl of template DNA, 5 µl of 10 × PCR buffer (CinnaGene Inc., Tehran, Iran), 1 µl MgCl
2, 0.2 mM each of dATP, dGTP, and dCTP, 0.4 mM dUTP (CinnaGene), 1.25 units of Tag DNA polymerase (CinnaGene) and 20
pmol of each P1 and P2 primers (CinnaGene) for PCR and double distilled H
2O were added up to 50 µl. The amplification conditions for
N. caninum included an initial enzyme activation of denaturation at 95℃ for 5 min, 40 cycles with denaturing at 94℃ for 60 sec, primer annealing at 63℃ for 60 sec and extension at 74℃ for 3.5 min, followed by final extension at 74℃ for 10 min. PCR Products were then chilled at 4℃. The final PCR products were subjected to electrophoresis in a 1.5% agarose gel with TBE buffer. Samples positive for
N. canium produced visible bands at 337 bp in the PCR product.
Two dairy calves (male, 5-7 months) were purchased from the farm of Faculty of Veterinary Medicine, Ferdowsi University of Mashhad. Each calf was confirmed to be seronegative for N. canium using a commercial ELISA kit (Bio-x Diagnostics, Belgium). Then, 2 fecal samples positive in PCR were selected for inoculation to calves. Prior to inoculation, potassium dichromate was washed out with tap water after 3 times centrifugation (1,100 g, 10 min) and the pellet was resuspended in 10 ml tap water. Finally, 2 calves were orally inoculated with approximately 50-100 HNLO oocysts. Blood was collected from the jugular vein every 2 weeks for 4 months. For preparing the serum, blood was centrifuged at 1,000 g for 10 min, and the serum was collected and stored at -20℃ until use. The collected sera were tested by a commercial ELISA kit (Bio-x Diagnostics).
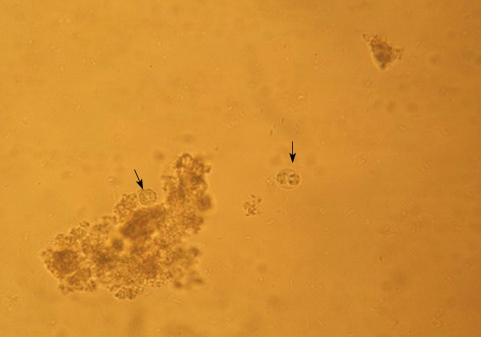
From 174 dogs examined, HNLO was microscopically detected in 4 fecal samples (3.48%) (
Fig. 1). The number of HNLO in examined fecal samples was low (5-10 oocysts per gram). Four samples with HNLO were tested by
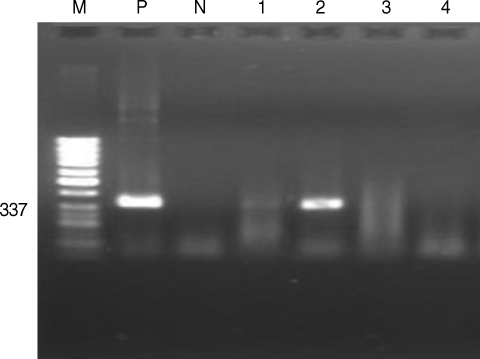
N. canium-specific PCR. Two samples were positive for
N. canium (
Fig. 2). The positive dogs were males and 1 of them was 2 years old and the other 4 months old. The calves fed sporulated HNLO remained healthy and
N. caninum-specific serological analysis of serum samples collected 3 to 16 weeks after infection was negative (
Table 1).
Many seroepidemiological studies were done for detecting
Neospora caninum infection in dogs worldwide [
2]. Seropositivity in dogs is a primarily indicator for a past or recent contact with the parasite, but cannot be correlated to shedding of oocysts. The majority of dogs shedding
N. caninum oocysts after experimental infection do not seroconvert in serologic examination [
12,
13,
18-
20]. Therefore, it is important to properly identify the
N. caninum oocysts in fecal samples. So far, there are only a few reports of
N. caninum oocyst shedding by naturally infected dogs [
21-
24]. In this study, HNLO were found only in 4 fecal samples of farm dogs, and also, DNA of
Neospora was detected in 2 of them by
N. caninum-specific PCR. The DNA of
Neospora or
N. caninum oocysts in the fecal samples may be due to feeding of fresh and uncooked infected meat. For confirmation, it was need to isolate parasites in bioassay examination or cell culture. Gerbils are highly susceptible to infection with oocysts of various
N. caninum isolates [
14]. Trees et al. [
25] observed that the inoculation of approximately 1
N. caninum oocyst was sufficient to induce a
N. caninum-specific antibody response in gerbils. However, because of unavailability of gerbils during the study, we used calves in bioassay examinations. Some studies were shown that challenge with 300-600 oocysts of
N. caninum could induce
N. caninum-specific antibodies in calves [
20,
25-
27]. In the present study, all calves were remained seronegative to
N. caninum before and after oral inoculation of oocysts from positive fecal samples. It seems that the dose of
Neospora oocysts may have been too low to induce immune responses or there may have been no
Neospora oocysts in the given fecal samples.
In conclusion, HNLO were found only in farm dogs and 2 of fecal samples revealed positive reactions to Neospora-PCR. This result shows that the presence of farm dogs may be a risk factor for N. caninum infection in dairy farms in Mashhad area, Iran. However, to confirm this hypothesis, a further molecular characterization of extracted DNA and isolation of parasites in gerbil bioassay or in cell culture is needed.
ACKNOWLEDGEMENTS
We thank Mr. H. Eshrati for his technical assistance. Special thanks are to Dr. Garrousi, Dr. Fallah, Mr. Azari for helping to collect fecal samples of dogs from dairy farms of Mashhad area. This project was financially supported (grant number 20039) by the Office of Research Affairs in Ferdowsi University of Mashhad, Iran.
References
- 1. Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol 1996;67:1-59.
- 2. Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum. Clin Microbiol Rev 2007;20:323-367.
- 3. Conraths FJ, Gottstein B. In Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D eds, Neosporosis: General considerations. Protozoal Abortion in Farm Ruminants. 2007, Wallingford, England. CAB International; pp 42-45.
- 4. Wouda W. In Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D eds, Neosporosis: Biology, transmission and clinical signs. Protozoal Abortion in Farm Ruminants. 2007, Wallingford, England. CAB international; pp 46-53.
- 5. McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol 1998;28:1473-1478.
- 6. Gondim LF, McAllister MM, Pitt WC, Zemlicka DE. Coyotes (Canis latrans) are definitive hosts of Neospora caninum. Int J Parasitol 2004;34:159-161.
- 7. Sadrebazzaz A, Haddadzadeh H, Esmailnia K, Habibi GR, Vojgani G, Hashemi-Fesharaki R. Serological prevalence of Neospora caninum in healthy and aborted dairy cattle in Mashhad, Iran. Vet Parasitol 2004;124:201-204.
- 8. Razmi GR, Mohammadi GR, Garrosi T, Farzaneh N, Fallah AH, Maleki M. Seroepidemiology of Neospora caninum infection in dairy cattle herds in Mashhad area, Iran. Vet Parasitol 2006;135:187-189.
- 9. Haddadzadeh HR, Sadrebazzaz A, Malmasi A, Talei Ardakani H, Khazraii Nia P, Sadreshirazi N. Seroprevalence of Neospora caninum infection in dogs from rural and urban environments in Tehran, Iran. Parasitol Res 2007;101:1563-1565.
- 10. Malmasi A, Hosseininejad M, Haddadzadeh HR, Badii A, Bahonar A. Serologic study of anti-Neospora caninum antibodies in household dogs and dogs living in dairy and beef cattle farms in Tehran, Iran. Parasitol Res 2006;100:1143-1145.
- 11. Razmi GR, Maleki M, Farzaneh N, Talebkhan Garoussi M, Fallah AH. First report of Neospora caninum-associated bovine abortion in Mashhad area, Iran. Parasitol Res 2007;100:755-757.
- 12. Lindsay DS, Dubey JP, Duncan RB. Confirmation that the dog is a definitive host for Neospora caninum. Vet Parasitol 1999;82:327-333.
- 13. Schares G, Heydorn AO, Cüppers A, Conraths FJ, Mehlhorn H. Hammondia heydorni-like oocysts shed by a naturally infected dog and Neospora caninum NC-1 cannot be distinguished. Parasitol Res 2001;87:808-816.
- 14. Schares G, Pantchev N, Barutzki D, Heydorn AO, Bauer C, Conraths FG. Oocysts of Neospora caninum, Hammondia heydorni, Toxoplasma gondii and Hammondida hammondi in faeces collected from dogs in Germany. Int J Parasitol 2005;35:1525-1537.
- 15. Schares G. In Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D eds, Neosporosis: Oocyst detection and differentiation. Protozoal Abortion in Farm Ruminants. 2007, Wallingford, England. CAB International; pp 54-58.
- 16. Mattsson JG, Müller N. In Ortega-Mora LM, Gottstein B, Conraths FJ, Buxton D eds, Neosporosis: Polymerase chain reaction. Protozoal Abortion in Farm Ruminants. 2007, Wallingford, England. CAB International; pp 59-63.
- 17. Müller N, Zimmermann V, Hentrich B, Gottstein B. Diagnosis of Neospora caninum and Toxoplasma gondii infection by PCR and DNA hybridization immunoassay. J Clin Microbiol 1996;34:2850-2852.
- 18. Sager HC, Moret CS, Müller N, Staubli D, Esposito M, Schares G, Hässig M, Stärk K, Gottstein B. Incidence of Neospora caninum and other intestinal protozoan parasites in populations of Swiss dogs. Vet Parasitol 2006;139:84-92.
- 19. Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW. Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine placenta but not after ingestion of colostrums spiked with Neospora caninum tachyzoites. Int J Parasitol 2001;31:747-752.
- 20. Gondim LF, Gao L, McAllister MM. Improved production of Neospora caninum oocysts, cyclical oral transmission between dogs and cattle, and in vitro isolation from oocysts. J Parasitol 2002;88:1159-1163.
- 21. Basso W, Venturini L, Venturini MC, Hill DE, Kwok OCH, Shen SK, Dubey JP. First isolation of Neospora caninum from the feces of a naturally infected dog. J Parasitol 2001;87:612-618.
- 22. Šlapeta JR, Modrý D, Kyselová I, Horejš R, Lukeš J, Koudela B. Dog shedding oocysts of Neospora caninum: PCR diagnosis and molecular phylogenetic approach. Vet Parasitol 2002;109:157-167.
- 23. McGarry JW, Stockton CM, Williams DJL, Trees AJ. Protracted shedding of oocysts of Neospora caninum by a naturally infected foxhound. J Parasitol 2003;89:628-630.
- 24. McInnes LM, Irwin P, Palmer DG, Ryan UM. In vitro isolation and characterisation of the first canine Neospora caninum isolate in Australia. Vet Parasitol 2006;137:355-363.
- 25. Trees AJ, McAllister MM, Guy CS, McGarry JW, Smith RF, Williams DJL. Neospora caninum: Oocyst challenge of pregnant cows. Vet Parasitol 2002;109:147-154.
- 26. De Marez T, Liddell S, Dubey JP, Jenkins MS, Gasbarre L. Oral infection of calves with Neospora caninum oocysts from dogs: Humoral and cellular immune responses. Int J Parasitol 1999;29:1647-1657.
- 27. Björkman C, Gondim LFP, Nöslund K, Trees AJ, McAllister MM. IgG avidity pattern in cattle after ingestion of Neospora caninum oocysts. Vet Parasitol 2005;128:195-200.
Fig. 1Unsporulated (left arrow) and sporulated (right arrow) oocysts of Hammondia Neospora-like oocysts (HNLO). ×400.

Fig. 2Detection of Neospora caninum by PCR with positive bands at 337 bp. M, marker of 50 bp ladder; P, positive control; N, negative control; lanes 1-4, samples.

Table 1.Diagnostic techniques to detect Neospora caninum oocysts in fecal samples of dogs
Table 1.
|
Fecal examination
|
N. caninum-specific PCR
|
Bioassay in calves
|
|
No. examined |
No. HNLO* positive |
No. examined |
No. positive |
No. examined |
Results |
|
Farm dogs |
89 |
4 |
4 |
2 |
2 |
Negative |
|
Household dogs |
85 |
0 |
0 |
0 |
0 |
0 |
|
Total |
174 |
4 |
4 |
2 |
2 |
Negative |