Abstract
The prevalence of fish-borne trematodes (FBT), including Clonorchis sinensis, is still high in riverside areas of the Republic of Korea. The author reviewed the detection and identification methods, differential keys, fish intermediate hosts, and morphological characteristics of FBT metacercariae. FBT metacercariae found in freshwater fish are classified mainly into 4 families, i.e., Opisthorchiidae, Heterophyidae, Echinostomatidae, and Clinostomidae. The metacercariae of C. sinensis, found in 40 species of freshwater fish, are elliptical and 0.15-0.17 × 0.13-0.15 mm in size, have nearly equal sized oral and ventral suckers, brownish pigment granules, and an O-shaped excretory bladder. Their general morphologies are similar to those of Metorchis orientalis (except in the thickness of the cyst wall). Metagonimus spp. (M. yokogawai, M. takahashii, and M. miyatai) metacercariae are subglobular or disc-shaped, and 0.14-0.16 mm in diameter. They have yellow-brownish pigment granules, a ventral sucker deflectively located from median, and a V-shaped excretory bladder. The metacercariae and fish intermediate hosts of Centrocestus armatus, Clinostomum complanatum, and 3 echinostomatid flukes (Echinostoma hortense, E. cinetorchis, and Echinochasmus japonicus) were summarized. FBT metacercariae detected in brackish water fish are mainly members of the Heterophyidae. The morphological characters, identification keys, and fish intermediate hosts of 7 species (Heterophyes nocens, Heterophyopsis continua, Pygidiopsis summa, Stellantchasmus falcatus, Stictodora fuscata, Stictodora lari, and Acanthotrema felis) were also reviewed. The contents treated in this study will provide assistance at the laboratory bench level to those working on recovery of metacercariae from fish hosts and identifying them.
-
Key words: fish-borne trematode (FBT), metacercaria, freshwater fish, brackish water fish, identification, differential key
INTRODUCTION
The fish-borne trematode (FBT) infections affect the health of more than 18 million people around the world, particularly in Asian countries. Humans are mainly infected with FBT when they eat raw or inadequately cooked fish contained infective larvae, metacercariae. These flukes provoke remarkable morbidity and cause serious damage to aquaculture, which is a valuable source of food and employment in developing countries. Freshwater and brackish water fish play a major role as the source of human infections with foodborne trematodes, which are receiving increasing attention as the information on their diversity and prevalence emerges in some Asian countries [
1-
3]. Whereas, some other kinds of animals, i.e., marine fish, mollusca, crustaceae, insects, amphibia and reptiles, act less commonly as the infection source or second intermediate hosts of trematodes. Accordingly, elimination of these parasites from the food supply, especially fish, is a very important work in epidemiological points of view.
The liver flukes,
Clonorchis sinensis and
Opisthorchis spp. (not existing in Korea), have been known as the representative FBT. In addition, many species of intestinal flukes, mainly those belonging to the Heterophyidae and Echinostomatidae are also contracted to humans by eating raw fish. In the Republic of Korea, many species of FBT including
C. sinensis have been reported.
C. sinensis is still prevalent in riverside areas and is the most important helminth species in aspects of public health. About 11 species of the Heterophyidae and 3 species of the Echinostomatidae have been reported as the intestinal flukes infected by eating raw fish meat [
2-
5].
Recent trends of helminthic infection in the Republic of Korea are characterized by a remarkable decrease of soil-transmitted nematodiases and moderate endemicity of foodborne trematodiases. Especially, the prevalence of FBT infections, such as
C. sinensis and intestinal flukes, has maintained at relatively high levels in riverside areas [
5-
7]. Therefore, it is worthwhile to pay attention to the infective stage and source of human infections with FBT in epidemiological points of view. In this paper, the FBT metacercariae of medical importance in the Republic of Korea are briefly reviewed to provide assistance at the laboratory bench level to those working for recovery of metacercariae from the second intermediate hosts.
DETECTION OF METACERCARIAE
Examination of FBT metacercariae in the second intermediate hosts is commonly done by 2 methods, i.e., muscle compression and pepsin-HCl artificial digestion techniques. The compression method is done by following step-by-step procedures.
Identify the species of fish.
Weigh and measure the size of each fish and record.
Take some of the flesh from different parts of fish (e.g., head, gill, muscles, fin, scale, intestine, other viscera).
Weigh each sample to estimate metacercarial density.
Compress each sample between 2 glass slides (repeat 2-3 times to increase the detection rate).
Observe and identify metacercariae under stereomicroscopy.
Count the number of metacercariae and calculate density.
On the other hand, the artificial digestion method is more complicated, and its step-by-step procedures are as follows.
Identify the species of fish.
Weigh and measure the size of each fish and record.
Take some of the flesh from different parts of fish (e.g., head, gill, muscles, fin, scale, viscera). Whole fish can be examined at one time.
Grind fish flesh one by one (or small fish can be grouped to be ground at one time) in a mortar with pestle.
Transfer the ground sample into a beaker containing artificial gastric juice (conc. HCl 8 ml + pepsin 1:10,000 6 g + distilled water 1,000 ml).
Mix well and place in a 37℃ incubator for 2 hr (or longer for hard parts like fin or scale) with occasional stirring.
Remove the larger particles (bones, scales, fins, and undigested materials) by the filtration (1 × 1 mm of mesh) of digested materials.
Add 0.85% saline, and let it stand for a while.
Discard supernatant very carefully and keep the sediment.
Repeat procedures 8 and 9 several times until the supernatant becomes clear.
Transfer the sediment a small bit into a Petri dish containing 6-7 ml physiological saline.
Observe and identify metacercariae using a stereomicroscope and light microscope (see Identification Method).
Isolate the metacercariae and put into a small dish.
Count the number of metacercariae of each fluke species.
Prepare for experimental infection to laboratory animals.
Store in a refrigerator until use.
Advantages of the compression method include being able to know the exact location and infection site of metacercariae in fish examined and economical without use of expensive reagents, such as pepsin. Whereas, in the digestion method, a large amount of samples can be dealt with, metacercariae can be isolated and purified, excellent morphologies can be obtained and more easily identified, and exact numbers of metacercariae can be prepared for experimental infections.
IDENTIFICATION OF METACERCARIAE
For the identification of FBT metacercariae, the following general procedures will be help. First of all, collect separately similar-shaped metacercariae based on the general feature in a small Petri dish. Second, move them with a spoid on a glass slide, cover a cover slip, and observe detailed morphology under a light microscope. Finally, identify the metacercariae based on characteristic features and dimensions. As the characteristic features, the shape of cysts, presence and size of suckers, and shape and contents of excretory bladder are important.
On the other hand, if the morphological features of metacercariae are not obvious and difficult to see, it should be excysted using the techniques described below. When the cyst wall is very thin, metacercariae can easily be liberated by giving only a slight pressure on the cover slip. Otherwise (when the cyst wall is thick and elastic), the artificial digestion methods are recommended. There are several published procedures for digesting the cyst and freeing the metacercariae. Basically, they involve incubating the metacercaiae in trypsin or bile at 37℃ for a brief interval [
8-
12].
More than 16 species of digenetic trematode metacercariae in 7 families have been found in freshwater fish from the Republic of Korea (
Table 1). Among them, 8 species in 4 families, Opisthorchiidae (
Clonorchis sinensis), Heterophyidae (3
Metagonimus spp. and
Centrocestus armatus), Echinostomatidae (
Echinostoma hortense,
E. cinetorchis, and
Echinochasmus japonicus) and Clinostomidae (
Clinostomum complanatum), are infected by eating raw flesh of freshwater fish in the Republic of Korea.
The metacercariae of
C. sinensis are elliptical and 0.15-0.17 × 0.13-0.15 mm in size, have nearly equal sized oral and ventral suckers, brownish pigment granules, and an O-shaped excretory bladder. Their general morphologies are similar to those of
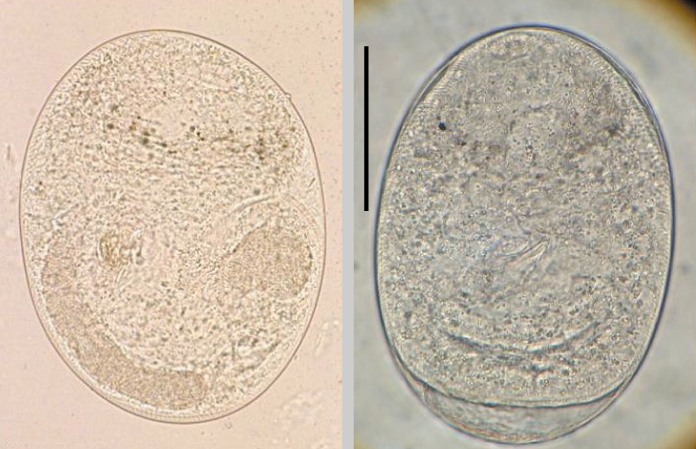
Opisthorchis viverrini, which is prevalent in Thailand, Vietnam, and Lao PDR, except the size of cysts (0.19-0.25 × 0.15-0.22 mm) (
Fig. 1) and to those of
Metorchis orientalis (0.156-0.188 × 0.138-0.170 mm in size) except the thickness of the cyst wall (5-19 µm) (
Fig. 2). The metacercariae of
C. sinensis can be differentiated from those of
Metagonimus spp., although the size and shape of cysts are similar each other; the size and location of suckers are somewhat different (
Fig. 3).
A large number of
Metagonimus sp. metacercariae are encysted in the fin and scale of fish intermediate hosts. They are subglobular or disc-shaped and 0.14-0.16 mm in diameter, and have yellow-brownish pigment granules, a ventral sucker deflectively located from median, and a V-shaped excretory bladder. The metacercariae of
Metagonimus spp. cannot be differentiated morphologically at a species level. Due to host specificity,
M. yokogawai metacercariae are dominantly collected from sweetfish,
Plecoglossus altivelis,
M. miyatai metacercariae are detected in the pale chub,
Zacco platypus and dark chub,
Z. terminckii, and
M. takahashii metecercariae are found in the crucian carp,
Carassius auratus (
Fig. 4). The metacercariae of another heterophyid fluke,
Centrocestus armatus, are frequently encysted in the viscera of fish hosts. They are elongated and elliptical, 0.20-0.25 × 0.10-0.12 mm in size, have 40-44 circumoral spines arranged in 2 rows around the oral sucker, and X-shaped excretory bladder (
Fig. 5).
Three species of echinostome metacercariae, i.e.
Echinostoma hortense,
E. cinetorchis, and
Echinochasmus japonicus, are found in freshwater fish. They are characterized by an oral sucker surrounded by prominent collar spines and excretory granules in 2 rows of excretory tubes.
E. hortense metacercariae are globular or elliptical, 0.17-0.19 × 0.15-0.16 mm in size, and have a double layered cyst wall, 27-28 collar spines, and a ventral sucker transversely elliptical, 2-fold as larger as the oral sucker (
Fig. 6).
E. cinetorchis metacercariae are rounded, 0.13-0.14 mm in diameter, and have 37 collar spines around the oral sucker and a ventral sucker lying in the middle of the body and larger than the oral sucker (
Fig. 7).
E. japonicus metacercariae are mainly encysted in the gill filaments of fish intermediate hosts. They are elliptical and very small, 0.077-0.080 × 0.054-0.060 mm in size, have a transparent and double layered cyst wall, 24 dorsally interrupted collar spines, and a ventral sucker lying median at posterior 1/3 of the body and somewhat smaller than the oral sucker (
Fig. 8).
The metacercariae of
C. complanatum have a very thin cyst wall, and thus they are easily liberated from the cysts during the process of collection. The excysted metacercariae are big (3.28-4.27 × 0.94-1.46 mm), tongue-shaped, and progenetic (
Fig. 9).
1.a. Brownish pigment granules present in 2
1.b. Brownish pigment granules absent in body 5
2.a. Cyst wall thin or thick; ventral sucker prominent, approximately same size as oral sucker 3
2.b. Cyst wall relatively thin; ventral sucker deflectively located from median Metagonimus spp.
3.a. Cyst wall relatively thin Clonorchis sinensis
3.b. Cyst wall very thick 4
4.a. Outer cyst wall below 30 µm thickness Metorchis orientalis
4.b. Outer cyst wall over 50 µm thickness M. taiwanensis
5.a. Spines present around the oral sucker 6
5.b. Spines absent around the oral sucker 9
6.a. Spines arranged in 2 rows just around the oral sucker, excretory bladder x-shaped Centrocestus armatus
6.b. Spines arranged in a single or 2 row, granules in excretory bladder arranged in 2 rows of tube 7
7.a. Arrangement of collar spines dorsally interrupted Echinochasmus spp.
7.b. Arrangement of collar spines not interrupted 8
8.a. No. of collar spines: 27 Echinostoma hortense
8.b. No. of collar spines: 37 Echinostoma cinetorchis
9.a. Excretory bladder distributed in the whole body 10
9.b. Excretory bladder mainly located in the posterior body 11
10.a. Rounded, with thick cyst wall and w-shaped excretory bladder Cyathocotyle orientalis
10.b. Elliptical, with thin cyst wall and 8-shaped excretory bladder Holostephanus nipponicus
11.a. Excretory bladder occupying large part of posterior body, and V-shaped Matacercaria hasegawai
11.b. Excretory bladder Y or V-shaped, and it's arms extended to pharynx-level 12
12.a. Excretory bladder V-shaped, with a pair of eye spots Exorchis oviformis
12.b. Excretory bladder Y-shaped Pseudexorchis major
Metacercariae in brackish water fish in the Republic of Korea
More than 10 species of digenetic trematode metacercariae in 3 families have been found in brackish water fishes in the Republic of Korea (
Table 2). Among them, 9 species in 2 families, Heterophyidae (
Hetrophyes nocens,
Heterophyopsis continua,
Pygidiopsis summa,
Sellantchasmus falcatus,
Metagonimus takahashii,
Stictodora fuscata,
Stictodora lari, and
Acanthotrema felis), and Echinostomatidae (
Echinostoma hortense), are listed as FBT in the Republic of Korea.
The metacercariae of
H. nocens are round or elliptical, 0.13-0.22 × 0.08-0.17 mm in size, with brownish pigment scattered throughout body, a ventral sucker elliptical and larger than the oral sucker, a genital sucker elliptical and lying closely right lateral posteriorly to the ventral sucker, and an excretory bladder O-shaped (
Fig. 10A).
H. continua metacercariae are rounded, 0.38 mm in diameter, and have a cyst wall of about 6 µm thick, a ventral sucker larger than the oral sucker, a genital sucker elliptical and lying dextroposteriorly to the ventral sucker, and an excretory bladder Y-shaped (
Fig. 10B).
P. summa metacercariae are elliptical, 0.24-0.29 × 0.23-0.28 mm in size, and have a pair of eyespots, genital apparatus crescent formed and lying to the dextroanterior margin of the ventral sucker, and an X-shaped excretory bladder (
Fig. 11A).
S. falcatus metacercariae are elliptical, 0.15-0.20 × 0.13-0.15 mm in size, and have a ventral sucker lying dextrally to median between 2 ceca, a genital atrium lying to the dextrolateral margin of the ventral sucker (
Fig. 11B).
S. fuscata macercariae are elliptical, 0.19-0.52 × 0.16-0.38 mm in size, and have a very thin and transparent cyst wall and an elliptical gonotyl armed with 12-15 chitinous spines (
Fig. 12A).
S. lari metacercariae are long elliptical, 0.39-0.43 × 0.32-0.35 mm in size, and have a very thin and transparent cyst wall and a gonotyl armed with 60-80 small spines (
Fig. 12B).
Acanthotrema felis metacercariae are elongated elliptical, 0.22-0.25 × 0.15-0.19 mm in size, and have a cyst wall very thin and transparent and a ventrogenital sac which is elliptical and equipped with fork-like sclerites (
Fig. 13).
1.a. Excretory bladder black and distinctly present 2
1.b. Excretory bladder vague or absent 5
2.a. Ventral sucker prominent, genital sucker present 3
2.b. Ventral sucker smaller, genital sucker absent 4
3.a. Elliptical, with thin cyst wall and brownish pigment Heterophyes nocens
3.b. Rounded, with thick cyst wall Heterophyopsis continua
4.a. Genital apparatus located at anterior margin of ventral sucker, x-shaped excretory bladder Pygidiopsis summa
4.b. Genital atrium with long expulser (seminal vesicle) Stellantchasmus falcatus
5.a. Long elliptical, 0.39-0.43 × 0.32-0.35 mm in size, cyst wall very thin and transparent, gonotyl elliptical, armed with 60-80 small spines Stictodora lari
5.b. Elliptical, 0.19-0.52 × 0.16-0.38 mm in size, cyst wall very thin and transparent, gonotyl elliptical, armed with 12-15 chitinous spines Stictodora fuscata
5.c. Long elliptical, 0.22-0.25 × 0.15-0.19 mm in size, cyst wall very thin and transparent, ventrogenital sac elliptical, enclosed fork-like sclerites Acanthotrema felis
The differential keys for digenetic trematode metacercariae are intended to assist identification of metacercariae recovered in fish from the Republic of Korea. It is not complete to cover all members of trematodes. However, it can be helpful for the identification of FBT metacercariae encountered in fish in the Republic of Korea.
FISH HOSTS
FBT metacercariae encysted in freshwater fish mainly belong to 4 families, Opisthorchiidae, Heterophyidae, Echinostomatidae and Clinostomidae. Nowadays,
C. sinensis of family Opisthorchiidae is the most important FBT in the Republic of Korea. The metacercariae of
C. sinensis have been detected in 42 species of freshwater fish (31 genera in 6 families). However, according to the FishBase site in internet [
12],
Culter brevicauda and
Erythroculter erythropterus are treated as the synonym and then their scientific names were changed into
Chanodichthys erythropterus.
Hemiculter eigenmanni is treated as the same species as
H. leucisculus, and
Acheilognathus lanceolata is changed into
Tanakia lanceolata. Therefore, total 40 species of freshwater fish (31 genera in 6 families) act as the second intermediate hosts of
C. sinensis in the Republic of Korea (
Table 3). In China, including Taiwan, total 102 species of freshwater fish of 59 genera in 15 families have been reported as the second intermediate hosts for
C. sinensis [
30].
As members of the Heterophyidae,
3 Metagonimus spp. (
M. yokogawai,
M. takahashii, and
M. miyatai) and
Centrocestus armatus are prevalent in freshwater fish in the Republic of Korea, and their fish intermediate hosts are listed in
Table 4. Only 3 fish species (
Plecoglossus altivelis,
Tribolodon taczanowskii, and
Lateolabrax japonicus) have been reported as the second intermediate hosts for
M. yokogawai, and 4 (
Carassius auratus,
P. altivelis,
T. taczanowskii, and
L. japonicus) and 2 fish species (
Zacco platypus and
Z. temminckii) listed as hosts for
M. takahashii and
M. miyatai, respectively. The fish intermediate hosts of
M. yokogawai are the same species as those of
M. takahashii, except
C. auratus.
M. miyatai revealed a host-specificity at the selection of the second intermediate host. Many fish species have been listed as hosts of unknown
Metagonimus sp. Studies on differentiation of
Metagonimus sp. metacercariae are needed.
There are 3 species of echinostomatid metacercariae, i.e.,
Echinostoma hortense,
Echinostoma cinetorchis, and
Echinochasmus japonicus, found from freshwater fish in the Republic of Korea (
Table 5).
Clinostomum complanatum metacercariae were found in 12 species of freshwater fish,
Acheilognathus koreensis,
Acheilognathus rhombea (=
A rhombeus),
Acheilognathus yamatsutae,
Carassius auratus,
Cobitis sinensis,
Microphysogobio yaluensis,
Pseudorasbora parva,
Pungtungia herzi,
Rhodeus uyekii,
Squalidus chankaesis tsuchigae (=
S. gracilis gracilis),
Squalidus gracilis maejimae, and
Zacco temminkii, as reported by Chung et al. [
49].
FBT metacercariae detected in brackish water fish are mainly those of the Heterophyidae, including
Heterophyes nocens,
Heterophyopsis continua,
Pygidiopsis summa,
Stellantchasmus falcatus,
Stictodora fuscata,
S. lari, and
Acanthotrema felis. Their second intermediate hosts are designated in
Table 6.
CONCLUSION
The detection and identification methods, differential keys, the second intermediate hosts, and morphological characteristics of fish-borne zoonotic trematode metacercariae in the Republic of Korea were briefly reviewed. The items treated in this paper will be useful to understand the epidemiology of fish-borne trematodiasis in the Republic of Korea, and to provide assistance at the laboratory bench level to those who work on metacercariae in fish hosts.
References
- 1. WHO. Food-borne trematode infections in Asia. Report of Joint WHO/FAO Workshop. 2002. Hanoi, Vietnam.
- 2. Chai JY, Murrell KD, Lymbry A. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 2005;35:1233-1254.
- 3. Chai JY. In Murrell KD, Fried B eds, Intestinal flukes. Food-Borne Parasitic Zoonoses: Fish and Plant-Borne Parasites. 2007, World Class Parasites, Vol. 11:New York, USA. Springer; pp 53-115.
- 4. Rim HJ. Epidemiology and control of clonorchiasis in Korea. Southeast Asian J Trop Med Public Health 1997;28(suppl):47-50.
- 5. Cho SH, Lee KY, Lee BC, Cho PY, Cheun HI, Hong ST, Sohn WM, Kim TS. Prevalence of clonorchiasis in southern endemic areas of Korea in 2006. Korean J Parasitol 2008;46:133-137.
- 6. Hong ST, Chai JY, Choi MH, Huh S, Rim HJ, Lee SH. A successful experience of soil-transmitted helminth control in the Republic of Korea. Korean J Parasitol 2006;44:177-185.
- 7. Korea Association of Health Promotion and Korean Center for Disease Control and Prevention. Prevalence of Intestinal Parasites in Korea. The 7th Report. 2004, Seoul, Korea. Art Motion.
- 8. Komiya K. In Morishita K, Komiya Y, Matsubayashi H eds, Metacercariae in Japan and adjacent territories. Progress of Medical Parasitology in Japan. 1965, Vol. II:Tokyo, Japan. Meguro Parasitological Museum; pp 14-20.
- 9. Fried B. In Fried B, Graczyk TK eds, Maintenance, cultivation, and excystation of echinostomes. Echinostomes as Experimental Models for Biological Research. 2000, Dordrecht. Kluwer Academic Publishers; pp 99-118.
- 10. Sukantason KL, Sukantason K, Kuntalue B, Boonsriwong N, Piangjai S, Chaithong U, Vanittanakom P. Surface ultrastructure of excysted metacercariae of Haplorchis taichui (Trematoda: Heterophyidae). Southeast Asian J Trop Med Public Health 2000;31:747-754.
- 11. Ooi HK, Chen CI, Oku Y. Excystation of Haplorchis taichui metacercariae could be elicited by change in pH. Jpn J Vet Res 2002;50:3-7.
- 12. Li S, Chung YB, Chung BS, Choi MH, Yu JR, Hong ST. The involvement of the cysteine proteases of Clonorchis sinensis metacercariae in excystment. Parasitol Res 2004;93:36-40.
- 13. Search FishBase. http://www.fishbase.org/search.php
- 14. Kobayashi H. On the life history and morphology of the liver distoma (Clonorchis sinensis). Mitt Med Hochsch Keijo 1917;1:251-289.
- 15. Lee SK, Kim KS. An epidemiological study of Clonorchis sinensis. J Taegu Med J 1958;1:1-7.
- 16. Seo BS. The in vitro excystation of metacercariae of Clonorchis sinensis. New Med J 1959;2:1389-1397.
- 17. Chun SK. Studies on some trematodes whose intermediate hosts are fishes in the Naktong River. Bull Pusan Fish Coll 1962;4:21-38.
- 18. Shin DS. Epidemiological studies of Clonorchis sinensis prevailed in the peoples of Kyungpook Province. Korean J Parasitol 1964;2:1-13.
- 19. Lee JT. Studies on the metacercariae from freshwater fishes in the Kumho River. Korean J Parasitol 1968;6:77-99.
- 20. Kim DC. Ecological studies of Clonorchis sinensis. Endemicity and propagation of clonorchiasis in high and low endemic areas in Korea. Yonsei Rep Trop Med 1974;5:3-44.
- 21. Choi DW. Clonorchis sinensis in Kyungpook province, Korea 2. Demonstration of metacercaria of Clonorchis sinensis from fresh water fish. Korean J Parasitol 1976;14:10-16.
- 22. Joo CY. Epidemiological studies of Clonorchis sinensis in vicinity of River Taewha, Kyungnam Province, Korea. Korean J Parasitol 1980;18:199-214.
- 23. Hwang JT, Choi DW. Changing pattern of infestation with larval trematodes from freshwater fish in river Kumho, Kyungpook Province, Korea. Kyungpook Uni Med J 1980;21:460-475.
- 24. Rhee JK, Lee HI, Baek BK, Kim PG. Survey on encysted cercariae of trematodes from freshwater fishes in Mangyeong riverside area. Korean J Parasitol 1983;21:187-192.
- 25. Rhee JK, Rim MH, Baek BK, Lee HI. Survey on encysted cercariae of trematodes from freshwater fishes in Tongjin riverside areas in Korea. Korean J Parasitol 1984;22:190-202.
- 26. Rim HJ. The current pathobiology and chemotherapy of clonorchiasis. Korean J Parasitol 1986;24(suppl):1-141.
- 27. Joo CY, Hong YA. Epidemiological studies of Clonorchis sinensis in the vicinity of River Ahnseong, Kyungpook Province, Korea. Jpn J Parasitol 1991;40:542-552.
- 28. Nam HS, Sohn WM. Infection status with trematode metacercariae in pond smelts, Hypomesus olidus. Korean J Parasitol 2000;38:37-39.
- 29. Moon HD, Na BK, Sohn WM. Infection status of trematode in residents of riverside villages and in freshwater fishes from Gyeonghogang (River) in Sancheong-gun, Gyeongsangnam-do, Korea. Gyeongsang Institute Health Sci J 2007;3:187-204.
- 30. Xu LQ, Yu SH, Chen YD. In Arizono N, Chai JY, Nawa Y, Takahashi Y eds, Clonorchiasis sinensis in China. Food-Borne Helminthiasis in Asia. 2004, Asian Parasitology, Vol. 1:Chiba, Japan. Federation of Asian parasitologists; pp 1-26.
- 31. Chun SK. A study on Metagonimus yokogawai from Plecoglossus altivelis in the Miryang River. Bull Pusan Fish Coll 1960a;3:24-32.
- 32. Choi DW, Lee JT, Hwang HK, Shin YD. Studies of the larval trematodes from brackish water fishes. 2. Observation on Metagonimus yokogawai Katsurada, 1912. Korean J Parasitol 1966;4:33-37.
- 33. Ahn YK. Lateolabrax japonicus, a role of second intermediate host of Metagonimus yokogawai. New Med J 1983;26:135-139.
- 34. Chun SK. A study on the metacercaria of Metagonimus takahashii and Exorchis oviformis from Carassius carassius. Bull Pusan Fish Coll 1960b;3:31-39.
- 35. Chai JY, Sohn WM, Kim MH, Hong ST, Lee SH. Three morphological types of the genus Metagonimus encysted in the dace, Tribolodon taczanowskii, caught from Sumjin River. Korean J Parasitol 1991;29:217-225.
- 36. Rim HJ, Kim KH, Joo KH. Classification and host specificity of Metagonimus spp. from Korean freshwater fish. Korean J Parasitol 1996;34:7-14.
- 37. Kim DG, Kim TS, Cho SH, Song HJ, Sohn WM. Heterophyid metacercarial infections in brackish water fishes from Jinju-man (Bay), Kyongsangnam-do, Korea. Korean J Parasitol 2006;44:7-13.
- 38. Saito S, Chai JY, Kim KH, Lee SH, Rim HJ. Metagonimus miyatai sp. nov. (Digenea: Heterophyidae), a new intestinal trematode transmitted by freshwater fishes in Japan and Korea. Korean J Parasitol 1997;35:223-232.
- 39. Kim CH. Study on the Metagonimus sp. in Gum River Basin, Chungchung-nam Do, Korea. Korean J Parasitol 1980;18:215-228.
- 40. Kim JH, Choi DW. Infestation with larval trematodes from freshwater fish in natural and fish breeding ponds. Korean J Parasitol 1981;19:157-166.
- 41. Kim CH, Kim NM, Lee CH, Park JS. Studies on the Metagonimus fluke in the Daecheong reservoir and upper stream of Geum River. Korean J Parasitol 1987;25:69-82.
- 42. Hong SJ, Woo HC, Kim IT. Study on Centrocestus armatus in Korea. I. Infection status of Zacco platypus and Z. temminckii with the metacercariae of C. armatus. Korean J Parasitol 1989;27:41-46.
- 43. Chai JY, Hong SJ, Sohn WM, Lee SH, Seo BS. Studies on intestinal trematode in Korea. XVI. Infection status of loaches with the metacercariae of Echinostoma hortense. Korean J Parasitol 1985;23:18-23.
- 44. Ryang YS, Ahn YK, Lee KW, Kim TS, Hahn MH. Two cases of natural human infection by Echinostoma hortense and its second intermediate host in Wonju area. Korean J Parasitol 1985;23:33-40.
- 45. Ahn YK, Ryang YS. Experimental and epidemiological studies on the life cycle of Echinostoma hortense Asada, 1926 (Trematoda: Echinostomatidae). Korean J Parasitol 1986;24:121-136.
- 46. Lee SK, Chung NS, Ko IH, Sohn WM, Hong ST, Chai JY, Lee SH. An epidemiological survey of Echinostoma hortense infection in Chongsong-gun, Kyongbuk Province. Korean J Parasitol 1988;26:199-206.
- 47. Rim HJ, Kim KH, Joo KH, Kim SJ, Eom KS, Chung MS. The infestation states and changing patterns of human infecting metacercariae in freshwater fish in Kyongsang-do and Kyonggi-do, Korea. Korean J Parasitol 1996;34:95-105.
- 48. Sohn WM, Na BK, Cho SH. Echinostoma hortense and heterophyid metacercariae encysted in yellowfin goby, Acanthogobius flavimanus, from Shinan-gun and Muan-gun (Jeollanam-do), Korea. Korean J Parasitol 2009;47:307-310.
- 49. Seo BS, Park YH, Chai JY, Hong SJ, Lee SH. Studies on intestinal trematodes in Korea. XIV. Infection status of loaches with metacercariae of Echinostoma cinetorchis and their development in albino rats. Korean J Parasitol 1984;22:181-189.
- 50. Chung DI, Kong HH, Moon CH. Demonstration of the second intermediate hosts of Clinostomum complanatum in Korea. Korean J Parasitol 1995;33:305-312.
- 51. Seo BS, Cho SY, Chai JY, Hong ST. Studies on intestinal trematodes in Korea. II. Identification of the metacercariae of Heterophyes heterophyes nocens in mullets of three southern coastal areas. Seoul J Med 1980;21:30-38.
- 52. Seo BS, Hong ST, Chai JY, Cho SY. Studies on intestinal trematodes in Korea. IV. Geographical distribution of Pygidiopsis and Heterophyes metacercariae. Seoul J Med 1981;22:236-242.
- 53. Sohn WM, Kim JA, Song HJ. Two species of goby, Boleophthalmus pectinirostris and Scartelaos sp., as the new second intermediate host of heterophyid flukes in Korea. Korean J Parasitol 2005;43:161-164.
- 54. Kim DG, Kim TS, Cho SH, Song HJ, Sohn WM. Heterophyid metacercarial infections in brackish water fishes from Jinju-man (Bay), Kyongsangnam-do, Korea. Korean J Parasitol 2006;44:7-13.
- 55. Chun SK. A study on some trematodes whose intermediate host are brackish water fish. (1) The life history of Heterophyes continus, the intermediate host of which is Laterolabrax japonicus. Bull Pusan Fish Coll 1960c;3:40-44.
- 56. Seo BS, Lee SH, Chai JY, Hong SJ. Studies on intestinal trematodes in Korea. XIII. Two cases of natural human infection by Heterophyopsis continua and the status of metacercarial infection in brackish water fishes. Korean J Parasitol 1984;22:51-60.
- 57. Sohn WM, Han GG, Kho WG, Chai JY, Lee SH. Infection status with the metacercariae of heterophyid flukes in the brackish water fish from Haenam-gun, Chollanam-do, Korea. Korean J Parasitol 1994;32:163-169.
- 58. Cho SY, Kim SI. Plecoglossus altivelis as a new fish intermediate host of Heterophyopsis continua. Korean J Parasitol 1985;23:173-174.
- 59. Kim KH, Choi ES, Rim HJ. Conger myriaster, a new second intermediate host of Heterophyopsis continua. Korean J Parasitol 1996;34:283-285.
- 60. Chun SK. A study on some trematodes whose intermediate host are brackish water fish. (II) The life history of Pygidiopsis summa, the intermediate host of which is Mugil cephalus. Bull Pusan Fish Coll 1963;5:1-5.
- 61. Seo BS, Hong ST, Chai JY, Cho SY. Studies on intestinal trematodes in Korea IV Geographical distribution of Pygidiopsis and Heterophyes metacercariae. Seoul J Med 1981;22:236-242.
- 62. Chai JY, Sohn WM. Identification of Stellantchasmus falcatus metacercariae encysted in mullets in Korea. Korean J Parasitol 1988;26:65-68.
- 63. Sohn WM, Chai JY, Lee SH. Stictodora fuscatum (Heterophyidae) metacercariae encysted in gobies, Acanthogobius flavimanus. Korean J Parasitol 1994;32:143-148.
- 64. Chai JY, Park SK, Hong SJ, Choi MH, Lee SH. Identification of Stictodora lari (Heterophyidae) metacercariae encysted in the brackish water fish, Acanthogobius flavimanus. Kisaengchunghak Chapchi 1989;27:253-259.
- 65. Sohn WM, Han ET, Seo M, Chai JY. Identification of Acanthotrema felis (Digenea: Heterophyidae) metacercariae encysted in the brackish water fish Acanthogobius flavimanus. Korean J Parasitol 2003;41:101-105.
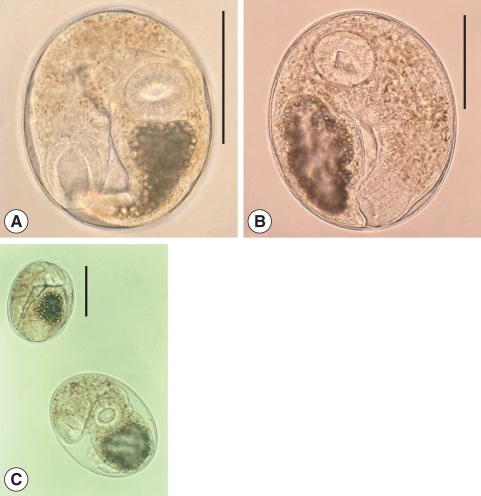
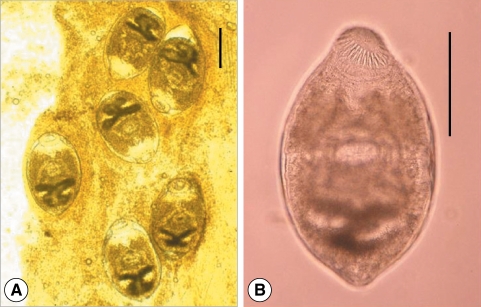
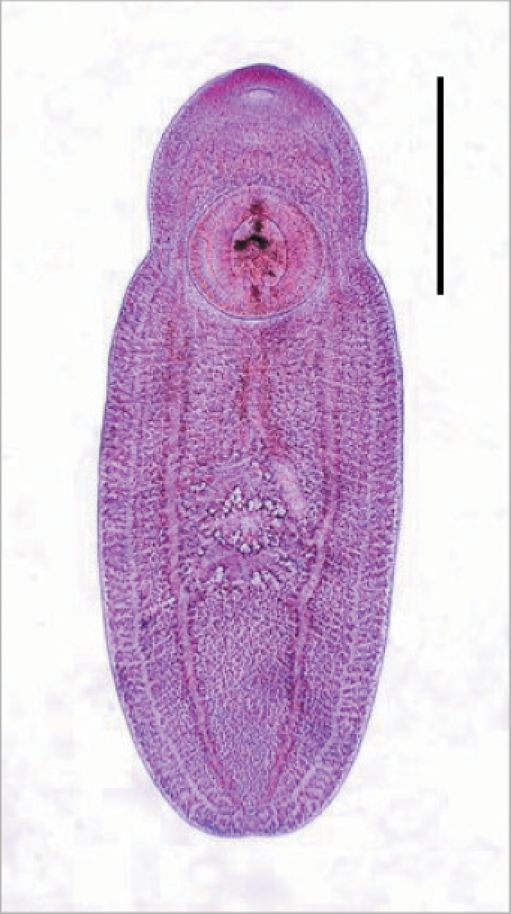
Fig. 1Metacercariae of Clonorchis sinensis (A: Korea) and Opisthorchis viverrini (B: Lao PDR). They have morphological similarities, such as elliptical shape, nearly equal sized oral and ventral suckers, brownish pigment granules, and an O-shaped excretory bladder. (C) The metacercaria of C. sinensis (upper) is comparatively smaller than that of O. viverrini (lower). Scale bar = 100 µm.

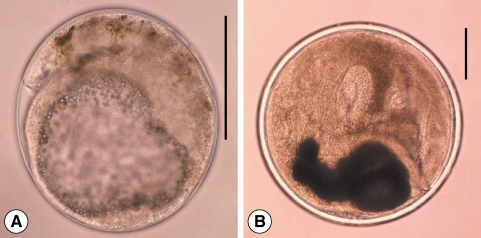
Fig. 2Metacercariae of Clonorchis sinensis (A) and Metorchis orientalis (B). (C) They are morphologically very similar except the thickness of the cyst wall. M. orientalis metacercaria (lower) is globular, 0.16-0.18 mm in diameter with a double layered cyst wall (outer cyst wall being 4 times as thick as that of C. sinensis; upper), and have nearly equal sized oral and ventral suckers and an O-shaped excretory bladder. Scale bar = 100 µm .

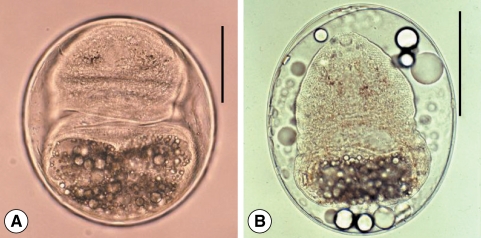
Fig. 3Metacercariae of Clonorchis sinensis (A) and Metagonimus sp. (B). (C) They are similar in size, and have yellow-brownish pigment granules in the body. However, the size of the ventral sucker and excretory bladder are somewhat different. Scale bar = 100 µm.

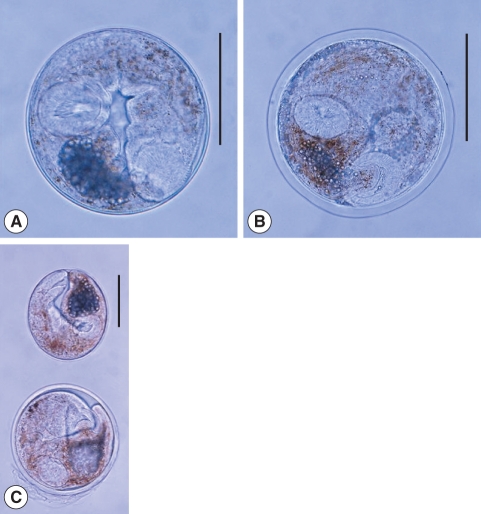
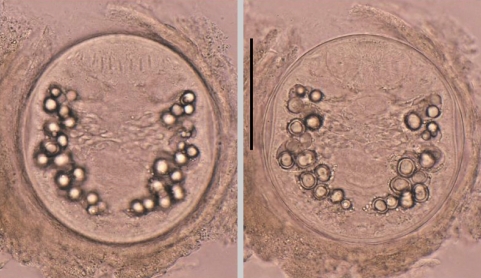
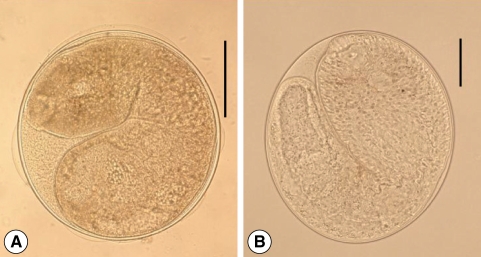
Fig. 4
Metagonimus spp. metacercariae. (A) Numerous metacercariae of M. yokogawai encysted on a scale of sweetfish, Plecoglossus altivelis. Scale bar = 200 µm. (B) A M. yokogawai metacercaria detected from a sweetfish. Scale bar = 100 µm. (C) A M. miyatai metacercaria collected from a pale chub, Zacco platypus. Scale bar = 100 µm. (D) A M. takahashii metacercaria detected from a crucian carp, Carassius auratus. They are subglobular or disc-shaped, and have yellow brownish pigment granules, a ventral sucker deflectively located from median and a V-shaped excretory bladder. Scale bar = 100 µm.

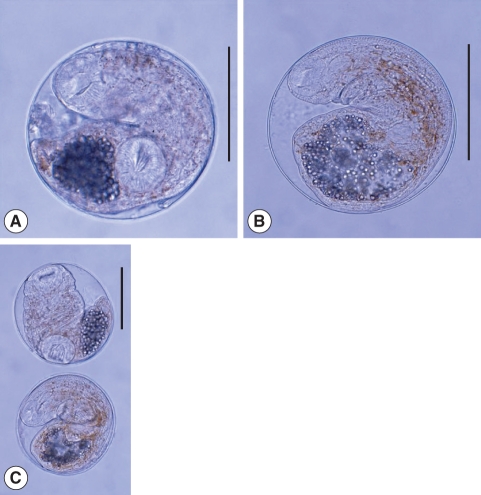
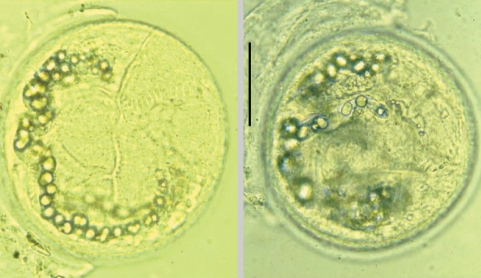
Fig. 5(A) Centrocestus armatus metacercariae encysted in the viscera of the pale chub, Z. platypus. (B) They are elongated elliptical and have 40-44 circumoral spines around the oral sucker arranged in 2 rows and an X-shaped excretory bladder. Scale bar = 100 µm.

Fig. 6(A) Echinostoma hortense metacercaria detected from a yellowfin goby, Acanthogobius flavimanus. It is globular or elliptical in shape, have a double layered cyst wall, 27-28 collar spines and a ventral sucker transversely elliptical, 2-fold as larger as the oral sucker. Scale bar = 100 µm.

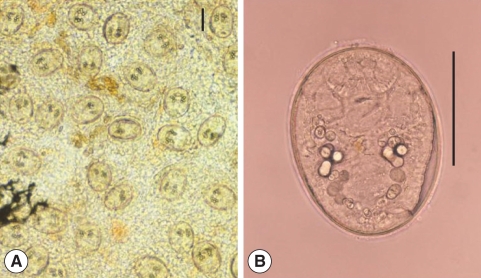
Fig. 7
Echinostoma cinetorchis metacercariae colleceted from a muddy loach, Misgurnus anguillicaudatus. They are rounded and have 37 collar spines around the oral sucker and a ventral sucker lying in the middle of the body and being larger than the oral sucker. Scale bar = 50 µm.

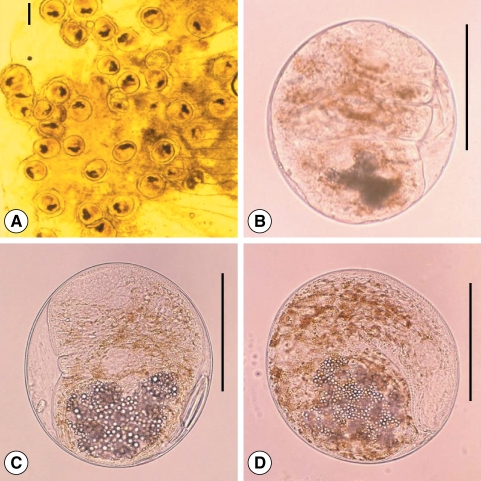
Fig. 8(A) Echinochasmus japonicus metacercariae encysted on the gills of Pseudorasbora parva. (B) They are elliptical and very small, have a transparent and double layered cyst wall, 24 dorsally interrupted collar spines, and a ventral sucker lying median at posterior 1/3 of the body. Scale bars = 50 µm.

Fig. 9Excysted metacercaria of Clinostomum complanatum found from a short barbel gudgeon, Squalidus japonicus coreanus (Semichon's acetocarmin stained), and is big and progenetic. It has a very thin cyst wall, and thus it is easily liberated from the cyst during the process of collection. Scale bar = 100 µm.

Fig. 10(A) Heterophyes nocens metacercaria detected from a yellowfin goby, A. flavimanus. It is round or elliptical, and has brownish pigment scattered throughout body, a ventral sucker larger than the oral sucker, an elliptical genital sucker lying closely right lateral posteriorly to the ventral sucker, and an O-shaped excretory bladder. Scale bar = 100 µm. (B) Heterophyopsis continua metacercaria collected from a yellowfin goby. It is rounded, and has a thick cyst wall (about 6 µm thickness), a ventral sucker larger than the oral sucker, an elliptical genital sucker lying dextroposteriorly to the ventral sucker, and a Y-shaped excretory bladder. Scale bar = 100 µm.

Fig. 11(A) Pygidiopsis summa metacercaria detected from a mullet, Mugil cephalus. It is elliptical, and has a pair of eyespots, a genital apparatus lying to the dextroanterior margin of the ventral sucker and an X-shaped excretory bladder. Scale bar = 100 µm. (B) Stellantchasmus falcatus metacercaria detected from a mullet. It is elliptical, and has a ventral sucker lying dextrally to median between 2 ceca and a genital atrium lying to the dextrolateral margin of the ventral sucker. Scale bar = 100 µm.

Fig. 12(A) Stictodora fuscata metacercaria detected from a yellowfin goby. It is elliptical, 0.19-0.52 × 0.16-0.38 mm in size, have a very thin and transparent cyst wall and an elliptical gonotyl armed with 12-15 chitinous spines. Scale bar = 100 µm. (B) Stictodora lari metacercaria detected from a yellowfin goby. It is elongated and elliptical, 0.39-0.43 × 0.32-0.35 mm in size, have a very thin and transparent cyst wall and a gonotyl armed with 60-80 small spines. Scale bar = 100 µm.

Fig. 13
Acanthotrema felis metacercaria detected from a yellowfin goby. It is elongated elliptical and has a very thin and transparent cyst wall and a ventrogenital sac enclosing fork-like sclerites. Scale bar = 100 µm.

Table 1.Zoonotic metacercariae encysted in freshwater fish in the Republic of Korea
Table 1.
|
Family |
Species |
|
Opisthorchiidae |
Clonorchis sinensis, Metorchis orientalis, M. taiwanensis
|
|
Heterophyidae |
Metagonimus yokogawai, M. miyatai, M. takahashii, Centrocestus armatus
|
|
Echinostomatidae |
Echinostoma hortense, E. cinetorchis, Echinochasmus japonicus
|
|
Clinostomidae |
Clinostomum complanatum
|
|
Cyathocotylidae |
Cyathocotyle orientalis, Holostephanus nipponicus
|
|
Cryptogonimidae |
Exorchis oviformis, Pseudoexorchis major
|
|
Bucephalidae |
Dollfustrema echinatum
|
Table 2.Zoonotic metacercariae encysted in brackish water fish in the Republic of Korea
Table 2.
|
Family |
Species |
|
Heterophyidae |
Heterophyes nocens, Heterophyopsis continua, Pygidiopsis summa, Sellantchasmus falcatus, Metagonimus takahashii, Stictodora fuscata, Stictodora lari, Acanthotrema felis
|
|
Echinostomatidae |
Echinostoma hortense
|
|
Bucephalidae |
Prosorhynchus uniporus
|
Table 3.The fish intermediate hosts of Clonorchis sinensis in the Republic of Korea
Table 3.
|
Family |
Genus |
Speciesa) (= valid name) |
|
Cyprinidae |
Abbottina
|
A. rivularis14), A. springeri25)
|
|
Acanthorhodeus
|
A. gracilis25) (= A. chankaensis)
|
|
|
A. asmussi17) (= Acheilognathus asmussi)
|
|
Acheilognathus
|
A. lanceolata26) (= Tanakia lanceolata), A. signifer 26), A. rhombeus21), A. yamatsutae26)
|
|
Aphyocypris
|
A. chinensis24)
|
|
Carassius
|
C. auratus14)
|
|
Coreoleuciscus
|
C. splendidus24)
|
|
Culter
|
C. brevicauda17) (= Chanodichthys erythropterus)
|
|
Cyprinus
|
C. carpio18)
|
|
Erythroculter
|
E. erythropterus21) (= Chanodichthys erythropterus)
|
|
Gnathopogon
|
G. strigatus24)
|
|
Hemiculter
|
H. eigenmanni24) (= H. leucisculus), H. leucisculus20)
|
|
Hemibarbus
|
H. labeo19), H. longirostris18)
|
|
Microphysogobio
|
M. koreensis26), M. yaluensis24)
|
|
Opsariichthys
|
O. uncirostris amurensis26) (= O. uncirostris)
|
|
Phoxinus
|
P. oxycephalus24)
|
|
Pseudogobio
|
P. esocinus17)
|
|
Pseudorasbora
|
P. parva14)
|
|
Puntungia
|
P. herzi15)
|
|
Rhodeus
|
R. ocellatus20) (= R. ocellatus ocellatus)
|
|
Saurogobio
|
S. dabryi27)
|
|
Sarcocheilichthys
|
S. nigripinnis morii26), S. variegatus wakiyae18)
|
|
Squaliobarbus
|
S. curriculus26)
|
|
Squalidus
|
S. japonicus coreanus15), S. gracilis majimae16)
|
|
Tribolodon
|
T. hakonensis17)
|
|
Zacco
|
Z. platypus17), Z. temminckii17)
|
|
Bagridae |
Coreobagrus
|
C. brevicorpus22)
|
|
Pristigasteridae |
Ilisha
|
I. elongata26)
|
|
Osphronemidae |
Macropodus
|
M. chinensis23) (= M. opercularis)
|
|
Percichthyidae |
Coreoperca
|
C. herzi23)
|
|
Siniperca
|
S. scherzei29)
|
|
Osmeridae |
Hypomesus
|
H. olidus28)
|
Table 4.The fish intermediate hosts of heterophyid trematodes in Korea
Table 4.
|
Trematode species |
The second intermediate hosts (References)a)
|
|
Metagonimus yokogawai
|
Plecoglossus altivelis (Chun, 1960a)31); Tribolodon taczanowskii (Choi et al., 1966)32); Lateolabrax japonicus (Ahn, 1983)33)
|
|
Metagonimus takahashii
|
Carassius auratus (Chun, 1960b)34); T. taczanowskii (Chai et al., 1991)35); P. altivelis (Rim et al., 1996)36) L. japonicus (Kim et al., 2006)37)
|
|
Metagonimus miyatai
|
Zaccoplatypus, Zacco temminckii (Saito et al., 1997)38)
|
|
Metagonimus spp. |
C. auratus, Coreoleuciscus splendidus, Cyprinus carpio, Hemibarbus labeo, Pseudogobio esocinus, Pseudorasbora parva, Puntungia herzi, Z. platypus, Sarcocheilichthys variegatus wakiyae, Squalidus chankaensis tsuchigae (= S. gracilis gracilis), Z. platypus (Lee, 1968)19); Coreobagrus brevicorpus, Lepomis macrochirus, Macropodus chinensis (= M. operculars), Acheilognahtus rhombeus, Z. temminckii (Joo, 1980)22); Acheilognahtus lanceolata (= Tanakia lanceolata), Coreoperca herzi, Gobiobotia brevibarba, Gobiobotia andersonii, Odontobutis macrocephala, Hemibarbus longirostris, Liobagrus platycephala, Pelteobagrus fulvidraco, Pseudopuntungia nigra, Siniperca scherzeri (Kim, 1980)39); Moroco oxycephalus (= Phoxinus oxycephalus) (Kim and Choi, 1981)40); Abbotina revularis, A. springeri, Acanthorhodeus gracilis (= A. chankaensis), A. yamatsutae, A. chinensis, Cobitis koreensis (= Iksookimia koreensis), Cobitis taenia, Hemiculter eigenmanni (= H. leucisculus), S. gracilis majimae, G. strigatus, Rhinogobius brunneus, Liobagrus mediadiposalis, M. yaluensis, Rhodeus uyekii, R. ocellatus ocellatus, S. nigripinnis morii (Rhee et al., 1983)24); Misgurnus anguillicaudatus, Acanthorhodeus asmussi(= Acheilognathus asmussi) (Rhee et al., 84)25); Opsariichthys bidens (Kim et al., 1987)41)
|
|
Centrocestus armatus
|
A. chinensis, C. auratus, C. splendidus, M. yaluensis, R. uyekii, P. parva, Z. platypus (Rhee et al., 1983)24); P. fulvidraco, R. ocellatus ocellatus (Rhee et al., 1984)25); Z. temminckii (Hong et al., 1989)42)
|
Table 5.The fish intermediate hosts of echinostomatid trematodes in Korea
Table 5.
|
Trematode species |
The second intermediate hosts (References)a)
|
|
Echinostoma hortense
|
Misgurnus anguillicaudatus (Chai et al., 1985)43); Moroco oxycephalus (= Phoxinus oxycephalus) (Ryang et al., 1985)44); Odontobutis obscura interrupta (= O. interrupta) (Ahn and Ryang, 1986)45); Squalidus coreanus (= S. japonicus coreanus) (Lee et al., 1988)46); Rhinogobius brunneus, Acanthorhodeus macropterus (= Acheilognathus macropterus) (Rim et al., 1996)47); Acanthogobius flavimanus (Sohn et al., 2009)48)
|
|
Echinostoma cinetorchis
|
Misgurnus anguillicaudatus (Seo et al., 1984)49)
|
|
Echinochasmus japonicus
|
Pseudorasbora parva, Hypomesus olidus (Rhee et al., 1983)24); Squalidus japonicus coreanus, Gnathopogon strigatus, Squalidus gracilis majimae, Pungtungia herzi, Zacco platypus, Abbottina springeri, Acheilognathus lanceolata (= Tanakia lanceolata), Aphyocypris chinensis, Carassius auratus, Hemibarbus longirostris, Moroco oxycephalus (= Phoxinus oxycephalus), Pseudogobio esocinus, Rhodeus uyekii, Acanthorhodeus gracilis (= A. chankaensis), Pelteobagrus fulvidraco (Rhee et al., 1984)25)
|
Table 6.The second intermediate hosts of heterophyid trematodes in Korea
Table 6.
|
Trematode species |
The second intermediate hosts (References)a)
|
|
Heterophyes nocens
|
Mugil cephalus (Seo et al., 1980)51); Acanthogobius flavimanus (Seo et al., 1981)52); Boleophthalmus pectinirostris, Scartelaos sp. (Sohn et al., 2005)53); Chelon haematocheilus (Kim et al. 2006)54)
|
|
Heterophyopsis continua
|
Laterolabrax japonicus (Chun, 1960c)55); A. flavimanus (Seo et al., 1984)56); Clupanodon punctatus (Sohn et al., 1994a)57); Plecoglossus altivelis (Cho and Kim, 1985)58); Conger myriaster (Kim et al., 1996)59); B. pectinirostris, Scartelaos sp. (Sohn et al., 2005)53)
|
|
Pygidiopsis summa
|
M. cephalus (Chun, 1963)60); A. flavimanus (Seo et al., 1981)61); C. haematocheilus (Kim et al. 2006)54)
|
|
Stellatchasmus falcatus
|
M. cephalus (Chai and Sohn, 1988)62)
|
|
Stictodora fuscata
|
A. flavimanus (Sohn et al., 1994b)63); B. pectinirostris (Sohn et al., 2005)53)
|
|
Stictodora lari
|
A. flavimanus (Chai et al., 1989)64)
|
|
Acanthotrema felis
|
A. flavimanus (Sohn et al., 2003)65)
|