Abstract
Toxoplasma gondii KI-1, a recent new isolate from Korea, shows similar pathogenicity and infectivity to mice compared to the virulent RH strain. To understand characteristics of host immunity, including immune enhancement or suppression, we investigated proliferative responses and phenotypes of spleen cells. In addition, kinetics of IFN-γ, a Th1 cytokine, was examined in BALB/c mice up to day 6 post-infection (PI). Intraperitoneal injection of mice with 103 KI-1 tachyzoites induced significant decreases (P < 0.05) in proliferative responses of spleen cells. This occurred at days 2-6 PI even when concanavalin A (con A) was added and when stimulated with KI-1 antigen, suggesting suppression of the immunity. CD4+ T-cells decreased markedly at day 2 PI (P < 0.05), whereas CD8+ T-cells, NK cells, and macrophages did not show significant changes, except a slight, but significant, increase of CD8+ T-cells at day 6 PI. The capacity of splenocytes to produce IFN-γ by con A stimulation dropped significantly at days 2-6 PI. These results demonstrate that intraperitoneal injection of KI-1 tachyzoites can induce immunosuppression during the early stage of infection, as revealed by the decrease of CD4+ T-cells and IFN-γ.
-
Key words: Toxopalsma gondii, Korean Isolate-1 (KI-1 isolate), splenocyte (murine), intraperitoneal infection, IFN-γ, host immunity
Toxoplasma gondii is a protozoan parasite that is controlled by the host immune system [
1]. Although infection with
T. gondii is generally asymptomatic in healthy adults, it may result in abortion, death, and severe neurologic sequelae in neonates, and life-threating lesions in AIDS patients [
2,
3].
T. gondii infects a variety of vertebrate species, including humans, and domestic and wild cats serve as the definitive host for this parasite [
3]. Transmission of
T. gondii can occur by ingestion of oocysts in feline feces, tachyzoites in blood or body fluid of infected animals, and cysts (bradyzoites) in chronically infected tissues of pigs, or by vertical transmission from mothers to newborns [
2].
Toxoplasmosis is a common protozoan infection in the United States with seroprevalence of about 15.8% among the age-adjusted population (12-49 years of age) and 14.9% among women [
4]. In the Republic of Korea, the seroprevalence was reported to be around 2-7% [
5], and clinical toxoplasmosis cases have been reported [
6]. Recently, tachyzoites of
T. gondii successfully isolated from blood of an ocular patient have been maintained in the laboratory and designated as KI-1 isolate [
7]. Its characteristics, including the morphology, virulence, infectivity, cell culture characteristics, and genetic properties, were identical to those of RH strain, a well-known virulent strain originating from a child who suffered from encephalitis [
7,
8].
One of the most distinctive immunologic features of
T. gondii infection is strong and persistent cell-mediated immunity which protects the host from the rapid tachyzoite growth and consequent pathology [
2,
3]. The highly effective resistance is thought to be mediated mainly by T-lymphocytes, in particular, CD4
+ (helper) T-cells and CD8
+ (cytotoxic) T-cells [
9,
10]. Resistance is associated with highly polarized Th1-type cytokine expressions; for example, IFN-γ plays a major role in acquired immunity to acute infection and in the control of parasite growth in chronically infected hosts [
11,
12]. However,
T. gondii subverts the host immune system [
13] and can induce host immunosuppression [
3,
14-
16]. Host immunosuppression may cause variable negative clinical effects in humans and mice, including prolonged pregnancy, retarded embryonic growth, and increased prevalence of chromosomal anomalies [
17]. Immunologic characteristics of KI-1, including immunosuppression of the host, have never been studied. Therefore, the present study focused on understanding the immune responses of BALB/c mice, including immunosuppression, after intraperitoneal infection with KI-1 tachyzoites.
Female BALB/c mice, 5-8 week-old, were purchased from SPF animal center (Koatech Comp., Gyeonggi-do, Republic of Korea). KI-1 tachyzoites were maintained every 5-6 days by intraperitoneal injection into BALB/c mice [
13]. The peritoneal exudate was harvested, washed with sterile PBS, and pelleted by centrifugation for 10 min at 3,000 rpm [
20]. Tachyzoites were purified using 40% Percoll (Pharmacia Biotech, Uppsala, Sweden) in PBS [
20]. The purified tachyzoites were disrupted by 5 cycles of freezing and thawing, and homogenated. The homogenates were centrifuged at 12,000 rpm at 4℃ for 30 min and the supernatants were used as the KI-1 antigen after filtration through a 0.45 µm membrane (Advantec MFS Inc., Pleasanton, California, USA).
Five mice in each group were infected with 10
5 KI-1 tachyzoites by intraperitoneal injection. At days 0 (control), 2, 4, and 6 post-infection (PI), the mice were sacrificed under ether anesthesia. The spleens were removed aseptically and kept in cold complete RPMI 1640 media (Gibco BRL, Grand Island, New York, USA) containing 10% heat-inactivated FBS, 2 mM L-glutamine, and 100 IU/ml of penicillin G, and 100 µg/ml of streptomycin. Spleen tissues were prepared to single cell suspension and RBC was lysed using a hypotonic buffer containing NH
4Cl [
20]. Finally, cells were resuspended in complete RPMI 1640 media and viable cells were counted by trypan blue exclusion test. Concanavalin A (con A) (Sigma-Aldrich, St. Louis, Missouri, USA), at a final concentration of 5 µg/ml, was prepared and stored at -70℃ A 40 µl (4 × 10
5 cells) of the spleen cell suspension was used for cell proliferation assay and a 100 µl (1 × 10
6 cells) cell suspension was for fluorescence-activated cell sorter (FACS) staining. To avoid non-specific binding of fluorescein-conjugated antibody, Fc blocker (anti-CD16/32 (Fcγ III/II receptor) (eBioscience, Boston, Massachusetts, USA) was used prior to labeling of anti-mouse CD3, CD4, CD8, DX5, or F4/80 antibodies. Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (eBioscience), phycoerythrin (PE)-conjugated anti-CD4, cyanin 5-phycoerythrin (Cy5-PE)-conjugated anti-CD8, PE-conjugated anti-DX5, and Cy5-PE-conjugated anti-F4/80 (eBioscience). Phenotypic analysis of CD4
+ or CD8
+ T-cells, NK cells, and macrophages was performed by FACScan flow cytometer (Becton-Dickinson, Rutherford, New Jersey, USA). Gates were set to exclude non-viable cells and adjusted to detect specifically stained cells [
19].
Cell proliferation assays were carried out in triplicate in 96-well flat-bottomed plates (NUNC Int., Rochester, New York, USA) in a 0.2 ml volume under the following conditions: 4 × 105 cells per well in 0.2 ml of culture media alone or in media containing 100 µg/ml KI-1 lysate antigen (TLA,) or 5 µg/ml con A. Plates were incubated for 72 hr in 5% CO2 at 37℃ and viable cells were converted to a colored product using XTT solution [2,3-Bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide] (Roche Diagnostics GmbH, Mannheim, Germany). A mixture of 1 µl phenazine methosulphate (PMS; electron-coupling reagent) and 50 µl of the XTT labeling reagent was added to each well and incubated at 37℃ during the last 4 hr of culture. Absorbance of the colored culture media was read at 450 nm with a reference wavelength of 650 nm (ELx808) (BioTek Instruments, Winooski, Vermont, USA). IFN-γ levels were measured using sandwich ELISA after the spleen cells were cultured with con A or TLA. The standard concentrations of cytokines were measured serially at 1/2-1/32 dilutions on murine recombinant IFN-γ (eBioscience). Plates were incubated for 2 hr at room temperature, and after 5 washes, biotinylated-anti-mouse IFN-γ (eBioscience) was added in each well and the plates were further incubated for 1 hr. Avidin-HRP (at 1/250) (eBioscience) was added to each well, and the plates were incubated for 30 min. Absorbance was measured at 450 nm using the Precision Microplate Reader (Molecular Devices, Sunnyvale, California, USA). Data are shown as mean ± SD and statistically analyzed by the Student's t-test. Values of P < 0.05 were considered statistically significant.
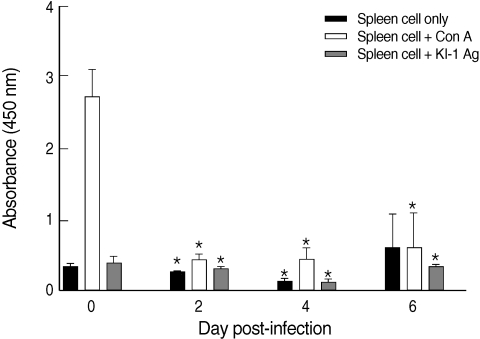
The proliferative capacity of the splenocytes of uninfected mice (day 0 PI) increased after the addition of con A (
Fig. 1). However, the proliferative capacity of the splenocytes of KI-1 infected mice decreased significantly at day 2 (
P = 0.000), day 4 (
P = 0.000), and day 6 PI (
P = 0.0011) after the addition of con A compared with uninfected controls, suggesting immunosuppression (
Fig. 1). Similarly, the splenocytes of the infected mice had significantly lowered responses to stimulation with KI-1 antigen at day 2 (
P = 0.0008), day 4 (
P = 0.000), and day 6 PI (
P = 0.0388), compared to the uninfected controls which also suggests suppression of immune responses (
Fig. 1).
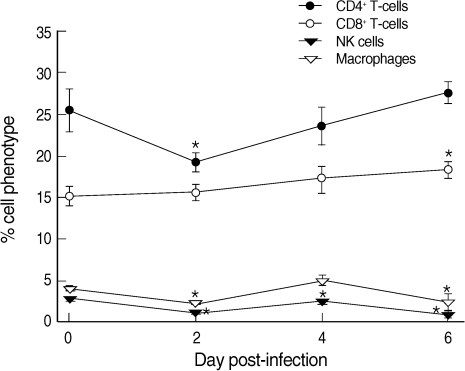
CD4
+ T-cells were decreased from 25.4% at day 0 PI to 19.1% at day 2 PI and slowly recovered to the level of uninfected mice at day 6 PI (
Fig. 2). In contrast, CD8
+ T-cells increased slightly but steadily during the whole infection period, though statistical significance was shown only at day 6 PI (
Fig. 2). Macrophages and NK cells showed only slight changes during the infection period, though at days 2, 4 (NK cells only), and 6 PI their levels were significantly lower than those of uninfected controls (
P < 0.05) (
Fig. 2).
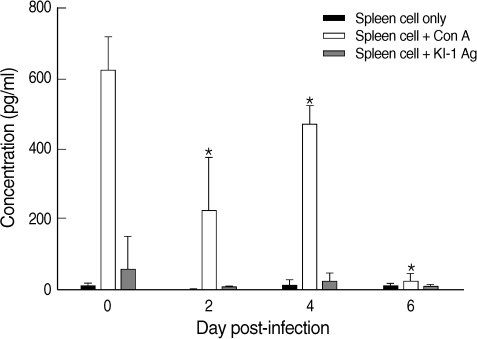
Con A stimulation of the splenocytes induced production of over 600 pg/ml of IFN-γ in uninfected mice (
Fig. 3). However, the splenocytes of infected mice showed significantly (
P < 0.05) depressed production of IFN-γ despite con A stimulation at days 2-6 PI compared with the controls (
Fig. 3). On the other hand, the splenocytes of infected mice showed no significant responses to KI-1 antigen stimulation at days 2-6 PI (
Fig. 3).
The results of the present study have demonstrated that intraperitoneal infection with T. gondii KI-1 tachyzoites can induce severe immunosuppression of BALB/c mice. The splenocytes of KI-1 infected mice did not respond to in vitro stimulation with con A or KI-1 antigen up to day 6 PI. In addition, the proportion of CD4+ T-cells among the splenocytes and IFN-γ production by the splenocytes were both significantly decreased in infected mice compared to the controls.
During the early stages of
Toxoplasma infection, macrophages and NK cells become activated and produce cytokines, including IFN-γ, which induce cell-mediated immune responses [
3]. CD4
+ T-cells are essential for inflammation responses against
T. gondii infection and have an important role in the maintenance of CD8
+ T-cell immunity against this protozoan parasite [
21]. In our study, the proliferative capacity of the splenocytes by con A or
Toxoplasma antigen stimulation was markedly suppressed in KI-1 infected mice at days 2-6 PI. In addition, the ratios of CD4
+ T-cells, macrophages, and NK cells among the splenocytes of KI-1 infected mice were significantly down-regulated up to day 6 PI. However, CD8
+ T-cells steadily increased during the infection period, suggesting that these cells may be important in response to
T. gondii infection [
21]. CD8
+ T-cells can lyse tachyzoites released from cells and are directly cytotoxic to infected host cells [
21]. Our data suggested that the increased CD8
+ T-cells may be important as a protective response against KI-1 infection. The importance of CD8
+ T-cells in protection against
T. gondii was also reported in mice vaccinated with dendritic cells [
22].
The host immunity against
T. gondii infection is known to be predominantly a cell-mediated type and characterized by highly polarized Th1 responses [
3]. Both CD4
+ and CD8
+ T-cells are known for their ability to produce high levels of IFN-γ in response to the parasite [
3], and IFN-γ has a crucial role in resistance to
T. gondii at both the acute and chronic stages of infection [
11,
12,
23]. However, our data showed only limited production of IFN-γ together with suppressed CD4
+ T-cell levels in the spleen of KI-1 infected mice, which may be related to the failure of these mice to survive beyond day 7 PI [
7]. CD4
+ T-cell suppression was also observed in
T. gondii-infected mouse retinal cells, and the suppression was mediated by the cell surface protein, programmed death ligand 1 (PD-L1) [
24].
Taken together, our study demonstrated that infection of BALB/c mice with T. gondii KI-1 tachyzoites resulted in marked suppression of the host immune responses, in particular in CD4+ T-cells and IFN-γ levels, during the early stages of infection. We suggest that this markedly suppressed immunity in KI-1 infected mice may be responsible for their early death before day 7 PI.
ACKNOWLEDGEMENTS
This work was supported by The Korea Research Foundation Grant funded by the Korean Government (KRF-2009-0076262).
References
- 1. Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol 1994;153:2533-2543.
- 2. Bhopale GM. Pathogenesis of toxoplasmosis. Comp Immunol Microbiol Infect Dis 2003;26:213-222.
- 3. Denkers EY, Gazzinelli RT. Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 1998;11:569-588.
- 4. Jones JL, Kruszon-Moran D, Wilson M. Toxoplasma gondii infection in the United States, 1999-2000. Emerg Infect Dis 2003;9:1371-1374.
- 5. Choi WY, Nam HW, Youn JH, Kim WS, Kim WK. Toxoplasma antibody titers by indirect latex agglutination test in patients of Kangnam St. Mary's Hospital and Cheju Medical Center. Korean J Parasitol 1989;27:171-175.
- 6. Choi WY, Nam HW, Kwak NH, Huh W, Kim YR, Kang MW, Cho SY, Dubey JP. Foodborne outbreaks of human toxoplasmosis. J Infect Dis 1997;175:1280-1282.
- 7. Chai JY, Lin A, Shin EH, Oh MD, Han ET, Nam HW, Lee SH. Laboratory passage and characterization of an isolate of Toxoplasma gondii from an ocular patient in Korea. Korean J Parasitol 2003;41:147-154.
- 8. Lin A, Shin EH, Kim TY, Park JH, Guk SM, Chai JY. Genetic characteristics of the Korean isolate KI-1 of Toxoplasma gondii. Korean J Parasitol 2005;43:27-32.
- 9. Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 1992;149:175-180.
- 10. Denkers EY. From cells to signaling cascades: manipulation of innate immunity by Toxoplasma gondii. FEMS Immunol Med Microbiol 2003;39:193-203.
- 11. Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 1988;240:516-518.
- 12. Suzuki Y, Conley FK, Remington JS. Importance of endogenous IFN-γ for prevention of toxoplasmic encephalitis in mice. J Immunol 1989;143:2045-2050.
- 13. Shin EH, Kim SB, Nam HW, Han ET, Park JH, Ahn HJ, Chai JY. Use of monoclonal antibodies for flow cytometric detection of intracellular Toxoplasma gondii in murine splenic lymphocytes. J Parasitol 2004;90:161-166.
- 14. Channon JY, Suh EI, Seguin RM, Kasper LH. Attachment ligands of viable Toxoplasma gondii induce soluble immunosuppressive factors in human monocytes. Infect Immun 1999;67:2547-2551.
- 15. Wei S, Marches F, Borvak J, Zou W, Channon J, White M, Radke J, Cesbron-Dclauw MF, Curiel TJ. Toxoplasma gondii-infected human myeloid dendritic cells induce T-lymphocyte dysfunction and contact-dependent apoptosis. Infect Immun 2002;70:1750-1760.
- 16. Lee EJ, Heo YM, Choi JH, Song HO, Ryu JS, Ahn MH. Suppressed production of pro-inflammatory cytokines by LPS-activated macrophages after treatment with Toxoplasma gondii lysate. Korean J Parasitol 2008;46:145-151.
- 17. Kaňková S, Holáň V, Zajicová A, Kodym P, Flegr J. Modulation of immunity in mice with latent toxoplasmosis-the experimental support for the immunosuppression hypothesis of Toxoplasma-induced changes in reproduction of mice and humans. Parasitol Res 2010;107:1421-1427.
- 18. Buzoni-Gatel D, Werts C. Toxoplasma gondii and subversion of the immune system. Trends Parasitol 2006;22:448-452.
- 19. Shin EH, Chai JY. 2007. T-helper-1 and T-helper-2 immune responses in mice infected with the intestinal fluke Neodiplostomum seoulense: their possible roles in worm expulsion and host fatality. J Parasitol 2007;93:1036-1045.
- 20. Choi WY, Nam HW, Youn JH, Kim DJ, Kong Y, Kang SY, Cho SY. Detection of antibodies in serum and cerebrospinal fluid to Toxoplasma gondii by indirect latex agglutination test and enzyme-linked immunosorbent assay. Korean J Parasitol 1992;30:83-90.
- 21. Casciotti L, Ely KH, Williams ME, Khan IA. CD8+ T-cell immunity against Toxoplasma gondii can be induced but not maintained in mice lacking conventional CD4+ T cells. Infect Immun 2002;70:434-443.
- 22. Guiton R, Zagani R, Dimier-Poisson I. Major role for CD8+ T cells in the protection against Toxoplasma gondii following dendritic cell vaccination. Parasite Immunol 2009;31:631-640.
- 23. Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Showe L, Grunvald E, Hieny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol 1996;157:4045-4054.
- 24. Charles E, Joshi S, Ash JD, Fox BA, Farris D, Bzik DJ, Lang ML, Blader IJ. CD4 T-cell suppression by cells from Toxoplasma gondii-infected retinas is mediated by surface protein PD-L1. Infect Immun 2010;78:3484-3492.
Fig. 1Proliferative responses of splenocytes isolated from uninfected (day 0 PI) and T. gondii KI-1 tachyzoite-infected (days 2, 4, and 6 PI) BALB/c mice. Proliferation was assayed by XTT method 72 hr after stimulation with 5 µg/ml con A (spleen cells + con A) or 100 µg/ml KI-1 lysate antigen (spleen cells + KI-1 antigen) and compared with the unstimulated group (spleen cells only). *Significantly lower (P < 0.05) than uninfected controls.
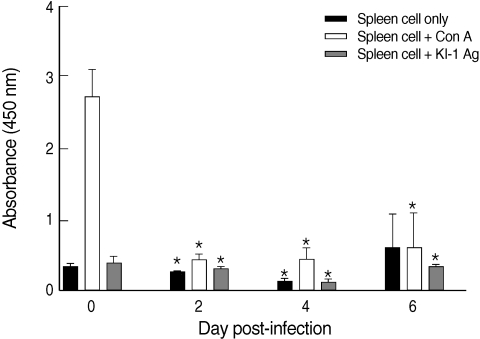
Fig. 2Proportions of CD4+ T-cells, CD8+ T-cells, NK cells, and macrophages among the phenotypes of splenocytes in uninfected (day 0 PI) and T. gondii KI-1 tachyzoite-infected (days 2, 4, and 6 PI) BALB/c mice. Cells were stained with phycoerythrin (PE)-conjugated anti-CD4, cyanin 5-phycoerythrin (Cy5-PE)-conjugated anti-CD8, PE-conjugated anti-DX5, and Cy5-PE-conjugated anti-F4/80, respectively. Phenotypic analysis was performed by a FAC-Scan flow cytometer. *Significantly lower (P < 0.05) than in uninfected controls (day 0 PI).
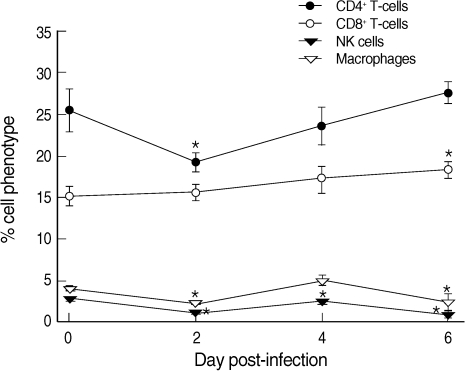
Fig. 3IFN-γ production by splenocytes isolated from uninfected (day 0 PI) and T. gondii KI-1 tachyzoite-infected (days 2, 4, and 6 PI) BALB/c mice, as measured by the sandwich ELISA. The levels were measured 72 hrs after stimulation with 5 µg/ml con A (spleen cells + con A) or 100 µg/ml KI-1 lysate antigen (spleen cells + KI-1 antigen) and compared with the unstimulated group (spleen cells only). *Significantly lower (P < 0.05) than in uninfected controls (day 0 PI).