Abstract
In the genus Cryptosporidium, there are more than 14 species with different sizes and habitats, as well as different hosts. Among these, C. parvum and C. hominis are known to be human pathogens. As C. parvum can survive exposure to harsh environmental conditions, including various disinfectants or high doses of radiation, it is considered to be an important environmental pathogen that may be a threat to human health. However, the resistance of other Cryptosporidium species to various environmental conditions is unknown. In this study, resistance against γ-irradiation was compared between C. parvum and C. muris using in vivo infection in mice. The capability of C. muris to infect mice could be eliminated with 1,000 Gy of γ-irradiation, while C. parvum remained infective in mice after up to 1,000 Gy of γ-irradiation, although the peak number of oocysts per gram of feces decreased to 16% that of non-irradiated oocysts. The difference in radioresistance between these 2 Cryptosporidium species should be investigated by further studies.
-
Key words: Cryptosporidium parvum, Cryptosporidium muris, mouse infectivity, radioresistance
Cryptosporidium parvum is a protozoan parasite that infects the gastrointestinal epithelial cells of many vertebrates, including humans [
1]. It causes watery diarrhea in infected individuals and can be fatal to immunocompromized individuals, e.g., AIDS patients [
1].
C. muris is another species of
Cryptosporidium that targets mammalian hosts. Although it typically parasitizes the stomach epithelium of murine species [
2],
C. muris has also been known to infect immunocompetent as well as immunocompromized humans [
3,
4].
C. parvum has been reported to be the most highly radioresistant organism ever known [
5,
6]. However, the resistance of other
Cryptosporidium species to various environmental stimuli, including radioresistance, is unknown. Therefore, in this study, we investigated the radioresistance of
C. muris and compared its radioresistance with that of
C. parvum.
The Animal Care and Use Committee of Konkuk University (Seoul, Republic of Korea) approved this study.
C. parvum (KKU isolate) oocysts were maintained in pathogen-free C57BL/6 female mice (8-9 week old) that had been immunosuppressed by adding 10 mg/ml disodium dexamethasone phosphate (Sigma-Aldrich, St. Louis, Missouri, USA) to their drinking water, which was provided ad libitum [
6].
C. muris (KKU isolate) oocysts were also maintained in pathogen-free C57BL/6 female mice (8-9 week old), but without immunosuppression. Oocysts were isolated as described by Petry et al. [
7]. Percoll purification was performed as described previously, with small modifications [
8].
In order to obtain a backscattered ray and simultaneously to reduce the temperature increase resulting from energy absorption during high-dose radiation, purified C. parvum or C. muris oocysts (2×107) were suspended in 1 ml of filtered (0.22 µm filter) water in 1.5-ml microcentrifuge tubes, which were placed within a 50-ml tube filled with distilled water. Irradiation, at doses of 50, 100, 300, 500, and 1,000 Gy, was performed with a 60Co IR-222 (Nordion Co., Canada) low intensity gamma irradiator, at room temperature (20℃), for 30 min. Non-irradiated oocysts were kept at room temperature for the same period. Room and irradiated sample tube temperatures were measured before and immediately following irradiation. Oocysts were then transported from the irradiation facility to the laboratory (6 hr), and used to infect mice.
Nine to 10 female C57BL/6, SPF, 3-week old mice were allocated to each experimental group; each mouse was infected orally with 2×106 non-irradiated or irradiated oocysts. Mice were then housed separately in a wire-bottom cage in a Clean Rack (Three Shine Co., Seoul, Korea); conventional food pellets and filtered (0.22 µm) water were freely supplied. Fresh feces (1 g) were collected from each mouse every other day, from 3 to 63 days post-infection (PI). The number of oocysts per gram of feces (OPG) was counted from a fecal smear stain, using the modified acid-fast staining method on feces concentrated by formalin-ether sedimentation. OPG data from the mice infected with irradiated and non-irradiaed oocysts were statistically compared by the t-test.
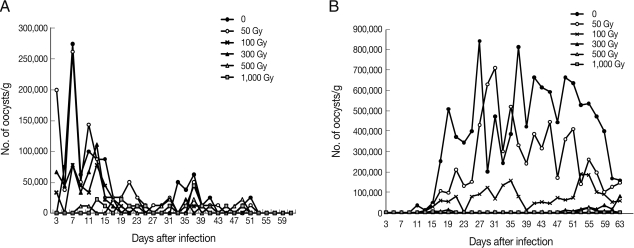
The temperatures of the irradiation room and sample tubes were the same (20℃) before and after irradiation. Mice infected with non-irradiated or irradiated (50 to 300 Gy)
C. parvum oocysts excreted oocysts from day 3 or 5 until day 51 PI, with a 46-48 day patent period (
Fig. 1). Mice infected with 500 or 1,000 Gy-irradiated
C. parvum oocysts commenced oocyst excretion a little later, i.e., day 9 or day 13, respectively, and the patent period was shortened to 36-40 days. The mean value of the peak OPG in control mice was 27.5×10
4 on the 7th day PI, and after this, the OPG decreased rapidly. The peak OPG value in the 50-Gy-irradiated group was similar to that of the non-irradiated control group, i.e., 26.3×10
4 at the 7th day PI, but mice irradiated with 100 to 1,000 Gy irradiation showed a reduced peak OPG value, reaching only 28% to 16% of that of the control group (
Table 1). Oocyst excretion continued until day 49-51 PI in all experimental groups and stopped thereafter. Significant OPG reduction (
P<0.05) was detected in mice infected with
C. parvum oocysts irradiated 500 or 1,000 Gy during day 9 to 13 PI.
On the other hand, mice infected with non-irradiated or irradiated (50 to 100 Gy)
C. muris oocysts excreted oocysts from day 11 or 13 until day 63 PI, with a 52-day patent period (
Fig. 1). In the mice infected with 300 to 500 Gy-irradiated oocysts, commencement of oocyst excretion was delayed up to day 49 or 53 PI. The mean peak OPG in control mice was 84.3×10
4 at the 27th day PI, and, thereafter, the OPG was maintained at 40×10
4 until day 59 PI. In the 50 Gy-irradiated group the peak OPG value (71.0×10
4 at the 31st day PI) reached 90% that of the non-irradiated control group, but it was reduced to only 2.6% that of the control group by 500 Gy irradiation (
Table 1). A statistically significant (
P<0.05) reduction of OPG in mice infected with
C. muris oocysts irradiated 300 to 1,000 Gy was observed from day 17 to 53 PI. The oocyst excretion continued until the end of the experiment (day 63 PI) in non-irradiated control groups as well as in those irradiated with 50 to 300 Gy. However, the oocyst excretion period of the 500 Gy-irradiated group was shorter, only 8 days (data not shown). In the mice infected with 1,000 Gy-irradiated oocysts, no oocysts were excreted.
The rate of reduction of oocyst production in mice [100-(number of infected mice/total number of experimental mice)×100] ranged from 0% to 22.0% in mice infected with 0 to 1,000 Gy-irradiated
C. parvum oocysts (
Table 1). On the other hand, the rate of reduction of oocyst production was 0% in mice infected with
C. muris oocysts that were irradiated with 0 to 300 Gy (
Table 1), whereas it was 100% in mice infected with 1,000 Gy-irradiated
C. muris oocysts (
Table 1).
Some of the helminthic parasites were reported to be controllable by γ-irradiation doses from 100 to 600 Gy [
9-
11]. Other coccidian protozoa, such as
Toxoplasma gondii and
Eimeria necatrix, are controlled by 600 to 2,000 Gy [
12-
14]. In the present study,
C. muris completely lost its infectivity in mice with 1,000 Gy irradiation, in contrast to
C. parvum, which retained its infectivity even with 1,000 Gy irradiation. A previous study found that
C. parvum remains infective to mice even after irradiation with up to 10 kGy and lost its infectivity only after 25 kGy irradiation [
6]. Thus, in the present study, we demonstrated that
C. muris required a 25-times lower dose of γ-irradiation to eliminate infectivity in mice, compared to that required for
C. parvum.
In the present study, we also noticed that the pattern of oocyst excretion in mouse feces differed substantially between C. parvum and C. muris. Oocyst excretion of C. parvum peaked as early as the 7th day PI, rapidly decreased thereafter, and tapered off until day 51 PI. In contrast, in C. muris infection, oocyst excretion commenced much later than that in C. parvum but was maintained over a longer period with a considerable OPG. This may underlie the excellent adaptation of C. muris to mice, explaining its categorization as a murine Cryptosporidium.
In our study, we found that there was a significant difference in radioresistance between C. parvum and C. muris; this difference should be further delineated by further studies.
ACKNOWLEDGMENT
This work was supported by Konkuk University in 2011.
References
- 1. O'Donoghue PJ. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol 1995;25:139-195.
- 2. Tzipori S, Ward H. Cryptosporidiosis: biology, pathogenesis and disease. Microbes Infect 2002;4:1047-1058.
- 3. Katsumata T, Hosea D, Ranuh IG, Uga S, Yanagi T, Kohno S. Short report: possible Cryptosporidium muris infection in humans. Am J Trop Med Hyg 2000;62:70-72.
- 4. Gatei W, Ashford RW, Beeching NJ, Kamwati SK, Greensill J, Hart CA. Cryptosporidium muris infection in an HIV-infected adult, Kenya. Emerg Infect Dis 2002;8:204-206.
- 5. Kato S, Jenkins MB, Ghiorse WC, Bowman DD. Chemical and physical factors affecting the excystation of Cryptosporidium parvum oocysts. J Parasitol 2001;87:575-581.
- 6. Yu JR, Park WY. The effect of gamma-irradiation on the viability of Cryptosporidium parvum. J Parasitol 2003;89:639-642.
- 7. Petry F, Robinson HA, McDonald V. Murine infection model for maintenance and amplification of Cryptosporidium parvum oocysts. J Clin Microbiol 1995;33:1922-1924.
- 8. Kilani RT, Sekla L. Purification of Cryptosporidium oocysts and sporozoites by cesium chloride and Percoll gradients. Am J Trop Med Hyg 1987;36:505-508.
- 9. Bickle QD, Dobinson T, James ER. The effects of gamma-irradiation on migration and survival of Schistosoma mansoni schistosomula in mice. Parasitology 1979;79:223-230.
- 10. Lee SH, Park YH, Sohn WM, Hong ST, Chai JY. The effects of gamma irradiation on the survival and development of Clonorchis sinensis metacercariae. Korean J Parasitol 1989;27:187-195.
- 11. Chai JY, Kim SJ, Kook J, Lee SH. Effects of gamma-irradiation on the survival and development of Metagonimus yokogawai metacercariae in rats. Korean J Parasitol 1995;33:297-303.
- 12. Singh J, Gill BS. Effect of gamma-irradiation on oocysts of Eimeria necatrix. Parasitology 1975;71:117-124.
- 13. Dubey JP, Jenkins MC, Thayer DW. Irradiation killing of Toxoplasma gondii oocysts. J Eukaryot Microbiol 1996;43:123S.
- 14. Song CC, Yuan XZ, Shen LY, Gan XX, Ding JZ. The effect of cobalt-60 irradiation on the infectivity of Toxoplasma gondii. Int J Parasitol 1993;23:89-93.
Fig. 1Number of oocysts per gram of feces from mice infected with Cryptosporidium parvum (A) and C. muris (B) oocysts irradiated with varying doses of γ-rays.
Table 1.Infection of C57BL mice with Cryptosporidium spp. oocysts exposed to γ-irradiation
Table 1.
|
Species |
Dose (Gy) |
Litter size |
No. (%) mice infected |
Peak no. OPGa [(mean±SD)×104] |
Date of peak OPG (PIb) |
% Reduction of oocyst production |
|
C. parvum
|
0 |
9 |
8 (88.9) |
27.5 ± 12.0 |
7 |
11.1 |
|
50 |
9 |
8 (88.9) |
26.3 ± 16.0 |
7 |
11.1 |
|
100 |
9 |
9 (100.0) |
7.7 ± 0.9 |
7 |
0.0 |
|
300 |
9 |
9 (100.0) |
11.1 ± 9.2 |
13 |
0.0 |
|
500 |
9 |
7 (77.7) |
2.2 ± 0.6 |
17 |
22.3 |
|
1,000 |
9 |
7 (77.7) |
4.4 ± 0.7 |
37 |
22.3 |
|
C. muris
|
0 |
9 |
9 (100.0) |
84.3 ± 6.3 |
27 |
0.0 |
|
50 |
10 |
10 (100.0) |
71.0 ± 4.8 |
31 |
0.0 |
|
100 |
9 |
9 (100.0) |
18.9 ± 4.1 |
53 |
0.0 |
|
300 |
10 |
10 (100.0) |
8.0 ± 3.3 |
63 |
0.0 |
|
500 |
9 |
2 (22.2) |
2.2 ± 0.6 |
55 |
77.8 |
|
1,000 |
10 |
0 (0.0) |
- |
- |
100.0 |