Abstract
The present study was performed to describe 2 human cases infected by the horsehair worm, Parachordodes sp., in Japan. Two gordiid worms were collected in the vomit and excreta of an 80-year-old woman in November 2009 in Kyoto city, and in the mouth of 1-year-old boy in December 2009 in Nara city, Japan, respectively. Both worms were males having bifurcated posterior ends and male gonads in cross sectional specimens. They were identified as Parachordodes sp. (Nematomorpha: Chordodidae) based on the characteristic morphologies of cross sections and areoles in the cuticle. DNA analysis on 18S rRNA partial sequence arrangements was also carried out and both worms were assumed to be close to the genus Paragordionus based on tree analysis, and far from Gordius sp. which has already been reported in humans in Japan. DNA sequencing of the Parachordodes worm does not appear on the database; therefore, more information on the gene sequences of the genus Parachordodes from humans, animals, or intermediates is required.
-
Key words: Parachordodes sp., horsehair worm, human infection, case report, morphology, DNA analysis
Nematomorpha is a phylum of animals that has been poorly studied. Despite their worldwide distribution, detailed life cycles of Nematomorpha have not been completely documented [
1].
Parachordodes or
Gordius worms belong to the phylum Nematomorpha (Gordiida, Gordioidea). Adults of
Parachordodes (Chordodidae: Parachordodinae),
Gordius (Gordiidae), or the genera of the subfamily Chordodinae (Chordodidae), also commonly known as hair worms, are free-living freshwater inhabitants, and their larvae are first free-living and then infect intermediate hosts. When intermediate hosts are eaten by the final hosts, larvae develop to juveniles and grow to adult size. The caudal end of the male is either bifurcated or has a dorsoventral groove in Gordiidae and Parachordodinae, but not in Chordodinae.
Parachordodes or
Gordius worms have parasitic larval stages, and final hosts include the mantis and some carnivorous or omnivorous species of Orthoptera (Acrididae: grasshoppers, Gryllidae: crickets, etc.) [
2], and Coleoptera (Carabidae: ground beetles).
Records of human accidental parasitism with
Parachordodes,
Paragordius, or
Gordius are uncommon in the literature, although many have been identified in different parts of the world from specimens recovered from the mouth, urethra, and anus [
3-
6]. Six human cases of
Gordius sp. have been reported in Japan [
7,
8]. In these cases, worms were vomited and excreted in the feces and from anus. A human case of a
Gordius worm found in the vomitus and another case of a
Parachordodes worm found in the urinary system have also been reported in Korea [
9,
10]. Moreover, at least 13 species of freshwater Nematomorpha have been recorded to date in Japan [
11-
13]. Additionally, some or new records of Nematomorpha in insects were recently reported from Shiga, Niigata, Nara, Gifu, Kyoto, and Fukuoka Prefectures, Japan [
11-
13], and molecular and morphological data have been used to analyze the systematic relationship of Nematomorpha [
14,
15]. Of the genus
Parachordodes, 2 species have been reported in Japan:
Parachordodes okadai [
16,
17] and
P. lestici [
18].
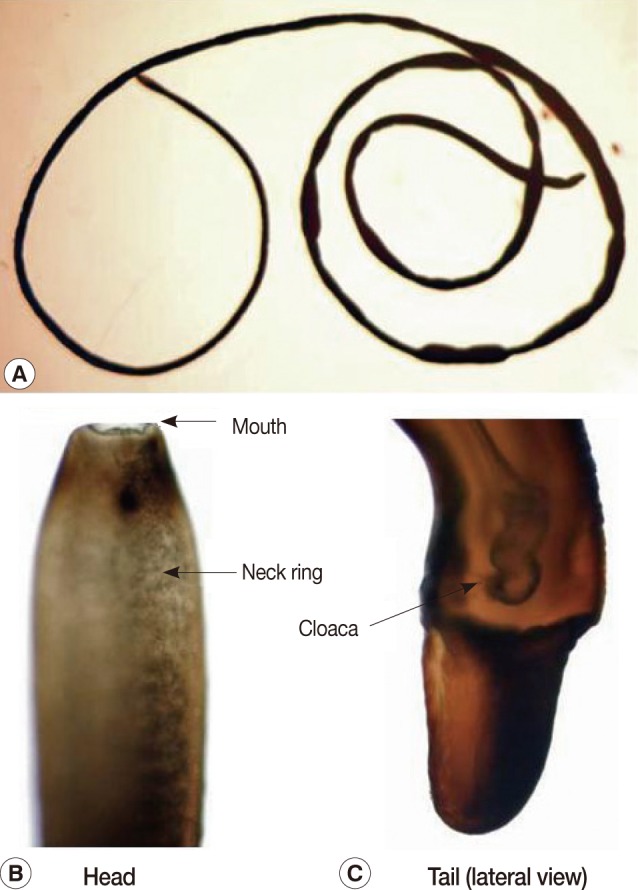
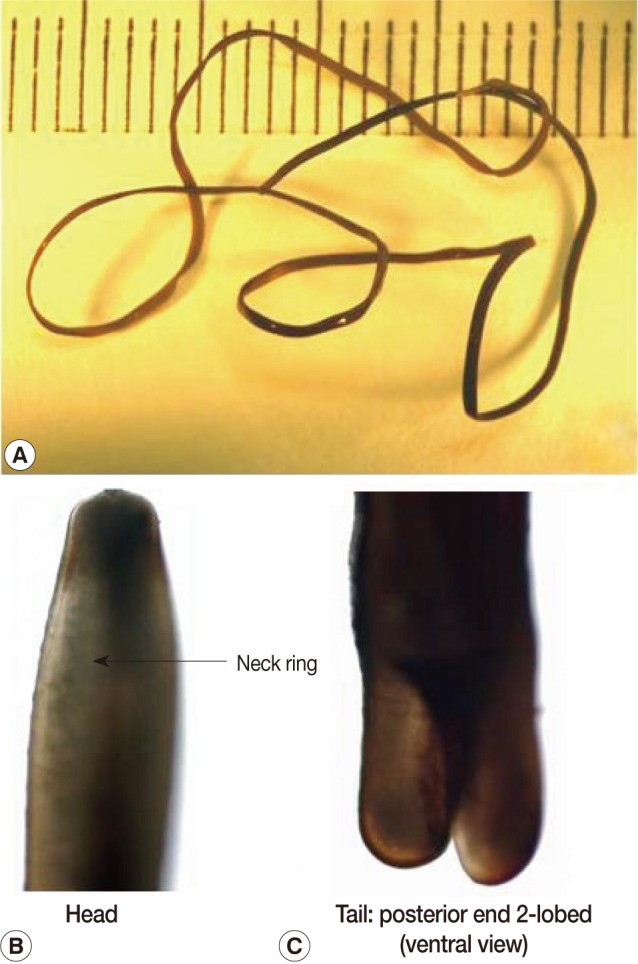
The present report deals with a species of hair worm collected from laboratory institutes in November and December, 2009, one of which was vomited from an 80-year-old woman and the other was collected from the mouth of a 1-year-old boy by his mother. The subjects lived in Kyoto city, Kyoto Prefecture, and Nara city, Nara Prefecture, Japan. The woman vomited a worm after gargling with a saline solution as she felt something was caught in her throat while she was lying in bed. The worm was preserved in 10% formalin before examination. She had eaten vegetables harvested from a private garden. The other worm from the mouth of a boy was removed by his mother. Both worms were examined with the naked eye and microscopically. They had the typical morphological features of nematomorphan worms. The specimens measured 13 cm and 12.5 cm in length and both had a uniform width of about 0.1 cm (
Figs. 1,
2). The bodies of both were black-brown with a neck ring (dark pigmentation) but with no ventral line nor dorsal line in the cuticle in the preserved condition (
Figs. 1,
2). The body was cylindrical and had the same diameter along its whole extent, though it somewhat narrowed anteriorly. The anterior end was rounded with a mouth opening. The posterior end was bifurcated. The tail lobes were 0.1 mm long and the ratio of the length of the lobe to the width was 0.5. A cloaca opening was found in 1 male worm (
Fig. 1). The posterior end of the male worms was spirally enrolled and furcated into 2 caudal lobes, which were nearly cylindrical, though somewhat concave on their medio-ventral surface. A postcloacal crescentic fold (cuticular ridge) was not present, which meant that these 2 human specimens did not belong to the genus
Gordius. Accordingly, the morphology of these worms was analyzed on tissue sections light-microscopically (
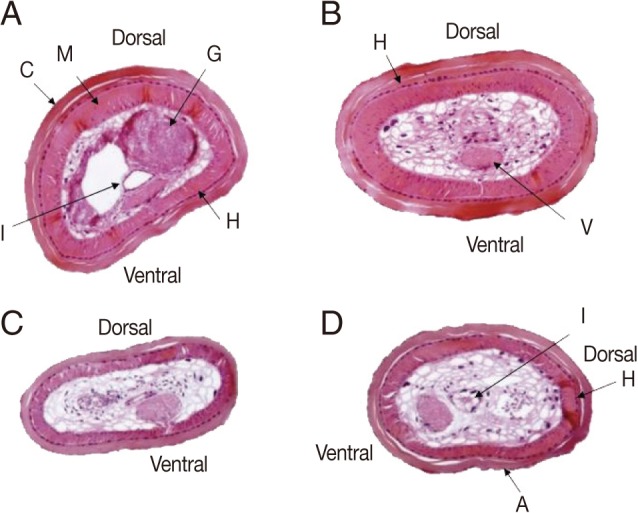
Fig. 3).
DNA (18S rRNA partial sequence) analyses were conducted using the GenBank database. For DNA analyses, the worm fixed with 10% formalin was washed 3 times using PBS, and a part of worm was cut using a clean cover glass and ruptured using microtube pestles (Scientific Specialties Inc., Lodi, California, USA) for DNA extraction. The DNA was extracted and purified using DNeasy Blood and Tissue Kits (Qiagen GmbH, Hilden, Germany) according to the manufacturer's instructions. The nuclear 18S ribosomal RNA gene (18S rDNA) region of both worms was amplified using 3F and 9R primers reported previously [
15]. PCR amplification was performed in a volume of 50 µl containing 1×PCR buffer, 2 mM MgCl
2, 250 µM of each dNTP, 0.5 µM of each primer, 1.25 units of TaKaRa Ex Taq Hot Start Version (TaKaRa Shuzo Co. Ltd., Otsu, Japan), and 3 µl of DNA sample. PCR reaction was performed under following conditions: Samples were denatured at 95℃ for 3 min; then they were subjected to 35 cycles of 94℃ for 30 sec, 55℃ for 30 sec, and 72℃ for 1 min, with final extension at 72℃ for 7 min on a thermocycler (GeneAmp PCR System 9700; Applied Biosystems, Foster City, California, USA). The PCR products were purified using a QIAquick PCR Purification Kit (Qiagen GmbH, Hilden, Germany), and were sequenced in both directions on an automated sequencer (ABI 3130; Applied Biosystems, Foster City, California, USA). Sequence chromatograms from each strand were inspected (Sequencher Version 4.1; Gene Codes Corp., Ann Arbor, Michigan, USA). The sequences obtained from both worms were aligned with the available nucleotide sequences of other nematomorpha species using Clustal X 2.0 (
http://www.clustal.org/) with initial fixed parameter values. A tree was constructed using the Neighbor-Joining algorithm based on evolutionary distances calculated using the Kimura 2-parameter model with 1,000 bootstrap sampling. The resulting tree was drawn using Njplot software (
http://pbil.univ-lyon.fr/software/njplot.html).
Tissue sections revealed that the body surface was covered with a cuticle of 25-50 µm thick, and lined with monolayer hypoderm of 5-10 µm thick with no lateral cord. The muscular layer was 25-60 µm thick (
Fig. 3). Parenchymatous cells filled the pseudocoel to near capacity, and a ventral nerve cord, a digestive tract, and a pair of male genital organs were identified in the parenchyma. The thickness of the muscle layer was 30-80 µm and the ventral nerve cord was 80×50 µm. The cuticular surface was a little rough and had small-sized areoles. After removal of muscle and hypodermis layers of the 2 worms from humans, the structure of the cuticle was found to be equal to that of the genus
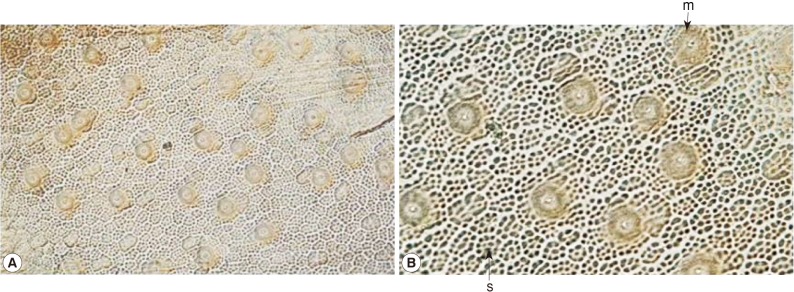
Parachordodes sp. containing 2 types of large and small areoles morphologically (
Figs. 3,
4). The small areoles with granules are the same characteristics as
Parachordodes lestici [
18] , but different from the small areoles of
P. okadai [
16,
17], which has smooth surface. The large areoles (megareoles) were almost round and those of the worm in case 1 were 23.9±2.5 (Mean±SD) µm in length and 23.5±2.7 µm in width, and those in case 2 were 23.3±1.2 µm in length and 23.1±1.0 µm in width. The megareoles are very different in these 2 species,
P. lestici and
P. okadai. They are almost round in
P. lestici, but elongate in
P. okadai. The present morphological examinations of human cases reveal that the present worms have the characteristics of
P. lestici, and could be justified to determine as
P. lestici. However, the areoles (2 types of small sized-areoles and megareoles) appeared on the cuticle in
Fig. 4 were very similar with those in Schmidt-Rhaesa et al. [
9]. The morphological characteristics and sizes of megareoles were also equal to those in Schmidt-Rhaesa et al. [
9]. Therefore, the 2 worms in the present study may be the same species as
Parachordodes megareolatus which was derived from the human urinary system in Korea [
9].
Members of the class Gordiacea belong to 2 recognized families, Gordiidae (
Gordius) and Chordodidae. The former has a smooth cuticle, but in some species of Gordiidae areoles do also occur. The cuticle in the second group is rough with true areoles. These subfamily groups include 18 genera (
Chordodes,
Paragordius,
Parachordodes,
Neochordodes,
Pseudogordius and others). There are also other genera with 2 types of areoles, but characteristic for
Parachordodes is that 1 type is very large and has a pore on top, and this type is called superareole [
19]. These characteristics of megareoles strongly suggest that the present worms can be diagnosed as
Parachordodes Camerano, 1897 [
19]. From Japan, 17 species of Nematomorpha (Gordiida) have been described to date [
11-
13] and 4 species of Nematomorpha have been also reported in Korea [
9,
10,
20].
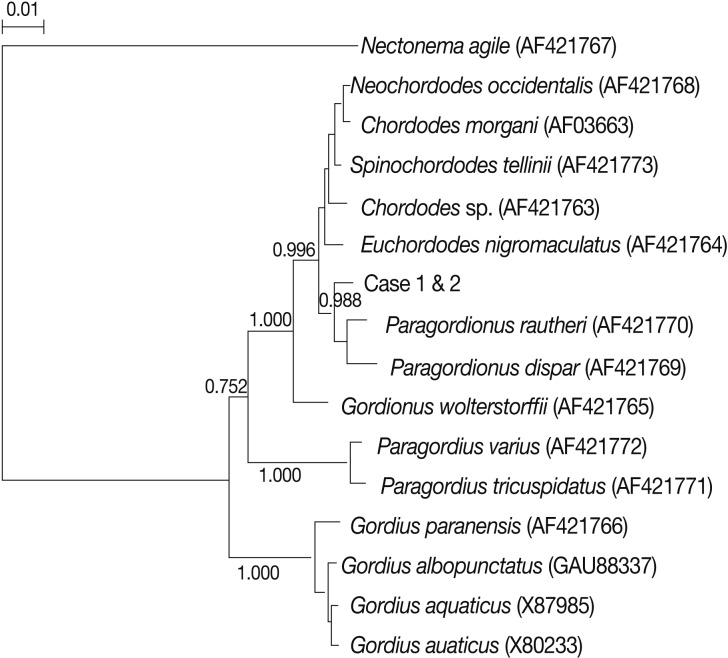
A phylogenetic relationship according to the 18S rRNA gene (1,414 bp) was conducted and the present worms were identified as a species that is genetically nearer to
Paragordionus species (GenBank no. AF421769 and AF421770) and were different from
Gordius worms (GenBank no. AF421766, GAU88337, X87985, and X80233) in a tree analysis (
Fig. 5). DNA sequencing of the
Parachordodes worm does not appear on the database, so more information on this species is required in the future.
As for the invasion route, the present human cases may have accidentally swallowed insects, such as a cricket or a beetle which is an intermediate host. The present specimens are the first recorded human hair worm, Parachordodes sp. in Japan.
References
- 1. Hanelt B, Thomas F, Schmidt-Rhaesa A. Biology of the phylum Nematomorpha. Adv Parasitol 2005;59:243-305.
- 2. Poulin R. Hairworms (Nematomorpha: Gordioidea) infecting New Zealand short-horned grasshoppers (Orthoptera: Acrididae). J Parasitol 1995;81:121-122.
- 3. Ali-khan FEA, Ali-khan Z. Paragordius varius (Leidy) (Nematomorpha) infection in man: A case report from Quebec (Canada). J Parasitol 1977;63:174-176.
- 4. Wei DX, Yang WY. Parachordodes sp. (Nematomorpha) human infestation of the lower urinary tract: The first case report in China. Acta Acad Med Wuhan 1981;1:40-45.
- 5. Saito Y, Inoue I, Hayashi F, Itagaki H. A hairworm, Gordius sp., vomited by a domestic cat. Nihon Juigaku Zasshi 1987;49:1035-1037.
- 6. Herter CD, Nesse RE. Pseudoparasitism with Gordius robustus. Am Fam Physician 1989;39:139-142.
- 7. Kagei N, Oshima T, Inoue I, Kumasaki T. First human case on gordid worm in Japan (Nematomorpha: Gordiidae). Jpn J Parasitol 1966;15:79-81. (in Japanese).
- 8. Uchikawa R, Akune K, Inoue I, Kagei N, Sato A. A human case of hair worm (Gordius sp.) infection in Kagoshima, Japan. Jpn J Parasitol 1987;36:358-360.
- 9. Schmidt-Rhaesa A, Chung PR, Sohn WM. Parachordodes megareolatus, a new species of horsehair worm (Nematomorpha: Gordioida: Gordea) from Korea. Korean J Syst Zool 2003;19:161-166.
- 10. Lee KJ, Bae YT, Kim DH, Deung YK, Ryang YS, Im KI, Yong TS. A Gordius worm found in a 3-year-old girl's vomitus. Yonsei Med J 2003;44:557-560.
- 11. Schmidt-Rhaesa A, Nishi H, Tanabe AS, Urabe M. New records of hairworms (Nematomorpha: Gordiida) from Japan. Species Diversity 2009;14:131-135.
- 12. Schmidt-Rhaesa A, Sato T. Gordionus chinensis and Gordionus kii sp.nov. (Nematomorpha: Gordiida), new reports of the genus Gordionus in Japan. Species Diversity 2009;14:61-67.
- 13. Schmidt-Rhaesa A, Urabe M. New records of Gordius specimens from Japan (Nematomorpha, Gordiida). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut 2009;106:1-6.
- 14. Bleidorn C, Schmidt-Rhaesa A, Garey JR. Systematic relationships of Nematomorpha based on molecular and morphological data. Invertebr Biol 2002;121:357-364.
- 15. Giribet G, Carranza S, Baguna J, Riutort M, Ribera C. First molecular evidence for the existence of a Tardigrada + Arthropoda clade. Mol Biol Evol 1996;13:76-84.
- 16. Heinze K. Weitere neue Parachordodes-Arten aus Asien. Zoologischer Anzeiger 1935;112:155-158.
- 17. Inoue I. Synopsis of Japanese Gordiacea, with a note on a new species. Bull Biogeogr Soc Jpn 1955;16-19:31-35. (in Japanese).
- 18. Inoue I. In Uchida T ed, Nematomorpha. Systematic Zoology. 1962, Vol. 4:Tokyo, Japan. Nakayama Shoten; pp 192-220 (in Japanese).
- 19. Schmidt-Rhaesa A. Are the genera of Nematomorpha monophyletic taxa? Zoologica Scripta 2002;31:185-200.
- 20. Baek KM, Noh YT. Two species of genus Gordius (Gordioidea: Nematomorpha) from Korea. Korean J Syst Zool 1992;8:223-230.
Fig. 1A male hairworm of Parachordodes sp. vomited from an 80-year-old woman. Whole body (A), anterior part (B), and posterior part (C).
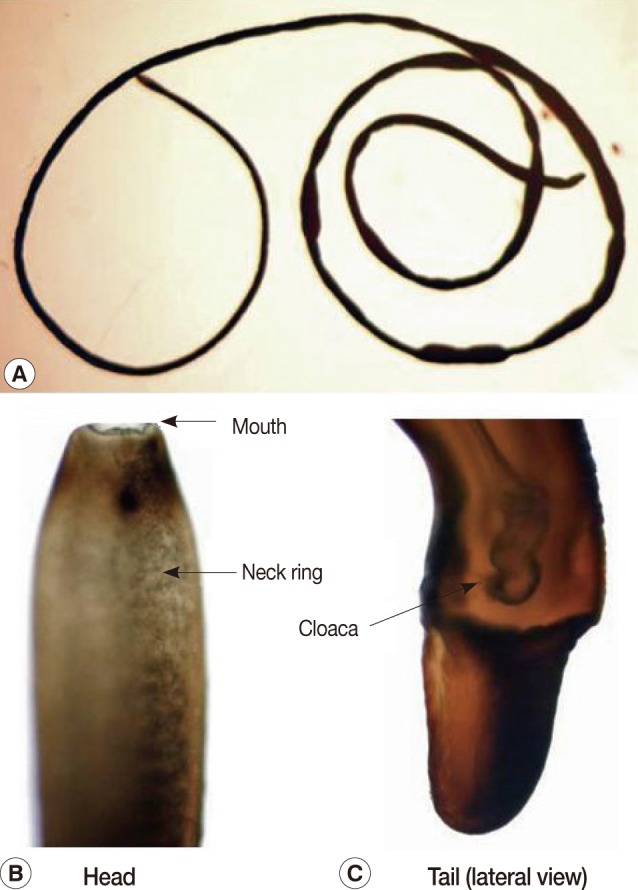
Fig. 2A male hairworm of Parachordodes sp. collected from the mouth of a 1-year-old boy by his mother. Whole body (A), anterior part (B), and posterior part (C).
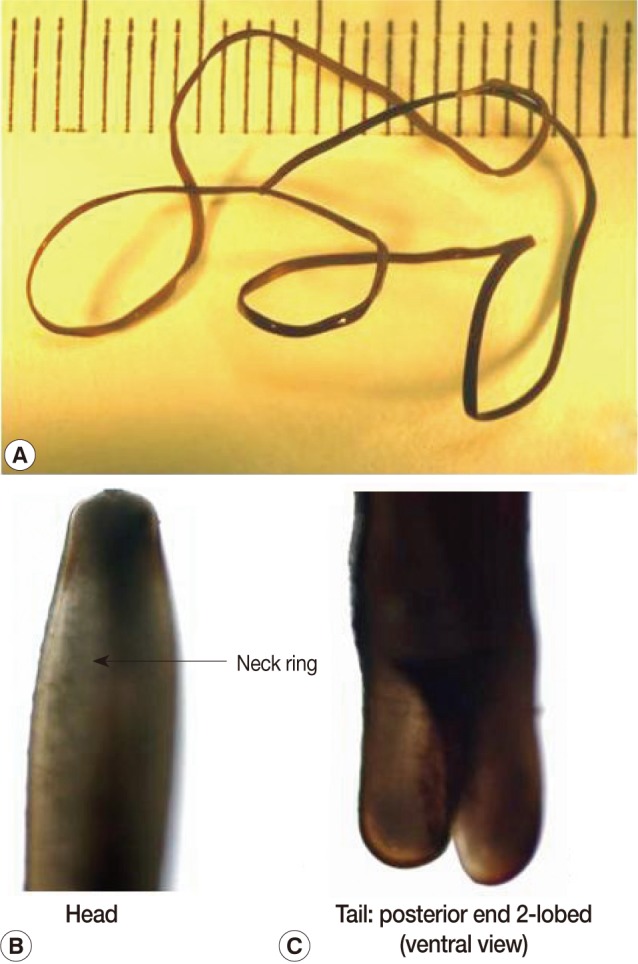
Fig. 3Cross sections of Parachordodes sp. hairworms derived from humans at about 5 mm from the posterior end. An 80-year-old woman (A and B) and a 1-year-old boy (C and D). A, areoles; C, cuticle; G, genital organs; H, hypodermis; I, intestine; M, muscle; V, ventral nerve cord.
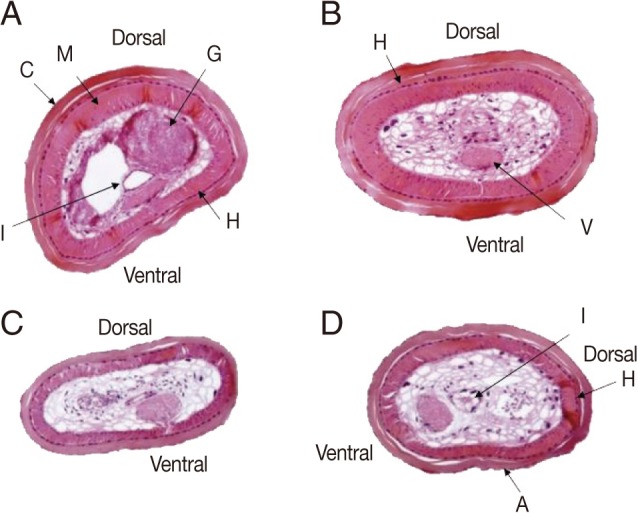
Fig. 4Two types of small sized-areoles (s) and megareoles (m) on the cuticle of Parachordodes sp. hairworms from humans (A: low magnification; B: high magnification).
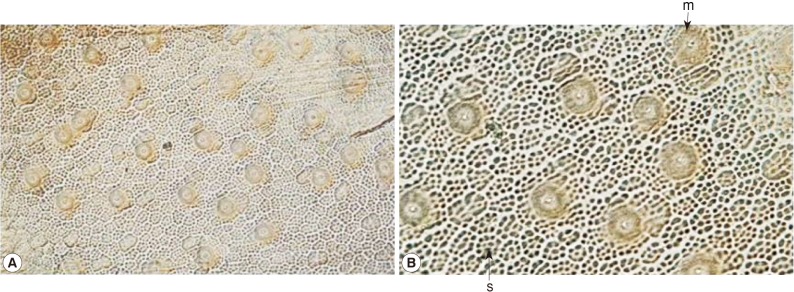
Fig. 5Phylogenetic analyses of hairworms (class Gordiacea) from humans based on 18S rRNA (1,414 bp). The evolutionary history was inferred using the Neighbor-Joining (NJ) method. The evolutionary distances were computed using the Kimura-2 parameter method and are in the units of the number of base substitutions per site. Bootstrap values are shown next to the branches. A high bootstrap value 0.988 (number of 1,000 replicates) was obtained at a junction of Paragordionus spp. and the present 2 worms.