Abstract
Several epidemiological surveys have reported the prevalence of Toxoplasma gondii infection in stray cats in Korea, but little information is available on T. gondii infection in household cats. The aim of the present study was to assess the prevalence and risk factors of T. gondii infection among household cats reared in Seoul, Korea. A total of 474 blood samples were collected from clinically healthy household cats. All samples were tested using ELISA and PCR. The risk factor analysis was based on a questionnaire filled out by the owners. The overall positive rate for ELISA and PCR assays was 2.2% (10/437) and 2.1% (10/474), respectively. With regard to the origin of cats, the positive rates among cats adopted from the animal shelter and veterinary clinic for stray cats were significantly different (P<0.05). Our study demonstrated that the positive rate of T. gondii infection in household cats was low and that this low prevalence was assumed to be associated with keeping the cats indoors and restriction of eating raw food and uncooked meat. Therefore, we suggest that the owners check the origin of the cats prior to adoption to prevent infection of other animals, including humans.
-
Key words: Toxoplasma gondii, risk factor, household cat, PCR, ELISA
Toxoplasma gondii is a zoonotic parasite that can infect humans and nearly all warm-blooded animals. Although almost all warm-blooded animals can serve as intermediate hosts, cats are essentially the only animal that acts as a definitive host and can excrete oocysts into the environment. Many studies have reported the prevalence of
T. gondii in cats as it is important in public health. Particularly, the prevalence of
T. gondii infection in household cats was 24.9-65.9% in the Western countries [
1-
3] and about 35% in the Middle East countries [
4]. Meanwhile, in Asia, its prevalence in cats was reported to be lower; 17.9% in Guangzhou, China [
5] and 8.7% in Japan [
6]. In Korea, several epidemiological surveys have been conducted on
T. gondii infection in stray cats [
7,
8]; however, the only survey for household cats was conducted by us on small populations in the capital city of Seoul [
9]. Recently, the companion pet market has increased in Korea and has led to an increase in the import of various cat species. In parallel to this trend, the number of household cats has also steadily increased, but no surveys have been conducted on
T. gondii infection among a large number of household cats. Therefore, the aim of this study was to investigate the prevalence of
T. gondii infection among household cats in Korea and its relation to the lifestyle of these household pets.
A total of 474 blood samples from the jugular vein of household cats were collected from April 2009 to June 2011. When cats were brought to the local veterinary clinic in Seoul for a regular health check or medical examination by the owners, blood sample collection and the owner completion of a questionnaire were conducted under the owner's agreement. The questionnaire included items on how the owner acquired the cat, the birth of origin, and the lifestyle of the pet in order to provide information on the possible routes for T. gondii infection. Sera were separated after blood cell sedimentation (centrifugation at 2,000 g for 5 min) and stored at -20℃ until use.
The total lysate antigen (TLA) was prepared from RH tachyzoites, and ELISA was performed as previously described [
10] with several modifications. Briefly, the tachyzoites were obtained from BALB/C mice, which were infected intraperitoneally 4 days earlier with
T. gondii. The tachyzoites were washed several times with PBS and were sonicated for 30 sec on ice to prepare the homogenate. The suspension was subsequently centrifuged at 15,000 g for 5 min at 4℃, and the supernatant was retained. The concentration of the lysate was measured by a Bio-Rad Protein assay kit (Bio-Rad Labs, Richmond, Virginia, USA), and stored at -70℃ until use. ELISA plates (Costa, Cambridge, Massachusetts, USA) coated with 2.5 µg/ml of TLA in coating buffer (pH 9.6) were incubated at 4℃ overnight and then washed 5 times with PBS-Tween 20 (0.05%). Next, the plates were filled with blocking buffer (5% skim milk in PBS-Tween 20), incubated at 37℃ for 1 hr and then washed 5 times. The sera diluted with the same solution were deposited at 200 µl/well (dilution of 1:100) and incubated for 60 min at 37℃. After the wells were washed 5 times, TLA-specific IgG was detected by using a horseradish peroxidase-conjugated anti-cat IgG (Jakson, Bar Harbor, Maine, USA) diluted 1:5,000 in blocking buffer and incubating buffer and incubation buffer, with the wells incubated at 37℃ for 1 hr. The colorimetric reaction was done with TMB and stopped with 4N H
2SO
4. Plates were read at 490 nm in a spectrophotometer, and the mean absorbance of the samples was 0.25 as a positive reaction. Genomic DNA was extracted from the blood of household cats using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Genomic DNA was finally resolved in 100 µl TE buffer and stored at -20℃. The target gene for amplification was a
T. gondii-specific B1 gene. The reaction was carried out with AmfiSure PCR Master mix (GenDEPOT, Barker, Texas, USA) and contained 1 µM of forward primer (5'-AGCAAACACCGACGAACTCT-3') and reverse primer (5'-CATGGTTTGCATTTTGTGG-3'). DNA samples and water were added to make a final reaction volume of 25 µl. The thermal cycle reactions were set to a first denaturing step of 94℃ for 5 min, then to 35 cycles of denaturation (94℃ for 60 sec), annealment (55℃ for 55 sec), and extension (72℃ for 60 sec), and finally an extension step at 72℃ for 4 min. Amplification products were resolved on ethidium bromide-stained 2% agarose gels by electrophoresis in Tris-acetate EDTA buffer. The PCR products were purified using the QIAquick PCR purification kit (Qiagen) and sequenced by a commercial laboratory.
The Chi square test was used to analyze the correlation between the prevalence of T. gondii and the various components of the cat lifestyle using SPSS 17.0K software (SPSS Inc., Chicago, Illinois, USA). A P-value of <0.05 was considered statistically significant.
The population of feral or stray cats and dogs has been increasing after the economic crisis in Korea. With the increase in feral cats, civil petitions regarding noise and other nuisance complaints to the Public Service Centers of the country's regional governments have also been gradually increasing. In addition, health professionals have raised concerns about the potential for human infection from
T. gondii, since this zoonotic pathogen has been found to be widespread among feral cats in Gyeonggi-do and Seoul [
7-
9]. Meanwhile, in our previous report on
T. gondii infection in household cats, no positive cases of
T. gondii infection were detected by ELISA or PCR [
9]. However, we noted that further studies using a larger number of household cats would be needed to examine the relationship between the infection rate and lifestyle in order to gain a better understanding of
T. gondii infection, since a small number of household cats were used in the previous study. For this reason, we surveyed the prevalence of
T. gondii in a relatively large population of household cats and evaluated the infection rate in terms of the pet lifestyle.
The prevalence of
T. gondii was 2.2% (10/437) and 2.1% (10/474) as determined by ELISA and PCR, respectively (
Table 1). It was relatively low compared to that reported in other countries. Generally, male cats were more likely than females to be positive for
T. gondii infection in previous reports. However, the prevalence between females and males in the current study was similar (
Table 2). In addition, as age increased, the positive rate of
T. gondii antibody was observed to increase [
11]. However, in the present study, the highest positive rate of
T. gondii infection was shown to be in the cats aged 6 months to 2 years, with the positive rate thereafter decreasing gradually with age. However, this trend was not statistically significant.
In terms of the lifestyle (
Table 2), most of the household cats primarily ate commercial cat food (over 90%) and were kept indoors (over 95%). Correspondingly, the cats were restricted from consumption of raw food or uncooked meat and primarily spent their time indoors. Although some cats occasionally had a chance to go outside, they were likely moved in the owner's bosom. According to the origin of the cats, the positive rate of household cats adopted from the animal shelter or veterinary clinic for stray cats was significantly higher than that of the other adopted origins, such as neighbors or other family members (
P<0.05). In addition, the cats identified to be positive by PCR would likely have shed oocysts within the previous 35 days [
12]. Hence, although fecal examinations were not performed in this study, the 10 cats with PCR-positive results were likely to have shed oocysts during the period. Through the survey questionnaire, we found that the 10 cats had lived with their owners for 1 month to 7 years. Therefore, the owners living with these infected cats might have contacted with oocysts shed from the cats. However, further studies are required to investigate the capacity of
T. gondii to infect the owners.
Feral and owned free-roaming cats also help spread pathogens such as
T. gondii to humans and livestock, as well as maintaining wildlife reservoirs [
13,
14]. Although no survey was conducted for
T. gondii infection in animal shelters in Korea, we assumed that the animals had a high chance of contacting
T. gondii, since many stray or feral animals had been gathered and lived together. Therefore, these positive cats may have been infected with
T. gondii when they lived in stray situations. In terms of the breeding environment, the positive rate of
T. gondii infection in household cats living with other cats was a little higher than that of cats living with other dogs or living alone, but not statistically different. The main infection route of
T. gondii to humans and animals is through the consumption of raw food and uncooked meat contaminated by oocysts of
T. gondii. However, in contrast to previous reports, no significant differences were observed in this study between infected and uninfected cats in regard to their eating habits. Based on the results of the questionnaire and test, we found household cats in Korea are strictly kept indoors, restricted from eating raw food and uncooked meat, and have no chance of contacting other wild animals or the ground. Therefore, we could assume that most of the household cats adopted from the animal shelter or clinic had already been exposure to
T. gondii infection outside and then had very few chances of exposure to
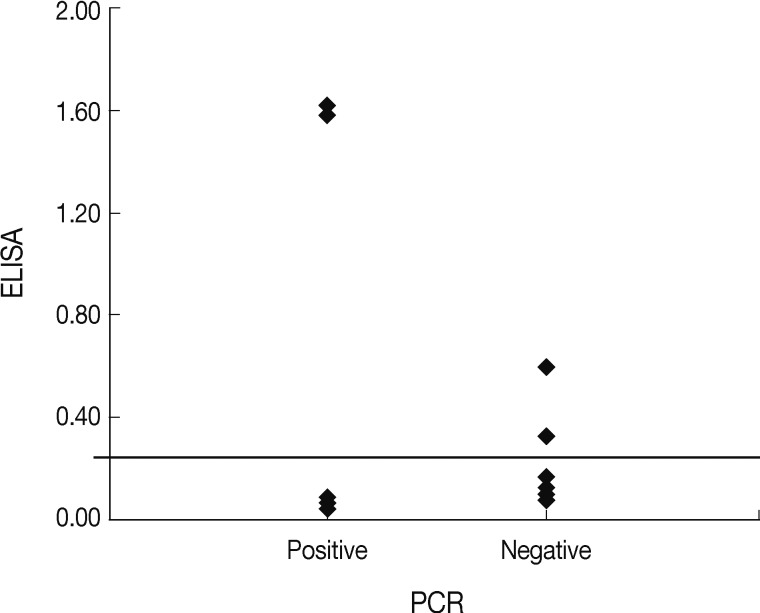
T. gondii infection after adoption. Only 2 of the cases were positive for both tests, and the ELISA values for these 2 cats were higher than those of the other ELISA-positive cats (
Fig. 1). Interestingly, both cats were originally stray but were discordant in regard to other examined factors. The reasons for differences in ELISA and PCR results for the household pets in this study may have a different detecting target with analysis, and affected by the period of infection and the host immune responses to
T. gondii.
In conclusion, our study demonstrated that the prevalence of T. gondii infection among household cats was low and that the low prevalence was caused by the cats being strictly kept indoors, restricted from eating raw food and uncooked meat, and having no chance to contact with other wild animals and the ground after adoption. Many of the household cats positive for T. gondii infection by ELISA and/or PCR were stray cats adopted from animal shelters and clinics. Therefore, the owner who wants to adopt a cat should identify the precise origin of the animal, and then keep it indoors and impose restrictions on its eating habits to protect from T. gondii and to maintain it as a safe companion animal.
ACKNOWLEDGMENT
This work was supported by funding (4847-302-210-13, 2011) from the Korea National Institute of Health, Korea Centers for Disease Control and Prevention.
References
- 1. Michalski MM, Platt-Samora JA, Mikulska-skupien E. Toxoplasma gondii antibodies in domestic cats in Olsztyn urban area, Poland. Wiad Parazytol 2010;56:277-279.
- 2. De Craeye S, Francart SA, Chabauty J, De Vriendt V, Van Gucht S, Leroux I, Jongert E. Prevalence of Toxoplasma gondii infection in Belgian house cats. Vet Parasitol 2008;157:128-132.
- 3. Miro G, Montoya A, Jimenez S, Frisuelos C, Mateo M, Fuentes I. Prevalence of antibodies to Toxoplasma gondii and intestinal parasites in stray, farm and household cats in Spain. Vet Parasitol 2004;126:249-255.
- 4. Haddadzadeh HR, Khazrainia P, Aslani M, Rezaeian M, Jamshidi S, Taheri M, Bahonar A. Seroprevalence of Toxoplasma gondii infection in stray and household cats in Tehran. Vet Parasitol 2006;138:211-216.
- 5. Zhang H, Zhou DH, Zhou P, Lun ZR, Chen XG, Lin RQ, Yuan ZG, Zhu XQ. Seroprevalence of Toxoplasma gondii infection in stray and household cats in Guangzhou, China. Zoonoses Public Health 2009;56:502-505.
- 6. Maruyama S, Hiraga S, Yokoyama E, Naoi M, Tsuruoka Y, Ogura Y, Tamura K, Namba S, Kameyama Y, Nakamura S, Katsybe Y. Seroprevalence of Bartonella henselae and Toxoplasma gondii infections among pet cats in Kanagawa and Saitama Prefectures. J Vet Med Sci 1998;60:997-1000.
- 7. Lee SE, Kim NH, Chae HS, Cho SH, Nam HW, Lee WJ, Kim SH, Lee JH. Prevalence of Toxoplasma gondii infection in feral cats in Seoul, Korea. J Parasitol 2011;97:153-155.
- 8. Kim HY, Kim YA, Kang SW, Lee HS, Rhie HG, Ahn HJ, Nam HW, Lee SE. Prevalence of Toxoplasma gondii in stray cats of Gyeonggi-do, Korea. Korean J Parasitol 2008;46:199-201.
- 9. Lee SE, Kim JY, Kim YA, Cho SH, Ahn HJ, Woo HM, Lee WJ, Nam HW. Prevalence of Toxoplasma gondii infection in stray and household cats in regions of Seoul, Korea. Korean J Parasitol 2010;48:267-270.
- 10. Choi WY, Nam HW, Youn JH, Kim DJ, Kong DJ, Kong Y, Kang SY, Cho SY. Detection of antibodies in serum and cerebrospinal fluid to Toxoplasma gondii by indirect latex agglutination test and enzyme-linked immunosorbent assay. Korean J Parasitol 1992;30:83-90.
- 11. Vollaire MR, Radecki SV, Lappin MR. Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. Am J Vet Res 2005;66:874-877.
- 12. Burney DP, Lappin MR, Spilker M, McReynolds L. A detection of Toxoplasma gondii parasitemia in experimenetally inoculated cats. J Parasitol 1999;85:947-951.
- 13. Frenkel JK, Hassanein KM, Hassanein RS, Brown E, Thulliez P, Quintero-Nunez R. Transmission of Toxoplasma gondii in Panama City, Panama: a five-year prospective cohort study of children, cats, rodents, birds, and soil. Am J Trop Med Hyg 1995;53:458-468.
- 14. Lehmann T, Graham DH, Dahl E, Sreekumar C, Launer F, Corn JL, Gamble HR, Dubey JP. Transmission dynamics of Toxoplasma gondii on a pig farm. Infect Genet Evol 2003;3:135-141.
Fig. 1Comparative distribution of the ELISA results among the nested PCR-positive and negative samples.

Table 1.The positive rate of Toxoplasma gondii infection in household cats using ELISA and PCR assay
Table 1.
|
Assay method |
No. examined |
No. of positives |
Positive rate (%) |
|
ELISA |
437 |
10 |
2.2 |
|
PCR |
474 |
10 |
2.1 |
|
ELISA & PCR |
435 |
2 |
0.4 |
Table 2.The results of a questionnaire for risk factor of Toxoplasma gondii infection in household cats based on detection methods
Table 2.
|
Variables |
ELISA
|
PCR
|
P-value |
|
Examined no. (%) |
Positive no. (%) |
Examined no. (%) |
Positive no. (%) |
|
Total |
437 |
10 (2.2) |
474 |
10 (2.1) |
|
|
Sex |
|
|
|
|
|
|
Male |
76 (17.4) |
3 (3.9) |
222 (46.8) |
2 (0.9) |
|
|
Female |
354 (81.0) |
7 (1.9) |
243 (51.3) |
8 (3.3) |
|
|
Unknown |
7 (1.6) |
0 |
9 (1.9) |
0 |
|
|
Age group |
|
|
|
|
|
|
<6 mo |
76 (17.4) |
0 |
57 (12.0) |
0 |
|
|
6 mo-2 yr |
207 (47.3) |
4 (1.9) |
254 (53.6) |
5 (2.0) |
|
|
2-5 yr |
86 (19.7) |
2 (2.3) |
90 (19.0) |
2 (2.2) |
|
|
>5 yr |
62 (14.2) |
4 (6.4) |
67 (14.1) |
3 (4.4) |
|
|
Unknown |
6 (1.4) |
0 |
6 (1.3) |
0 |
|
|
Analysis of life-style |
|
|
|
|
|
|
Eating habits |
|
|
|
|
|
|
Cat (commercial) food |
402 (91.9) |
9 (2.2) |
445 (93.9) |
10 (2.2) |
|
|
Mixed (raw+cat) food |
23 (5.3) |
1 (4.3) |
20 (4.2) |
0 |
|
|
Unknown |
12 (2.7) |
0 |
9 (1.9) |
0 |
|
|
Habitat |
|
|
|
|
|
|
Indoor |
422 (96.6) |
8 (1.9) |
454 (95.8) |
9 (2.0) |
|
|
Outdoor |
13 (3.0) |
2 (15.4) |
18 (3.8) |
1 (5.6) |
|
|
Unknown |
2 (0.4) |
0 |
2 (0.4) |
0 |
|
|
Adopted origin |
|
|
|
|
|
|
Neighbor/Other family |
313 (71.6) |
3 (0.9) |
365 (77.0) |
1 (0.2) |
|
|
Animal shelter/Clinic |
120 (27.5) |
7 (5.8) |
101 (21.3) |
9 (8.9) |
<0.05 |
|
Unknown |
4 (0.9) |
0 |
8 (1.7) |
0 |
|
|
Breeding environment |
|
|
|
|
|
|
Alone |
188 (43.0) |
2 (1.0) |
203 (42.8) |
2 (1.0) |
|
|
With other cats |
207 (47.4) |
7 (3.4) |
222 (46.8) |
8 (3.6) |
|
|
With other dogs |
17 (3.9) |
1 (5.9) |
17 (3.6) |
0 |
|
|
With other cats and dogs |
3 (0.7) |
0 |
7 (1.5) |
0 |
|
|
Other animals & Unknown |
22 (5.0) |
0 |
25 (5.3) |
0 |
|
|
Consumption of raw food |
|
|
|
|
|
|
Yes |
32 (7.3) |
1 (3.1) |
40 (8.4) |
1 (0.3) |
|
|
No |
385 (88.1) |
9 (2.3) |
427 (90.1) |
9 (2.1) |
|
|
Unknown |
20 (4.6) |
0 |
7 (1.5) |
0 |
|
|
Experience of a walk |
|
|
|
|
|
|
Yes |
72 (16.5) |
2 (2.8) |
51 (10.8) |
2 (3.9) |
|
|
No |
355 (81.2) |
8 (2.3) |
423 (89.2) |
8 (1.9) |
|
|
Unknown |
10 (2.3) |
0 |
0 |
0 |
|