Abstract
This study describes the first record of Bourgelatia diducta (Nematoda: Chabertiidae) from wild boars in the Republic of Korea (=South Korea). Gastrointestinal tracts of 87 Korean wild boars (Sus scrofa coreanus) hunted in mountains in the south-western part of South Korea between 2009 and 2012 were examined for their visceral helminths. B. diducta, as identified by morphological characteristics of the head and tail, were recovered from the large intestine of 47 (54%) wild boars. The average length of adult female worms was 11.3±0.87 mm and the thickest part of the body measured 0.54±0.04 mm in maximum width, while those of males were 9.8±0.72 and 0.45±0.03 mm, respectively. The characteristic J-shaped type II ovejector was observed in females, and the type II dorsal ray with 2 rami on each side of the median fissure was uniquely seen in males. The buccal capsule was small, relatively thin-walled, cylindrical, very short, and ring-shaped. The externodorsal ray arose from a common stem with the dorsal ray. The cervical groove was absent. The anterior extremity was equipped with 20-22 external corona radiata, 4 cephalic papillae and 2 lateral amphids around the mouth. The eggs were 66.0×38.9 µm in average size. By the present study, B. diducta (Nematoda: Chabertiidae) is recorded for the first time in South Korea. Additionally, morphological characteristics and identification keys provided in the present study will be helpful in the faunistic or taxonomic studies for strongylid nematodes related.
-
Key words: Bourgelatia diducta, wild boar, identification key, South Korea
INTRODUCTION
Bourgelatia diducta Ralliet, Henry and Bauche, 1919 is a small-sized, milky white-colored nematode parasite with a well-developed mouth and bursa. This nematode is a member of the subfamily Oesophagostominae, family Chabertiidae, and order Strongylida [
1]. The genus
Bourgelatia comprises a single species,
B. diducta, which was first found in the cecum and colon of pigs in 1919 [
2]. The species was formerly classified as
Phacochoerostrongylus Schwartz, but later was reclassified into the genus
Bourgelatia by differences in the morphology of buccal capsule, ovejecter, vagina length, and dorsal ray [
3]. Thus far, little is known about the pathogenesis and clinical symptoms of this parasite to its host,
Sus scrofa. The life cycle is speculated to be direct [
4].
Previous studies indicated that the infection of this worm is common among domestic pigs in India (21.6%) [
5], Papua New Guinea [
6,
7] and the Solomon Islands (13%) [
7]. Although the parasite was found in a relatively high prevalence among wild boars in Japan (95%) [
8], the parasite so far has not been reported in the Republic of Korea (South Korea). It has been reported that Korean domestic pigs are infected with
Oesophagostomum detatum only without
B. diducta in some surveys performed by fecal examination [
9-
12].
Recently, the population of wild boars in South Korea has gradually increased due to the absence of predators such as Panthera tigris. Therefore, the growing number of wild boars has been creating serious problems for human residential life and local agriculture industry. However, there has been little systematic approach to investigate the disease status among wild boars in South Korea. The purpose of this study, therefore, was to investigate parasitic diseases of wild boars in South Korea. In this study, we examined the gastrointestinal tract of 87 wild boars over a 4-year period in the southwestern part of South Korea and found that B. diducta was quite prevalent among wild boars. Together with light and scanning electron microscopic evidences, we provided the identification key and measurements of various parts of the worm.
MATERIALS AND METHODS
Animals
Gastrointestinal tracts of 87 Korean wild boars (Sus scrofa coreanus) hunted in mountains of Suncheon-si, Gwangyang-si, and Boseong-gun between 2009 and 2012 by members of local hunting associations were collected and examined for visceral helminths. The gastrointestinal tract of each wild boar was removed from the body and brought to the laboratory to examine the presence of parasites. The cecum and colon were slit open lengthwise and the mucosa and contents were examined carefully in a separate container.
Light microscopy
Worms were preserved in 70% ethanol, and were mounted on a slide glass using polyvinyl alcohol mounting medium [
13]. Measurements were made under a light microscope (AxioScop, Zeiss, City, Country) on body dimensions, lengths of corona radiata, eosophagus, spicules, and gubernaculum, width of pharynx and other body parts of adult male and female worms. Species identification of the worm was based on the description by Yamaguti [
14] and Lichtenfels [
3].
Scanning electron microscopy was used to identify
B. diducta as previously described by Yadav and Tandon [
15]. Briefly, worms were washed 3 times with 0.85% PBS for 20 min, fixed in 2.5% glutaraldehyde in 0.1M phosphate buffer (pH 7.2) for 24 hr in a refrigerator at 4℃ and were post-fixed in 1% OsO4 for 2 hr in a refrigerator at 4℃. After serial dehydration steps in 30, 50, 70, 80, 90, and 100% ethanol for 20 min each, critical-point drying in a Hitachi HCP-2 (Hitachi Ltd, Tokyo, Japan) and sputter-coating with gold-palladium in an Emitech K550 (Emitech Ltd, Ashford, Kent, England), digital photography of worms was taken using a Hitachi S-2400 scanning electron microscope (Hitachi Ltd, Tokyo, Japan).
RESULTS
The average length of adult females was 11.3±0.87 mm, and the thickest part of the body measured 0.54±0.04 mm in maximum width, while those of males were 9.8±0.72 and 0.45±0.03 mm, respectively. The average dimension of eggs was 66.0×38.9 µm. Female worms had a characteristic J-shaped type II ovejector (
Fig. 1G, H), and males had the type II dorsal ray with 2 rami (
Fig. 1C) on each side of the median fissure. The buccal capsule was small, relatively thin-walled, cylindrical, very short, and ring-shaped (
Fig. 1E). Externodorsal ray was arising from a common stem with the dorsal ray (
Fig. 1C). Although the oral collar was present (
Fig. 2C), the cervical groove that can be recognized in the genus
Oesophagostomum was absent (
Fig. 1F). Based on these morphological features, the worm was identified as
B. diducta.
The ovijector in the inside of the posterior extremity of females consisted of 3 parts as a thick-walled vestibule that connected to the vagina, a paired thick-walled sphincters and a thinner-walled infundibula which was connected to the uteri (
Fig. 1G, H). In addition, the infundibula were relatively smaller than the vestibule and sphincters. The dorsal ray was short and bifurcated deeper than proximal branches (
Fig. 1C). The anterior extremity was equipped with 20-22 external corona radiata, a well-developed leaf-crown structure surrounding the mouth, 4 cephalic papillae and 2 lateral amphids around the mouth (
Fig. 2A, B). There was an oral collar which was much deeper than transverse ridges of the body cuticle, and an excretory pore and a pair of cervical papillae were also present (
Fig. 2C-F).
The posterior extremity of female worms became narrow toward the end (
Fig. 3A). The vulva was shown as a prominent circular protrusion with a semicircular opening while the anus was shown as a rather flat semicircular hole (
Fig. 3A-C). The tapering tail bore a pair of minute caudal papillae and a spike (
Fig. 3D). The posterior extremity of males had a well-developed bursa that consist of a long dorsal and 2 slightly shorter lateral lobes (
Fig. 3E, F). There were 2 conspicuous protrusions at the end of externodorsal and anterolateral rays on its surface (
Fig. 3E). The genital cone was observed at the point of the spicules flowing in and out (
Fig. 3F).
From 87 Korean wild boars captured in the southwestern area of South Korea, 47 (54.0%) were found to harbour
B. diducta in the large intestine, mainly in the cecum. The worm was found in 5 of 13 wild boars (38.5%) from Suncheon-si, 38 of 70 (54.3) from Gwangyang-si, and all 4 (100%) from Boseong-gun. No differences between the gender were recognized on the rate of infection (
Table 1). A total of 938 worms (551 males and 387 females) of
B. diducta was collected from the cecum and colon with an average number of 20.0 per animal and the infection intensity being the highest in animals from Boseong-gun. The number of female worms present was 1.3 times more prevalent than the number of male worms (
Table 2).
DISCUSSION
From 87 Korean wild boars captured from mountains of the Suncheon-si, Gwangyang-si and Boseong-gun, South Korea spanning 2009 to 2012, the infection of
B. diducta, with an average number of 20 worms per animal was confirmed in 47 (54%) wild boars. Although this is the first report in South Korea, the prevalence of the parasite in pigs from other countries has been previously reported. For instance, the prevalence of
B. diducta in wild boars or domestic pigs reported from Japan [
8], India [
5], and the Solomon islands [
7] was 95%, 21.6%, and 13.0%, respectively. These reports suggest that the parasite may be distributed over a rather wide area ranging from Asia and Oceania. Up to now, however, there have been few reports on the intensity and pathogenesis of the infection with the parasite among pigs in the rest of the world.
Although we carefully observed the large intestine, pathologic changes in the mucosa was hardly recognized. In the infection with
Oesophagostomum detatum which belongs to the same subfamily Oesophagostominae as
Bourgelatia, infective larvae exsheath in the small intestine and enter the mucosa of the large intestine causing small nodules [
4]. Although the parasite is normally regarded as being only mildly pathogenic to pigs [
16,
17], the intestinal walls become edematous and strongly hyperemic in heavy burdens to develop into necrotic enteritis [
17,
18]. As observed during the autopsy in this study, the infection of
B. diducta did not elicit pathologic lesions in pigs at the population density of 20 worms per animal. There is a possibility that pathologic changes in the mucosa were not recognized due to the relatively low worm burden. Also, it is possible that the worm is relatively non-pathogenic in wild boars.
The infection of domestic pigs with the genus
Oesophagostomum spp. has been frequently reported in South Korea and the prevalences of
O. dentatum among domestic pigs based on fecal examination of eggs were 10.5% [
19], 14.8% [
12], 6.4% [
10], and 2.5% [
9]. However, eggs of the genus
Oesophagostomum and
Bourgelatia are indistinguishable by size and appearance, and therefore it is possible that previous surveys based on fecal examination might have made mistakes stemming from correct identification of the genus. Similarly, the prevalence of
B. diducta (17%) previously reported in pigs from Nigeria [
20] by fecal examination might not be based on correct identification. Therefore, the infection status of
O. dentatum among domestic pigs in Korea requires identification of adult worms for a correct faunistic record in Korea.
Previous studies on the morphological features of
B. diducta are remarkably consistent with those of our present study [
3,
4,
14,
15,
21]. The measurements of the worm body in this study were practically identical with the report by Yamaguti [
14] (
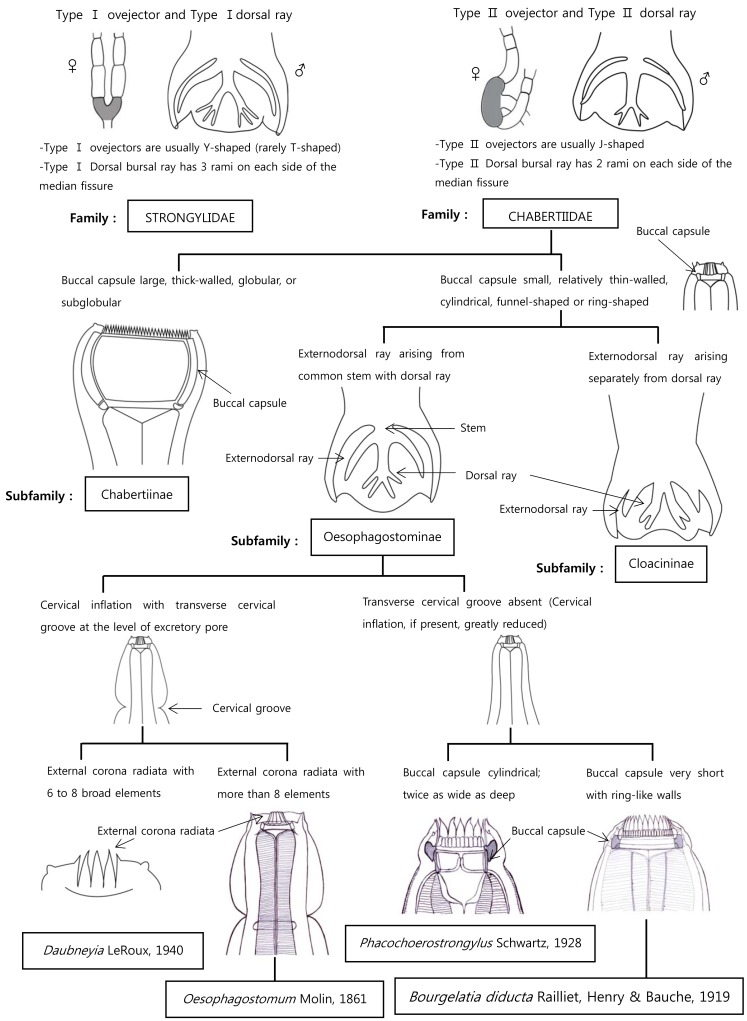
Table 4). Studies on SEM structures by Yadav [
15] on external corona radiata, oral collar, cephalic papilla, lateral amphid, bursa, genital cone, externodorsal and anterolateral rays, vulva, anus, and caudal papillae match structurally up with our findings. In other members of the subfamily Oesophagostominae in pigs, morphological structures are varied in shape and size. The cervical groove is present in Daubneyia LeRoux, 1940 and
Oesophagostomum Molin, 1861 while the buccal capsule of
Phacochoerostrongylus Schwartz, 1928 is twice as wide as deep [
3]. The
Bourgelatioides traguli differs from
B. diducta in lacking the anterior leaf-crown and in other details of the mouth capsule, in the presence of a groove and overlying flaps in the cervical region, and in the presence of terminal convoluted filaments on the spicules [
2]. We provided some identification key aspects regarding the morphological features of the Superfamily Strongyloidea and the genus
Bourgelatia (
Fig. 4). The identification keys in this article are adapted from Lichtenfels [
3].
Conclusively, in the present study, B. diducta (Nematoda: Chabertiidae) is recorded for the first time in South Korea. In addition, morphological characteristics and differential keys provided in the present study will be helpful in the faunistic and taxonomic studies of strongylid nematodes related.
References
- 1. Railliet A, Henry A, Bauche J. Un noveau Strongylide du porc. B Soc Pathol Exot 1919;12:324-332.
- 2. Chandler AC. New genera and species of nematode worms. P Natl Muse 1931;78:1-11.
- 3. Lichtenfels J. 5. Strongylida, Strongyloidea. In Anderson R, Chabaud A, Willmott S eds, Keys to the Nematode Parasites of Vertebrates: Archival Volume. London, UK. CABI; 2009, pp 69-109.
- 4. Soulsby E. Helminths, Arthropods and Protozoa of Domesticated Animals. Lea & Febiger; 1982, pp 183-195.
- 5. Yadav A, Tandon V. Nematode parasite infections of domestic pigs in a sub-tropical and high-rainfall area of India. Vet Parasitol 1989;31:133-139.
- 6. Talbot NT. Incidence and distribution of helminth and arthropod parasites of indigenous owned pigs in Papua New Guinea. Trop Anim Health Prod 1972;4:182-190.
- 7. De Fredrick DF. Pig production in the Solomon Islands. II. Diseases and parasites. Trop Anim Health Prod 1977;9:135-139.
- 8. Sato H, Suzuki K, Yokoyama M. Visceral helminths of wild boars (Sus scrofa leucomystax) in Japan, with special reference to a new species of the genus Morgascaridia Inglis, 1958 (Nematoda: Schneidernematidae). J Helminthol 2008;82:159-168.
- 9. Park SJ, Thak TS, Cha WS. A survey of swine internal parasites at the cement-floored and sawdust fermentative pigsty. Korean J Vet Serv 1992;15:121-127.
- 10. Kim WG, Lee HS, Yang HC, Yoon YB. Survey of swine parasitic infection rate in lri area. Korean J Vet Serv 1990;13:103-109.
- 11. Jang DH, Nah JW, Kang DW. Preliminary survey of swine internal parasites at the sawdust fermentation floor system. Korean J Vet Res 1991;31:509-513.
- 12. Kang YB, Wee SH. Fluctuation of internal parasite infections in industrialized piggeries in Korea. Korean J Vet Public Health 1989;13:15-19.
- 13. Downs WG. Polyvinyl alcohol: a medium for mounting and clearing biological specimens. Science 1943;97:539-540.
- 14. Yamaguti S. Parasitic worms mainly from Celebes. Part 10. Nematodes of Birds and Mammals. Acta Med Okayama 1954;9:143-144.
- 15. Yadav A, Tandon V. SEM structure of Bourgelatia diducta (Nematoda, Chabertiidae). Acta Parasitol 1993;38:128-130.
- 16. Stewart T, Gasbarre L. The veterinary importance of nodular worms (Olesophagostomum spp). Parasitol Today 1989;5:209-213.
- 17. Roepstorff A, Bjørn H, Nansen P, Barnes E, Christensen C. Experimental Oesophagostomum dentatum infections in the pig: Worm populations resulting from trickle infections with three dose levels of larvae. Int J Parasitol 1996;26:399-408.
- 18. Christensen C, Barnes E, Nansen P, Roepstorff A, Slotved HC. Experimental Oesophagostomum dentatum infection in the pig: Worm populations resulting from single infections with three doses of larvae. Int J Parasitol 1995;25:1491-1498.
- 19. Lee CG, Lee CY, Park YJ. A survey on the internal parasitism of swine in Chonnam area. Rural Dev Rev 1982;17:79-84.
- 20. Nwoha RIo, Daniel G. Prevalence of gastrointestinal nematode parasites in intensively managed pigs of different ages and sexes in Umuahia city of Abia state. Contl J Vet Sci 2011;5:11-17.
- 21. Yamaguti S. Systema Helminthum. Nematodes of Vertebrates (in 2 pts.), 1961. Interscience Publishers; 1961, pp 380-381.
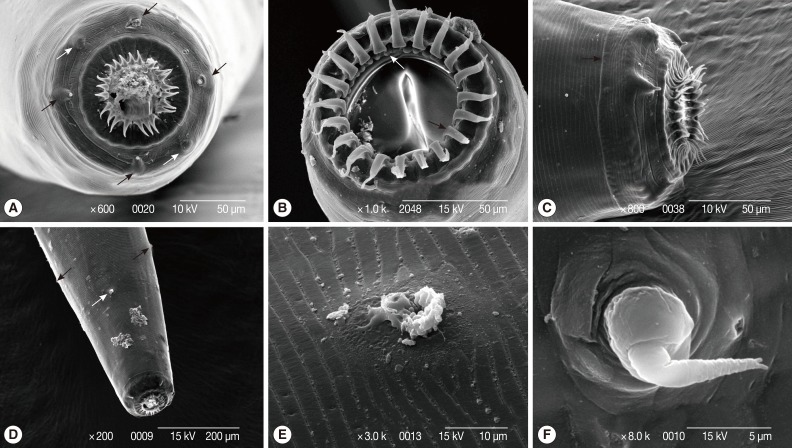
Fig. 1Light micrographs of Bourgelatia diducta isolated from the large intestine of wild boars (Sus scrofa coreanus) from South Korea. (A) Entire view of an adult female. (B) Entire view of an adult male. (C) Lateral view of the posterior end of a male, showing the spicule (SP), gubernaculum (G), dorsal ray (D), 2 rami (R), and the externodorsal ray (ED). (D) Egg of B. diducta. (E) Enlarged view of the anterior end, showing leaf-like structures of external corona radiata (ER), internal corona radiata (IR), buccal cavity and buccal capsule (BC). (F) Lateral view of the anterior end, showing the esophagus (ES) and excretory pore (EX). (G) Lateral view of the posterior end of a female, showing the ovejector (H), vulva (Vu), anus (An), and tail. (H) Enlarged view of the ovejector consisted of 3 parts; infundibula (in), sphincter (sp), vestibula (vs), and uteri (ut).

Fig. 2Scanning electron micrographs of Bourgelatia diducta isolated from the large intestine of wild boar (Sus scrofa coreanus) from South Korea.(A) Enface anterior end, illustrating 4 cephalic papillae (black arrows) and 2 lateral amphids (white arrows). (B) Anterior end with the oral cavity wide open, showing leaf-like structures of external corona radiata (black arrow) and internal corona radiata (white arrow). (C) Anterior end showing the oral collar (black arrow). (D) Anterior end showing the excretory pore (white arrow) and 2 cervical papilla (black arrows). (E) Enlarged view of the excretory pore. (F) Enlarged view of a cervical papillum.

Fig. 3Scanning electron micrographs of Bourgelatia diducta isolated from the large intestine of wild boar (Sus scrofa coreanus) from South Korea. (A) Caudal end of a female, illustrating the vulva (black arrow), anus (white arrow), and tail (white arrow head). (B) Enlarged view of the vulva. (C) Enlarged view of the anus. (D) Enlarged view of the female tail, illustrating 1 of a pair of caudal papilla (white arrow) and spike (black arrow). (E) Caudal end of a male, illustrating externodorsal (black arrow) and anterolateral (white arrow) rays. (F) Caudal end of a male, showing the bursa and a genital cone (white arrow).

Fig. 4Differential keys to the closely related genera of Strongyloidea.

Table 1.Infection status of Bourgelatia diducta in wild boars from the Republic of Korea
Table 1.
|
Infection status |
Locality and gender of wild boars
|
Suncheon-si
|
Gwangyang-si
|
Boseong-gun
|
Total
|
|
Male |
Female |
Total |
Male |
Female |
Total |
Male |
Female |
Total |
Male |
Female |
Total |
|
Infected |
3 |
2 |
5 |
24 |
14 |
38 |
0 |
4 |
4 |
27 |
20 |
47 |
|
Uninfected |
1 |
7 |
8 |
22 |
10 |
32 |
0 |
0 |
0 |
23 |
17 |
40 |
|
Total |
4 |
9 |
13 |
46 |
24 |
70 |
0 |
4 |
4 |
50 |
37 |
87 |
|
Infection rate (%) |
75.0 |
22.2 |
38.5 |
52.2 |
58.3 |
54.3 |
0.0 |
100.0 |
100.0 |
54.0 |
54.1 |
54.0 |
Table 2.Number of adult Bourgelatia diducta collected from wild boars in the Republic of Korea
Table 2.
|
Area |
Gender of wild boars |
Number of infected boars |
Number of adult worms
|
|
Male |
Female |
Total (no. worms/ wild boars) |
F/M ratio |
|
Suncheon-si |
Male |
3 |
4 |
10 |
14 (4.7) |
2.5 |
|
Female |
2 |
19 |
26 |
45 (22.5) |
1.4 |
|
Total |
5 |
23 |
36 |
59 (11.8) |
1.6 |
|
Gwangyang-si |
Male |
24 |
242 |
295 |
537 (22.4) |
1.2 |
|
Female |
14 |
118 |
132 |
250 (17.9) |
1.1 |
|
Total |
38 |
360 |
427 |
787 (20.7) |
1.2 |
|
Boseong-gun |
Male |
0 |
0 |
0 |
0 (-) |
- |
|
Female |
4 |
27 |
65 |
92 (23.0) |
2.4 |
|
Total |
4 |
27 |
65 |
92 (23.0) |
2.4 |
|
Total |
Male |
27 |
246 |
305 |
551 (20.4) |
1.2 |
|
Female |
20 |
164 |
223 |
387 (19.4) |
1.4 |
|
Total |
47 |
410 |
528 |
938 (20.0) |
1.3 |
Table 3.Body part measurements of adult Bourgelatia diducta isolated from wild boars of Korea
Table 3.
|
Anatomical location |
|
Categories |
Mesurement (µm)
|
|
Male |
Female |
|
General |
|
Number of worms |
9 |
13 |
|
|
Body length |
9,777 ± 723 |
11,272 ± 874 |
|
|
Body width |
452 ± 30 |
541 ± 37 |
|
Anterior extremity |
|
Length of external corona radiata |
30.7 ± 4.3 |
34.1 ± 4.2 |
|
|
Length of internal corona radiata |
20.7 ± 1.2 |
23 ± 2 |
|
|
Length Ratio of internal to external corona radiata |
1.5 ± 0.3 |
1.5 ± 0.1 |
|
|
Width of pharynx |
68.8 ± 7.9 |
76.0 ± 3.9 |
|
|
Length of oesophagus |
836.3 ± 37.8 |
932.7 ± 69.4 |
|
|
Proportion of oesophagus to body length from the anterior end |
11.9 ± 0.7 |
12.1 ± 0.9 |
|
|
Excretory pore from head end |
358.3 ± 10.4 |
385 ± 17.9 |
|
|
Number of external corona radiataa
|
21 |
20-22 |
|
Posterior extremity |
Female |
Length of vestibule |
- |
221.5 ± 24.9 |
|
|
Length of sphincters |
- |
200.2 ± 24.4 |
|
|
Length of infundibula |
- |
72.0 ± 14.9 |
|
|
Distance of vulva from anus |
- |
463.8 ± 69.3 |
|
|
Distance of anus from antererior end |
- |
418.1 ± 51.5 |
|
|
Egg size |
|
66.0 ± 4.19 × 38.9 ± 2.26 |
|
Male |
Length of spicules |
1,155.6 ± 191.7 |
- |
|
|
Length of gubernaculum |
140 ± 9 |
- |
|
|
Length of dorsal ray |
178.8 ± 28.1 |
- |
|
|
Length of branch of dorsal ray |
51.9 ± 10.7 |
- |
|
|
Length of externodorsal ray |
168.8 ± 14.4 |
- |
Table 4.Comparison of Bourgelatia diducta body parts from wild boars of Korea with those of previous reports (mm)
Table 4.
|
Railliet, Henry & Bauche (1919) |
Yamaguti (1935) |
Yamaguti (1954) |
This study |
|
Body of male |
9.3-12.5×0.4-0.6 |
8.8-12×0.5-0.65 |
8.9-9.7×0.42 |
8.7-11.0×0.41-0.50 |
|
Body of female |
11-13.5×0.5-0.64 |
9.3-10.1×0.58-0.6 |
10.3-10.5×0.56-0.61 |
10.2-12.9×0.48-0.59 |
|
Cervical papillae from head end |
0.42-0.55 |
0.42-0.53 |
0.44-0.48 |
0.48-0.62 |
|
Excretory pore from head end |
0.28-0.64 |
0.3-0.35 |
0.31-0.32 |
0.35-0.41 |
|
Esophagus |
0.65-0.85 |
0.7-0.9 |
0.71-0.77 |
0.79-1.03 |
|
Spicule |
1.25-1.33 |
1.1-1.25 |
1.1-1.2 |
0.93-1.60 |
|
Gubernaculum |
0.135 |
0.16 |
0.13 |
0.13-0.15 |
|
Tail of female |
0.4-0.425 |
045-0.5 |
0.29-0.295 |
0.34-0.50 |
|
Vulva from tail end |
0.95-1.06 |
0.9-1.0 |
0.74-0.83 |
0.69-1.01 |
|
Eggsa
|
69-77×38-42 |
- |
57-69×39-48 |
53-75×35-43 |