Abstract
Taenia pisiformis is one of the most important parasites of canines and rabbits. T. pisiformis cysticercus (the larval stage) causes severe damage to rabbit breeding, which results in huge economic losses. In this study, the genetic variation of T. pisiformis was determined in Sichuan Province, China. Fragments of the mitochondrial cytochrome b (cytb) (922 bp) gene were amplified in 53 isolates from 8 regions of T. pisiformis. Overall, 12 haplotypes were found in these 53 cytb sequences. Molecular genetic variations showed 98.4% genetic variation derived from intra-region. FST and Nm values suggested that 53 isolates were not genetically differentiated and had low levels of genetic diversity. Neutrality indices of the cytb sequences showed the evolution of T. pisiformis followed a neutral mode. Phylogenetic analysis revealed no correlation between phylogeny and geographic distribution. These findings indicate that 53 isolates of T. pisiformis keep a low genetic variation, which provide useful knowledge for monitoring changes in parasite populations for future control strategies.
-
Key words: Taenia pisiformis, cytochrome b, genetic variation, China
INTRODUCTION
The larval stage of the tapeworm
Taenia pisiformis (Cestoda: Taeniidae) is the causative agent of cysticercosis in rabbits, and is globally distributed [
1,
2].
T. pisiformis cysticercosis usually parasitizes the liver capsule, gastric omentum majus, and mesentery of rabbits, while adult
T. pisiformis establish and mature in the small intestine of dogs and foxes. China is the world's largest producer of rabbits [
3], and
T. pisiformis has become one of the most common parasites to severely affect rabbit breeding. It mainly causes autologous poisoning and emaciation, but can also weaken resistance to other diseases; it may even cause death [
4]. However, insufficient studies on genetic variation of
T. pisiformis in China have been carried out to date. Due to faster mutation rates of mitochondrial DNA (mtDNA) sequences than nuclear genes [
5] and the absence of host selection pressures [
6], mtDNA sequences are considered to be more suitable to discriminate between closely related organisms [
7].
Mitochondrial genes have successfully been used to study genetic variation, and enable a focus on the genetic origin, scope, and genotype of organisms [
8]. The structure and function of cytochrome
b (
cytb) have been verified in mtDNA sequences of cestodes and maintain a moderate evolutionary speed. Thus,
cytb has been used to study the population structure and genetic differentiation of several tapeworm species [
9,
10]. In this study, we determined the genetic variation of
T. pisiformis based on partial
cytb gene sequences from Sichuan Province, China.
MATERIALS AND METHODS
Sample collection
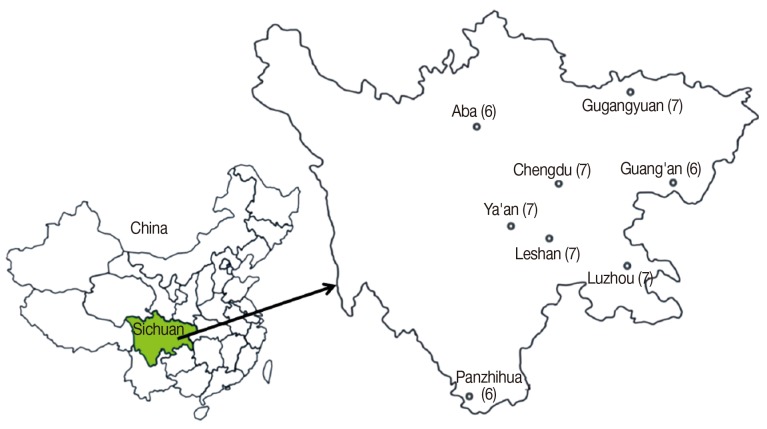
A total of 53 isolates were collected from routine autopsies in 8 geographical regions of Sichuan Province, China. The regions included Ya'an (7 isolates, YA1-YA7), Chengdu (7 isolates, CD1-CD7), Panzhihua (6 isolates, PZ1-PZ6), Leshan (7 isolates, LS1-LS7), Guangyuan (7 isolates, GY1-GY7), Luzhou (7 isolates, LZ1-LZ7), Guang'an (6 isolates, GA1-GA6), and Aba (6 isolates, AB1-AB6) (
Fig. 1). The maintenance and care of the rabbits used in this study was in strict accordance with good animal practice regulations.
Approximately 0.5 g genomic DNA was extracted from cysticerci using the phenol-chloroform extraction as described by Sambrook et al. [
11]. The DNA was resuspended in 50 µl Tris-EDTA (TE) buffer and stored at -20℃. To amplify the
cytb gene, PCR primers (forward: 5'-ATGGTTAGTTTATTACGTCGGA-3'; and reverse: 5'-TAAGAACTCTAAACACTTGACATAC-3') were designed by the program primer 5.0 using the mitochondrial genome sequence of
T. pisiformis, which is available at the National Center for Biotechnical Information (NCBI) database (GenBank no. NC013844). PCR reactions were carried out in a 50 µl reaction mixture containing 25 µl of PCR mixture (Tiangen, Beijing, China), 2 µl of each primer (forward and reverse), 1 µl of template DNA (approximately 100 ng), and 20 µl sterile water. The amplification conditions for
cytb consisted of an initial denaturation step at 94℃ for 5 min, followed by 30 cycles of denaturation at 94℃ for 55 sec, annealing at 54℃ for 55 sec, elongation at 72℃ for 50 sec, and a final extension step at 72℃ for 10 min. PCR products (50 µl) were separated by electrophoresis on a 1.0% agarose gel and stained with ethidium bromide. Amplicons were cloned into a pMD
19-t vector (TaKaRa, Dalian, China) according to the manufacturer's instructions. Purified PCR products and positive clones were sequenced 3 times in-house using an ABI PRISM™ 377XL DNA Sequencer (ABI, Foster City, USA) with universal forward and reverse primers, respectively.
The sequences of the
cytb gene were confirmed by a comparison with the published mitochondrial genome sequence of
T. pisiformis. Multiple alignments were performed in ClustalX 1.83. Aligned sequences (excluding primer sequences) were transformed to FASTA and MEGA files. The number of haplotypes, calculation of haplotype diversity (Hd), nucleotide diversity (π), gene flow (Nm), and neutrality tests (including Tajima's D, and Fu and Li's test) were performed using DnaSP 4.10.9 [
12]. To obtain the conserved and variable sites, global
FST value and AMOVA were analyzed using Arlequin v3.11 [
13]. Network 4.0 software [
14] was used to analyze the MJ-network of haplotypes. The phylogenies were reconstructed using the neighbor-joining (NJ) method in MEGA 4.0 [
15]. Parameters for tree construction included the Kimura-2-parameter index and 1,000 bootstrap resampling.
RESULTS
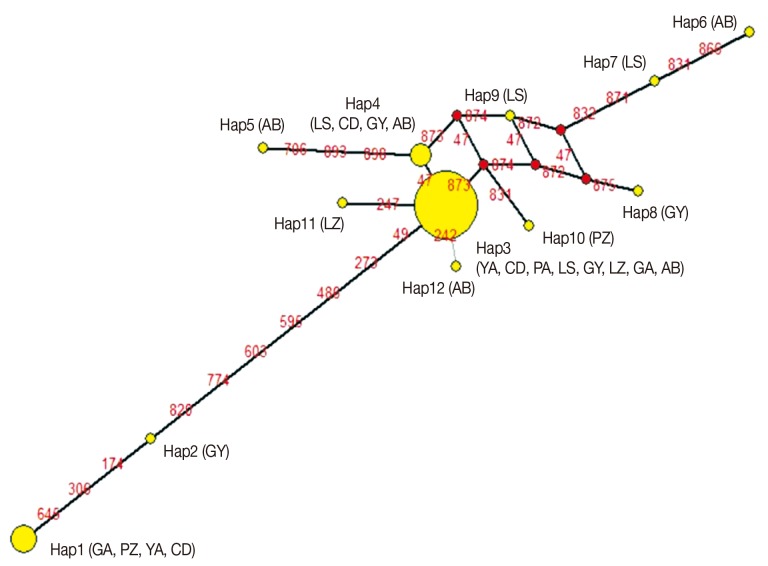
The partial sequence size of the cytb gene was 922 bp, which occupied 86.3% (922/1,068) of the whole length. The sequences of 53 isolates were submitted to GenBank under the accession numbers JN870153-JN870178 and JX535256-JX535282. The average base composition of cytb was 44.9% (T), 26.3% (A), 19.9% (G), and 8.9% (C), with AT-richness in the sequences. No insertions, deletions, or stop codons were detected. Twenty-four variable sites, including 17 parsimony informative sites and 7 singleton sites, were found in cytb. These included 14 transitions (12 A-G and 2 T-C), and 10 transversions (3 A-T, 2 A-C, 1 G-C, and 4 T-G). Twelve haplotypes were found in 53 isolates, giving an Hd value of 0.578 and a π value of 0.00371. As observed in this study, Hap3 was the most common haplotype (found in 35 isolates, 66.0%), followed by Hap1 (YA2, GA1, GA2, CD1, and CD2) and Hap4 (AB2, LS2, CD3, and GY3). Nine haplotypes were found only in a single isolate, including GY4 (Hap2), AB3 (Hap5), AB1 (Hap6), LS3 (Hap7), GY2 (Hap8), LS1 (Hap9), PZ3 (Hap10), LZ7 (Hap11), and AB6 (Hap12).
The AMOVA showed that 98.4% genetic variation was derived from intra-region, and 1.6% from inter-region. The global Nm and
FST of genetic differentiation across 53 samples were 1.46 and 0.0157, respectively. Analysis of the haplotype MJ-network showed that Hap3 was the most prominent haplotype, and the other haplotypes centered on it (
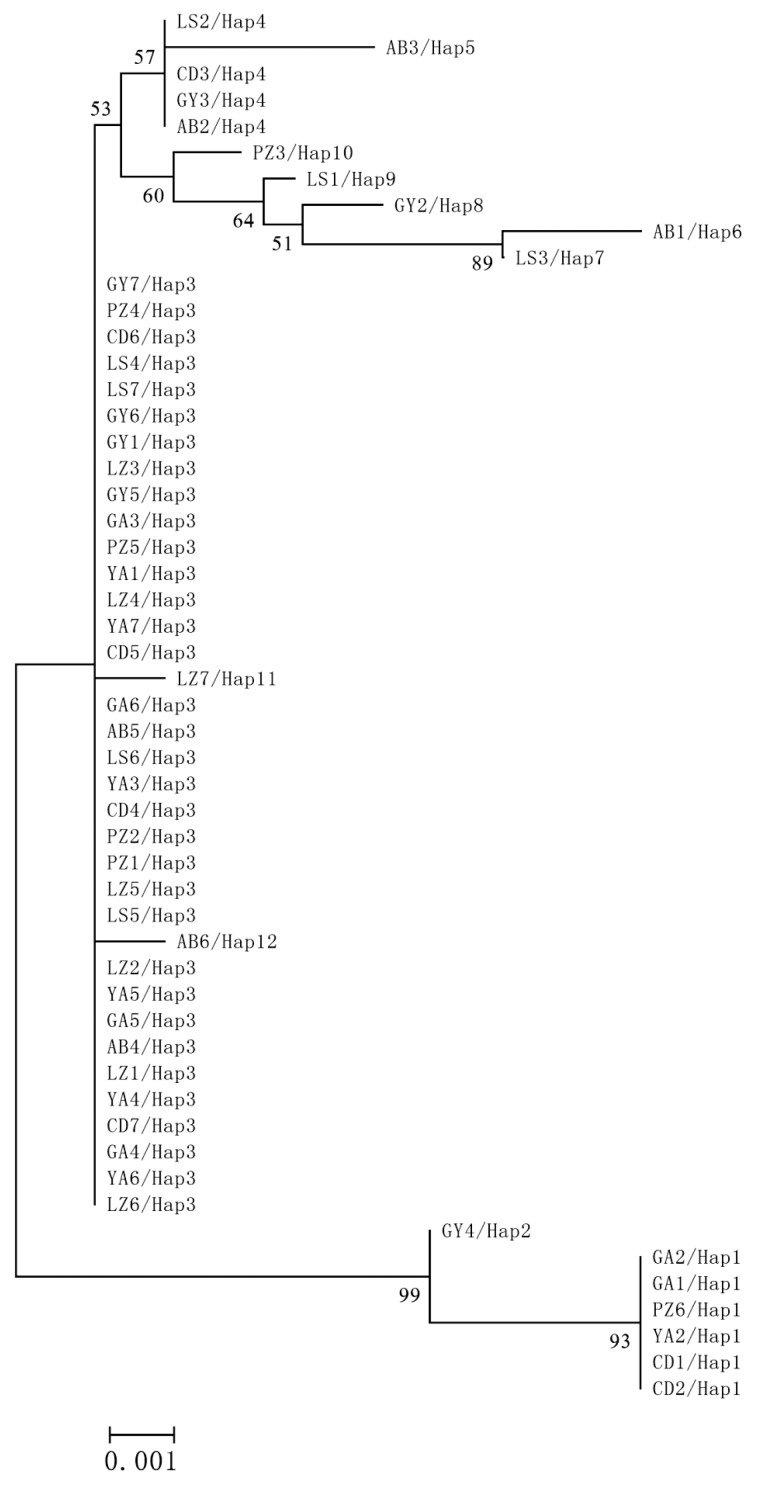
Fig. 2). No geographical clustering was observed from the NJ tree analysis (
Fig. 3). However, haplotype clustering was significant in the phylogenetic trees. The first cluster was Hap1 and Hap2, and the second was Hap3 to Hap12. The neutrality test showed that Tajima's D=-1.13902 (
P>0.1), Fu and Li's D
*=-0.52626 (
P>0.1), Fu and Li's F
*=-0.88276 (
P>0.1) were not significantly different.
DISCUSSION
In our study, the AT-richness of
cytb exceeded 70%, which was similar to observations by Jia et al. [
16] and Liu et al. [
17]. They suggested an AT-bias in the mitochondrial genomes of
T. taeniaeformis,
T. multiceps,
T. hydatigena, and
T. pisiformis. The π value is an important index to measure the level of genetic diversity. In most animals, a π value of >0.01 is considered to indicate a comparatively large variation [
18]. In this study, π value was lower than 0.01, suggesting that there was low genetic variation of 53 isolates from 8 regions. According to the different allele frequency, the genetic differentiation index,
FST, is often used to evaluate the proportion of genetic diversity [
19]. Nm>1 is considered enough to resist the function of genetic drift, and prevent the occurrence of genetic differentiation [
20]. Nm value (1.46) and
FST (0.01573) of global isolates demonstrated low levels of genetic diversity. The 53 geographcial isolates may belong to a larger population in Sichuan Province. There was no evidence demonstrating a geographical clustering from the haplotype MJ-network and phylogeny tree. Phylogenetic analyses revealed that there was no correlation between phylogeny and geographical distribution. In our study, the values of Tajima's D, Fu and Li's D, and Fu and Li's F scores proved that the evolution of
T. pisiformis followed a neutral mode.
Barbosa et al. [
21] used the wild rabbit (
Oryctolagus cuniculus) and a parasitic tapeworm (
T. pisiformis) as an example to study phylogeographic triangulation, and argued that the phylogeography of a parasite that needs 2 hosts to complete its life cycle should reflect population history traits of both hosts. Many macroparasites do not actively disperse their populations but rely on the movement of their intermediate or final hosts [
22,
23]. Following the increasingly frequent trade of canines and rabbits over long distances, the exchange of genetic material appears more likely, which may cause the low genetic variation of
T. pisiformis in Sichuan Province, China.
In conclusion, our results showed that low genetic variations were present in 53 isolates of T. pisiformis from Sichuan, China, which provided genetic evidence for future development of prevention and control measures. However, the relationship between T. pisiformis pathogenicity, drug resistance, and genetic structure requires further research to clarify the role of each.
ACKNOWLEDGMENT
This study was supported by a grant from the Program for Changjiang Scholars and Innovative Research Team in University, China (PCSIRT) (No. IRT0848).
References
- 1. Bagrade G, Kirjusina M, Vismanis K, Ozoliņs J. Helminth parasites of the wolf Canis lupus from Latvia. J Helminthol 2009;83:63-68.
- 2. Lahmar S, Sarciron ME, Rouiss M, Mensi M. Echinococcus granulosus and other intestinal helminths in semi-stray dogs in Tunisia: infection and re-infection rates. Tunis Med 2008;86:657-664.
- 3. Chen YF, Xie XP, Sun SP. The existing state and development strategy of rabbits production in China. Modern Agricult 2010;6-7.
- 4. Zhou YX, Du AF, Zhang XJ, Wu YM, Tong FY, Wu GY. Research of harmfulness of Cysticercus pisiformis in rabbit. J Zhejiang Agricult Sci 2008;3:372-373.
- 5. Saarma U, Jogisalu I, Moks E, Varcasia A, Lavikainen A, Oksanen A, Simsek S, Andresiuk V, Denegri G, Gonzalez LM, Ferrer E, Garate T, Rinaldi L, Maravilla P. A novel phylogeny for the genus Echinococcus, based on nuclear data, challenges relationships based on mitochondrial evidence. Parasitology 2009;136:317-328.
- 6. Gasser RB, Zhu X, McManus DP. NADH dehydrogenase subunit 1 and cytochrome c oxidase subunit I sequences compared for members of the genus Taenia (Cestoda). Int J Parasitol 1999;29:1965-1970.
- 7. Campbell G, Garcia HH, Nakao M, Ito A, Craig P. Genetic variation in Taenia solium. Parasitol Int 2006;55:S121-S126.
- 8. Nakao M, Xiao N, Okamoto M, Yanagida T, Sako Y, Ito A. Geographic pattern of genetic variation in the fox tapeworm Echinococcus multilocularis. Parasitol Int 2009;58:384-389.
- 9. Nakao M, Okamoto M, Sako Y, Yamasaki H, Nakaya K, Ito A. A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology 2002;124:657-662.
- 10. Martinez-Hernandez F, Jimenez-Gonzalez DE, Chenillo P, Alonso-Fernandez C, Maravilla P, Flisser A. Geographical widespread of two lineages of Taenia solium due to human migrations: can population genetic analysis strengthen this hypothesis? Infect Genet Evol 2009;9:1108-1114.
- 11. Sambrook J, Fristsch EE, Maniatis T. Molecular Cloning. A Laboratory Manual. 3 rd ed. New York, USA. Cold Spring Harbor Labtetory Press; 2002, pp 463-470.
- 12. Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 2003;19:2496-2497.
- 13. Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 2007;1:47-50.
- 14. Bandelt HJ, Forster P, Röhl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol 1999;16:37-48.
- 15. Tamura k, Dudley J, Nei M, Kumar S. MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 2007;24:1596-1599.
- 16. Jia WZ, Yan HB, Guo AJ, Zhu XQ, Wang YC, Shi WG, Chen HT, Zhan F, Zhang SH, Fu BQ, Littlewood DT, Cai XP. Complete mitochondrial genomes of Taenia multiceps, T. hydatigena and T. pisiformis: additional molecular markers for a tapeworm genus of human and animal health significance. BMC Genomics 2010;11:447.
- 17. Liu GH, Lin RQ, Li MW, Liu W, Liu Y, Yuan ZG, Song HQ, Zhao GH, Zhang KX, Zhu XQ. The complete mitochondrial genomes of three cestode species of Taenia infecting animals and humans. Mol Biol Rep 2011;38:2249-2256.
- 18. Neigel JE, Avise JC. Application of a random walk model to geographic distribution of animal mitochondrial DNA variation. Genetics 1993;135:1209-1220.
- 19. Holsinger KE, Weir BS. Genetics in geographically structured populations: defining, estimating and interpreting F (ST). Nat Rev Genet 2009;10:639-650.
- 20. Hamrick JL, Godt MJW, Sherman-Boyles SL. Gene flow among plant populations: evidence from genetic markers. In Hoch PC, Stephnon AG eds, Experimental and Molecular Approaches to Plant Biosystematics. Missouri, USA. Missouri Botanical Garden; 1995, pp 215-232.
- 21. Barbosa AM, Thode G, Real R, Feliu C, Vargas JM. Phylogeographic triangulation: using predator-prey-parasite interactions to infer population history from partial genetic information. PLoS One 2012;7:e50877.
- 22. Jefferies R, Shaw SE, Willesen J, Viney ME, Morgan ER. Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palaearctic and Nearctic ecozones. Infect Genet Evol 2010;10:561-568.
- 23. Baldwin RE, Rew MB, Johansson ML, Banks MA, Jocobson KC. Population structure of three species of Anisakis nematodes recovered from pacific sardines (Sardinops sagax) distributed throughout the California current system. J Parasitol 2011;97:545-554.
Fig. 1Eight collection sites of Taenia pisiformis in Sichuan Province, China. The number in curly brackets is the amount of collected worms from different regions.

Fig. 2Haplotype network for 8 regions of Taenia pisiformis. Circles and red numbers represent the frequency of haplotype distributions and the mutational site, respectively. The geographical distribution of each haplotype was presented in curly brackets (Ya'an, YA; Chengdu, CD; Panzhihua, PZ; Leshan, LS; Guangyuan, GY; Luzhou, LZ; Guang'an, GA; Aba, AB).

Fig. 3Neighbor-joining phylogenetic tree constructed by the Kimura-2-parameter index and 1,000 bootstrap resampling using 53 cytb sequences (922 bp) of T. pisiformis.