Abstract
Morphological characteristics of Mesocestoides lineatus tetrathyridia collected from Chinese snakes and their adults recovered from experimental animals were studied. The tetrathyridia were detected mainly in the mesentery of 2 snake species, Agkistrodon saxatilis (25%) and Elaphe schrenckii (20%). They were 1.73 by 1.02 mm in average size and had an invaginated scolex with 4 suckers. Adult tapeworms were recovered from 2 hamsters and 1 dog, which were orally infected with 5-10 larvae each. Adults from hamsters were about 32 cm long and those from a dog were about 58 cm long. The scolex was 0.56 mm in average width with 4 suckers of 0.17 by 0.15 mm in average size. Mature proglottids measured 0.29 by 0.91 mm (av.). Ovaries and vitellaria bilobed and located in the posterior portion of proglottids. The cirrus sac was oval-shaped and located median. Testes were follicular, distributed in both lateral fields of proglottids, and 41-52 in number per proglottid. Gravid proglottids were 1.84 by 1.39 mm (av.) with a characteristic paruterine organ. Eggs were 35 by 27 µm in average size with a hexacanth embryo. These morphological characteristics of adult worms were identical with those of M. lineatus reported previously. Therefore, it has been confirmed that the tetrathyridia detected in 2 species of Chinese snakes are the metacestodes of M. lineatus, and 2 snake species, A. saxatilis and E. schrenckii, play the role of intermediate hosts.
-
Key words: Mesocestoides lineatus, tetrathyridium, Chinese snakes, Agkistrodon saxatilis, Elaphe schrenckii
INTRODUCTION
Mesocestoides spp. are members of the tapeworm order Cyclophyllidea. However, they have some different characters from other groups of cyclophyllideans. In their life cycle, 3 hosts including 2 intermediate and 1 definitive hosts, are required. Adult worms live in the small intestines of carnivorous mammals, i.e., the dog, cat, fox, wolf, coyote, jackal, raccoon, badger, lynx, and some species of wild felines [
1-
11], and rarely birds and humans [
12-
17]. The tetrathyridium, the metacestode of
Mesocestoides spp., is found in the abdominal cavity of a great variety of intermediate hosts, such as amphibia, reptiles, birds, and mammals [
18,
19]. Meanwhile, the role of coprophagic arthropods as its first intermediate host is presumed as Padgett and Boyce [
20] detected
Mesocestoides sp. DNA from ants. There were experimental trials to infect insects with eggs; however, enough evidence has not yet been obtained [
21]. Morphologically, the median ventral position of the genital atrium and bipartite vitelline gland in these tapeworms are unique and differ from other groups of cyclophyllideans [
22].
In the Republic of Korea (=Korea), Kobayashi [
23] reported adult
Mesocestoides infection from dogs in Seoul in 1928. Thereafter, Cho et al. [
24] in 1982 detected tetrathyridia in a snake,
Elaphe rufodorsata, from Gangwon-do [
24]. Two cases of human infections with adult
Mesocestoides lineatus were reported by Choi et al. [
25] and Eom et al. [
16]. However, there have been few studies on the life cycle of
Mesocestoides tapeworms in Korea. Moreover, there is no information on biological relationships between the tetrathyridia in intermediate hosts and the adults in definitive hosts. In the present study, we succesfully obtained adult tapeworms of
M. lineatus from 2 hamsters and a dog which were experimentally infected with tetrathyridia collected from Chinese snakes. The morphological characteristics of the tetrathyridia and adult tapeworms are described herein.
MATERIALS AND METHODS
A substantial number of snakes obtained from the Incheon customs officer were transferred to the Parasitology Laboratory of Gyeongsang National University School of Medicine in November, 2003. Among them, 421 snakes which included
Agkistrodon saxatilis (n=60),
Elaphe schrenckii (n=80),
Dinodon rufozonatum (n=120),
Agkistrodon brevicaudus (n=111), and
Elaphe davidi (n=50) were examined to detect parasitic helminths including tetrathyridial larvae (
Table 1). The muscle and viscera of each snake were isolated after skinned off and artificially digested with pepsin-HCl solution in an incubator at 36℃ for 2-5 hr. The digested material was washed with 0.85% saline until the supernatant became clear, and the sediments were examined under a stereomicroscope. Some collected tetrathyridia were fixed in 10% formalin under a cover glass pressure, and 5-10 larvae per animal were orally infected to 3 rats, 5 hamsters, 2 cats, and 2 dogs to obtain the adult
Mesocestoides tapeworm. About 1 month later, experimental animals were killed after anesthesia, and their intestines were isolated and longitudinally opened with a pair of scissors in a beaker with 0.85% saline. The intestinal contents of each animal were washed with 0.85% saline until the supernatant cleared, and the sediments were examined with naked eyes and under a stereomicroscope. The adult tapeworms were fixed in 10% formalin under a slide glass pressure. The tetrathyridia and adults fixed with 10% formalin were stained with Semichon's acetocarmine and observed using a light microscope with a micrometer.
In order to observe the surface ultrastructure of the tetrathyridium, some worms were washed several times in 0.2 M cacodylate buffer (pH 7.2) and fixed in 2.5% glutaraldehyde at 4℃. After washing 3 times with the same buffer, they were dehydrated through a graded alcohol series (50%, 70%, 80%, 90%, 95%, and absolute alcohol), dried in a critical point dryer, coated with gold in the JFC-1100E ion sputtering device (JEOL, Tokyo, Japan), and observed using a scanning electron microscope (Philips XL-30S, Amsterdam, Netherlands) with an accelerating voltage of 20 kV.
RESULTS
Infection status of Chinese snakes with tetrathyridia and adult worm recovery
Tetrathyridial larvae were collected from 15 (25%)
A. saxatilis, 12 (30%)
E. schrenckii (yellow), and 4 (10%)
E. schrenckii (black/white). They were mainly detected in the mesentery of snakes, and their infection status was as shown in
Table 2. No tetrathyridia were found in
D. rufozonatum,
A. brevicaudus, and
E. davidi. Adult tapeworms were recovered from 2 (40%) hamsters and 1 (50%) dog. No worms were detected from 3 rats, 3 hamsters, 2 cats and 1 dog, which were experimentally infected with tetrathyridia.
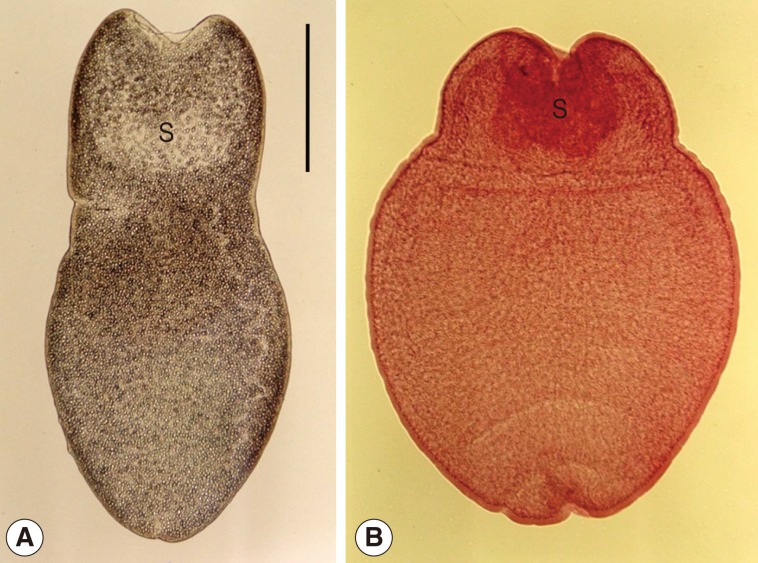
Tetrathyridia of
M. lineatus were oval or elongated with a somewhat pointed posterior end, slightly constricted at the anterior portion, longer than wide, 1,000-3,750 (1,730 in average) by 680-2,050 (1,020 in average) µm in size. They had an invaginated scolex with 4 suckers in the anterior constricted portion (
Fig. 1).
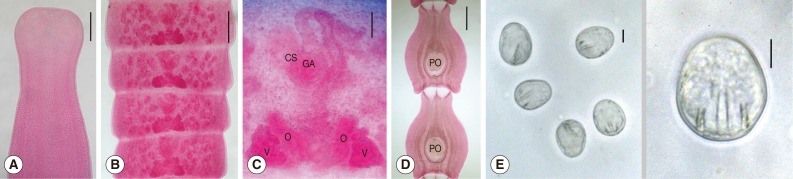
Adult worms from hamsters were about 32 cm (n=3) and from a dog was 58 cm (n=1) in length respectively. Scolices were transversely oval or slightly quadrigonal in shape and 480-680 (560) µm in width. They had 4 cup-like suckers of 160-180 (173) by 135-165 (150) µm size, without a rostellum (
Fig. 2A). Immature proglottids were wider than long and had no genital organs. Mature proglottids were also wider than long and 260-320 (290) by 730-1,210 (910) µm in size. Ovaries and vitellaria were bilobed and located in the posterior portion of the mature proglottid, and were 93-108 (102) by 45-75 (60) and 70-88 (79) by 60-70 (64) µm in size, respectively. The cirrus sac was oval-shaped, 75-95 (86) by 55-70 (61) µm in size and located median. Testes were follicular, 55-75 (61) by 38-50 (42) µm in size and distributed in both lateral fields of proglottids, and 41-52 in number per proglottid (
Fig. 2B, C). Gravid proglottids were longer than wide, 1,550-2,400 (1,840) by 1,280-1,700 (1,390) µm in size, and had a characteristic paruterine organ of 530-600 (578) by 440-550 (481) µm in size (
Fig. 2D). Eggs were elliptical with thin shell and a hexacanth embryo, and 33-36 (35) by 25-28 (27) µm in size (
Fig. 2E).
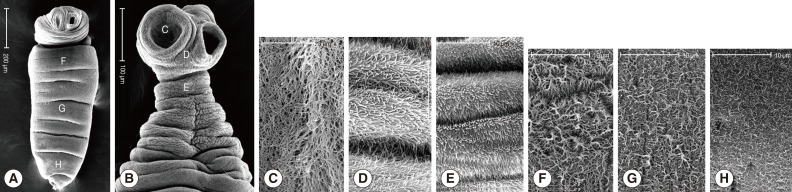
Tetrathyridia were elongated with a somewhat pointed posterior end and had an invaginated scolex at the anterior constricted portion. The whole body surface was covered with numerous microtriches (
Fig. 3A). The evaginated scolex with 4 cup-like suckers and the neck portion were also covered with microtriches (
Fig. 3B). Microtriches on the tegument were somewhat morphologically different according to the body locations. Long filamentous micritriches were distributed at the inner portion of suckers (
Fig. 3C). Numerous hair-like microtriches were compactly distributed between suckers in the scolex (
Fig. 3D). More or less stouter microtriches were seen on the tegument just below the scolex and neck portion (
Fig. 3E). On the surface of the posterior body, except for the scolex and neck, somewhat short microtriches were distributed and their length and density were decreased posteriorly (
Fig. 3F-H).
DISCUSSION
In the present study, tetrathyridia of
M. lineatus were detected in 2 species of snakes,
A. saxatilis and
E. schrenckii, from China. No tetrathyridia were found in the other 3 snake species,
D. rufozonatum,
A. brevicaudus, and
E. davidi. However, these 3 snake species have been reported as the intermediate hosts for
Gnathostoma hispidum [
26,
27]. Generally, foodborne parasite infections are related to the food-chain in the natural ecosystem. Snakes, as the predator or a transport host, are infected with gnathostome larvae or tetrathyridia by preying upon the other intermediate or transport hosts, such as amphibians, reptiles, avians, and small mammals. How are the real intermediate hosts infected with the tetrathyridia of
Mesocestoides spp. then? It is difficult to answer because of the link between the eggs and tetrathyridia (the first intermediate host necessary?) is obscure in the life cycle of
Mesocestoides spp. However, tetrathyridia have been detected in various kinds of animals, i.e., 22 reptilian spp., 15 avian spp., and 20 mammalian spp. [
18,
19]. Therefore, snakes as an upper-level predator may be easily infected by feeding their prey, i.e., reptiles, birds, and small mammals. As the snake intermediate hosts,
Agkistrodon halys and
E. rufodorsata were reported in Japan and Korea [
24,
28].
So far, about 30 human infections with
Mesocestoides spp. adults have been documented in Japan, China, Korea, USA, Ruanda-Urundi, and Greenland [
14-
17]. Human infections are acquired by ingesting raw wild animals containing tetrathyridia. Several species of snakes, such as
A. brevicaudus,
A. halys,
Elaphe quadrivirgata and
E. rufodorsata, and chickens were reported as the source of human infections in Japan and Korea [
14,
16,
24,
25,
28].
Adult
Mesocestoides tapeworms have been found in surveys of carnivorous mammals, such as, dogs, cats, foxes, wolves, coyotes, jackals, raccoons, badgers, lynxes, and some species of wild felines [
1-
11]. Among the carnivorous mammals, foxes were the most frequently examined at various localities of the world and their infection rate with
Mesocestoides spp. was relatively high [
9,
29-
31]. Recently, Hrčkova et al. [
9] detected
Mesocestoides tapeworms from 41.9% out of 3,157 red foxes in central Europe. Magi et al. [
29] found
M. lineatus in 45.4% of 129 red fox from central Italy. Saeed et al. [
30] also detected
Mesocestoides sp. tapeworms from 35.6% of 1,040 red foxes in Denmark. In these endemic areas, foxes play a crucial role as the definitive host in maintaining the life cycle. What kinds of wild animals are the natural definitive hosts for
M. lineatus in Korea? Adult
Mesocestoides tapeworms were found in dogs and 2 human cases in Korea [
16,
23,
25]; however, no reports are available on wild carnivorous mammals. Hence, keen observations are needed for detection of
Mesocestoides tapeworms in surveys of wild carnivorous mammals in Korea.
To obtain adult worms, we orally infected 5-10 tetrathyridial larvae to 3 rats, 5 hamsters, 2 cats and 2 dogs. However, no worms were recovered in 3 rats, 3 hamsters, 2 cats, and 1 dog, and the fate of uninfected larvae is uncertain. Most of them may have died and passed out of the digestive tract with feces. However, some survived larvae might have migrated into the other organs and provoked the tetrathyridiasis like the canine peritoneal larval cestodosis (CPLC) [
19]. Unfortunately, however, we examined only the small intestine of experimental animals to detect adult worms. It is a new finding in this study that hamsters are an experimental definitive host for
M. lineatus.
Mesocestoides sp. tapeworms have morphological characteristics, such as the median ventral position of the genital atrium and bipartite vitelline gland in the mature proglottid and a paruterine organ in the gravid proglottid, which differ from those of other cyclophyllidean tapeworms. Especially, the paruterine organ in gravid proglottids is unique and regarded as a diagnostic key in the subfamily Mesocestoidinae [
22]. On the other hand, morphological characteristics of adult worms observed in the present study were identical with those of
M. lineatus, which were previously reported by Kamegai et al. in 1967 [
32] and Eom et al. in 1992 [
16] (
Table 3). Morphological characteristics of the metacestode and adult of
M. lineatus observed in the present study will be helpful in taxonomic studies of
Mesocestoides tapeworms.
Ministry of Health and WelfareNIH 348-6111-215
Notes
-
We have no conflict of interest related with this study.
ACKNOWLEDGMENTS
This work was financially supported by Anti-Communicable Disease Control Program of the National Institute of Health (NIH 348-6111-215), National Research and Development Program, Ministry of Health and Welfare, the Republic of Korea. We thank staffs of Incheon Customs Officer for their help to provide the snakes distrained. We also thank Jung-A Kim and Soo-Jung Park, Department of Parasitology, Gyeongsang National University School of Medicine, Jinju, Korea, for her help in recovery of worms from snakes and experimental animals.
References
- 1. Kapel CM, Nansen P. Gastrointestinal helminths of Arctic foxes (Alopex lagopus) from different bioclimatological regions in Greenland. J Parasitol 1996;82:17-24.
- 2. Torres J, Garciá-Perea R, Gisbert J, Feliu C. Helminth fauna of the Iberian lynx, Lynx pardinus. J Helminthol 1998;72:221-226.
- 3. Torres J, Miquel J, Motjé M. Helminth parasites of the eurasian badger (Meles meles L.) in Spain: a biogeographic approach. Parasitol Res 2001;87:259-263.
- 4. Dalimi A, Sattari A, Motamedi G. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet Parasitol 2006;142:129-133.
- 5. Wang CR, Qiu JH, Zhao JP, Xu LM, Yu WC, Zhu XQ. Prevalence of helminthes in adult dogs in Heilongjiang Province, the People's Republic of China. Parasitol Res 2006;99:627-630.
- 6. Sato H, Suzuki K. Gastrointestinal helminths of feral raccoons (Procyon lotor) in Wakayama Prefecture, Japan. J Vet Med Sci 2006;68:311-318.
- 7. Bridger KE, Baggs EM, Finney-Crawley J. Endoparasites of the coyote (Canis latrans), a recent migrant to insular newfoundland. J Wildl Dis 2009;45:1221-1226.
- 8. Mohsen A, Hossein H. Gastrointestinal parasites of stray cats in Kashan, Iran. Trop Biomed 2009;26:16-22.
- 9. Hrčkova G, Miterpáková M, O'Connor A, Šnábel V, Olson PD. Molecular and morphological circumscription of Mesocestoides tapeworms from red foxes (Vulpes vulpes) in central Europe. Parasitology 2011;138:638-647.
- 10. Gallas M, Silveira EF. Mesocestoides sp. (Eucestoda, Mesocestoididae) parasitizing four species of wild felines in Southern Brazil. Rev Bras Parasitol Vet 2011;20:168-170.
- 11. Abdybekova AM, Torgerson PR. Frequency distributions of helminths of wolves in Kazakhstan. Vet Parasitol 2012;184:348-351.
- 12. Millán J, Gortazar C, Casanova JC. First occurrence of Mesocestoides sp. in a bird, the red-legged partridge, Alectoris rufa, in Spain. Parasitol Res 2003;90:80-81.
- 13. Sanmartín ML, Alvarez F, Barreiro G, Leiro J. Helminth fauna of Falconiform and Strigiform birds of prey in Galicia, Northwest Spain. Parasitol Res 2004;92:255-263.
- 14. Morisita T, Nagase K, Moriyama K, Matsumoto Y. The 11th case of human infection with Mesocestoides lineatus in Japan. Jpn J Parasitol 1975;24:353-356. (in Japanese).
- 15. Fan SQ. First case of Mesocestoides lineatus infection in China. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 1988;6:310.
- 16. Eom KS, Kim SH, Rim HJ. Second case of human infection with Mesocestoides lineatus in Korea. Korean J Parasitol 1992;30:147-150.
- 17. Fuentes MV, Galán-Puchades MT, Malone JB. Short report: a new case report of human Mesocestoides infection in the United States. Am J Trop Med Hyg 2003;68:566-567.
- 18. Witenberg G. Studies on the cestode genus Mesocestoides. Arch Zool Ital 1934;20:467-509.
- 19. Wirtherle N, Wiemann A, Ottenjann M, Linzmann H, van der Grinten E, Kohn B, Gruber AD, Clausen PH. First case of canine peritoneal larval cestodosis caused by Mesocestoides lineatus in Germany. Parasitol Int 2007;56:317-320.
- 20. Padgett KA, Boyce WM. Ants as first intermediate hosts of Mesocestoides on San Miguel Island, USA. J Helminthol 2005;79:67-73.
- 21. Loos-Frank B. One or two intermediate hosts in the life cycle of Mesocestoides (Cyclophyllidea: Mesocestoididae)? Parasitol Res 1991;77:726-728.
- 22. Rausch RL. Family Mesocestoididae Fuhrmann, 1907. In Khalil LF, Jones A, Bray RA eds, Keys to the Cestode Parasites of Vertebrates. Wallingford, UK. CAB International; 1994, pp 309-314.
- 23. Kobayashi H. On the animal parasites in Chosen (Korea). Second report. Acta Med Keijo 1928;11:109-124.
- 24. Cho SY, Song KW, Lee SH. Cestode parasites of terrestrial snakes in Korea. Chung-Ang J Med 1982;7:321-332. (in Korean).
- 25. Choi WY, Kim BC, Choi HS. The first case of human infection with tapeworms of the genus Mesocestoides in Korea. Korean J Parasitol 1967;5:60-64. (in Korean).
- 26. Sohn WM, Lee SH. The first discovery of larval Gnathostoma hispidum (Nematoda: Gnathostomatidae) from a snake host, Agkistrodon brevicaudus. Korean J Parasitol 1998;36:81-89.
- 27. Cho SH, Kim DS, Kong Y, Na BK, Sohn WM. Larval Gnathostoma hispidum detected in the red banded odd-tooth snake, Dinodon rufozonatum rufozonatum, from China. Korean J Parasitol 2007;45:191-198.
- 28. Kumada N, Mizuno S, Kato Y, Mizuno T, Oya H, Suzuki T, Hattori T. Eighth record of a human case of Mesocestoides lineatus (Cestoda: Cyclophyllidea) in Japan. Jpn J Parasitol 1972;21:336-345. (in Japanese).
- 29. Magi M, Macchioni F, Dell'omodarme M, Prati MC, Calderini P, Gabrielli S, Iori A, Cancrini G. Endoparasites of red fox (Vulpes vulpes) in central Italy. J Wildl Dis 2009;45:881-885.
- 30. Saeed I, Maddox-Hyttel C, Monrad J, Kapel CM. Helminths of red foxes (Vulpes vulpes) in Denmark. Vet Parasitol 2006;139:168-179.
- 31. Dalimi A, Sattari A, Motamedi G. A study on intestinal helminthes of dogs, foxes and jackals in the western part of Iran. Vet Parasitol 2006;142:129-133.
- 32. Kamegai S, Ichihara A, Nonobe H, Machida M. The 6th and 7th records of human infection with Mesocestoides lineatus (Cestoda) in Japan. Res Bull Meguro Parasitol Mus 1967;1:1-7.
Fig. 1Tetrathyridia (A: fresh worm; B: Semichon's acetocarmine-stained one) collected in the mesentery of a viper snake, Agkistrodon saxatilis, from China. They are slightly constricted in the anterior portion, oval or elongated in shape with a somewhat pointed posterior end, and have an invaginated scolex (S) with 4 suckers at the anterior constricted portion. Scale bar is 500 µm.
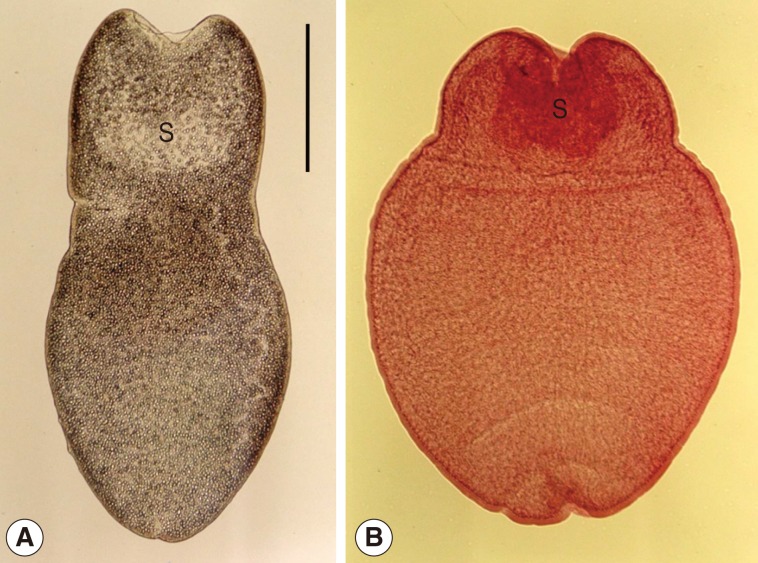
Fig. 2Adult Mesocestoides lineatus recovered in the small intestine of a dog experimentally infected with tetrathyridia. (A) Scolex with 4 cup-like suckers. Scale bar=250 µm. (B) Four mature proglottids. Scale bar=200 µm. (C) Magnified view of a mature proglottid with bilobed ovaries (O) and vitellaria (V) in the posterior portion, oval-shaped cirrus sac (CS) and genital atrium (GA) in the median portion, and follicular testes in both lateral fields of proglottids. Scale bar=50 µm. (D) Gravid proglottids with a characteristic paruterine organ (PO). Scale bar=500 µm). E. Eggs with a hexacanth embryo. Scale bar=10 µm.
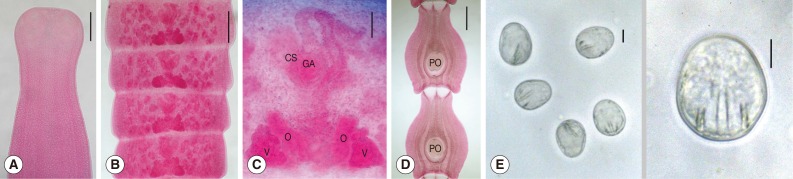
Fig. 3SEM findings of tetrathyridia collected in the mesentery of a viper snake, A. saxatilis. (A) A whole worm elongated in shape with a somewhat pointed posterior end has an invaginated scolex at the anterior constricted portion. The whole body surface is covered with numerous microtriches, of which length and density are different according to the body level; anterior (F), middle (G), and posterior (H) portions of the posterior body. (B) Evaginated scolex with 4 cup-like suckers and neck portion covered with microtriches, of which shapes are different according to the body locations; the inner portion of a sucker (C), between suckers (D), and neck portion (E). (C) Tegument of the inner portion of a sucker (C portion in Fig. 3B) showing numerous long filamentous microtriches. (D) Tegument between suckers (D in Fig. 3B) showing numerous hair-like microtriches. (E) Tegument just below the scolex and neck portion (E in Fig. 3B) showing numerous stouter microtriches. (F) Tegument at the anterior portion of the posterior body (F in Fig. 3A). (G) Tegument on the middle portion of the posterior body (G in Fig. 3A). (H) Tegument in the posterior portion of the posterior body (H in Fig. 3A) showing somewhat short microtriches, of which length and density are decreasing posteriorly.

Table 1.Snakes examined in this study
Table 1.
|
Species of snake |
No. of snakes examined |
Length (cm)
|
Weight (g)
|
|
Range (average) |
Range (average) |
|
Agkistrodon saxatilis
|
60 |
62-82 (72.4) |
93-273 (152.0) |
|
Agkistrodon brevicaudus
|
111 |
44-65 (55.2) |
26-94 (59.6) |
|
Dinodon rufozonatum
|
120 |
84-105 (93.8) |
73-253 (143.0) |
|
Elaphe davidi
|
50 |
65-87 (79.6) |
58-90 (74.5) |
|
Elaphe schrenckii (Y)a
|
40 |
148-195 (155.8) |
688-1,354 (824.0) |
|
Elaphe schrenckii (B/W)b
|
40 |
122-161 (138.8) |
237-633 (436.9) |
Table 2.Infection status of Chinese snakes with tetrathyridia of M. lineatus
Table 2.
|
Species of snake |
No. of snakes examined |
No. (%) of snakes infected |
No. of larvae detected
|
|
Total |
Range |
Average |
|
Agkistrodon saxatilis
|
60 |
15 (25.0) |
547 |
1-92 |
36.5 |
|
Elaphe schrenckii (Y) |
40 |
12 (30.0) |
309 |
1-87 |
25.8 |
|
Elaphe schrenckii (B/W) |
40 |
4 (10.0) |
7 |
1-3 |
1.8 |
Table 3.Comparison of the current Mesocestoides lineatus with previous reports
Table 3.
|
Item (unit) |
Present study (2013) |
Kamegai et al. (1967) |
Eom et al. (1992) |
|
Scolex width (mm) |
0.48-0.68 |
0.56 |
- |
|
Cirrus sac (μm) |
75-95 × 55-70 |
162-253 × 116-209 |
120-170 × 70-100 |
|
Testes number |
41-52 |
41-60 |
42-54 |
|
Testis (μm) |
55-75 × 38-50 |
38 |
35-89 × 30-68 |
|
Paruterine organ (μm) |
530-600 × 440-550 |
465-600 × 468-735 |
450-590 × 410-550 |
|
Egg (μm) |
33-36 × 25-28 |
31-34 × 24-29 |
28-32 × 20-24 |
|
Host |
dog, hamster |
dog, cat, fox |
human |