Abstract
To examine the infection status of freshwater fish with Gnathostoma spp. larvae in Myanmar, we purchased 15 snakeheads, Channa striatus, from a local market in a suburban area of Naypyidaw, the new capital city. Two larval gnathostomes were collected using an artificial digestion technique, and observed by a light microscope and a scanning electron microscope. The size of an intact larva was 2.65 mm long and 0.32 mm wide. The characteristic morphology of the larvae included the presence of a long esophagus (0.80 mm long), 2 pairs of cervical sacs (0.43 mm long), and a characteristic head bulb with 4 rows of hooklets. The number of hooklets in the 1st, 2nd, 3rd, and 4th row was 45, 48, 50, and 52, respectively. Based on these morphological characters, the larvae were identified as the advanced 3rd-stage larvae of Gnathostoma spinigerum. This is the first report of detection of G. spinigerum 3rd-stage larvae in the central part of Myanmar. Our study suggests that intake of raw meat of snakehead fish in Myanmar may result in human gnathostomiasis.
-
Key words: Gnathostoma spinigerum, advanced 3rd-stage larva, snakehead, Myanmar
Several species of the genus
Gnathostoma are clinically important, causing food-borne parasitic zoonoses in humans [
1]. More than 10
Gnathostoma species have been reported in various parts of Southeast Asia, including Japan and Thailand, Europe, Oceania, and Americas [
1]. A human infection with
Gnathostoma spinigerum was first documented in 1836 in Thailand [
1,
2], and then
Gnathostoma hispidum,
Gnathostoma nipponicum, and
Gnathostoma doloresi infections have been added to the species that are able to cause human infections [
3-
7]. The infection is clinically characterized by creeping eruption in subcutaneous tissues due to migrating larvae [
3]. However, the larvae are also known to invade the lungs, eyes, and even the brain, occasionally causing death [
3].
The adult worms of
Gnathostoma are found in the stomach or esophageal wall of definitive hosts including cats and dogs [
1]. When the feces of definitive hosts containing eggs are deposited in freshwater, free-swimming first-stage larvae are liberated and ingested by cyclops in which they molt twice to become early 3rd-stage larvae (L3). They then develop into advanced L3 in the second intermediate hosts, namely freshwater fish and amphibia. They are passed to a wide spectrum of paratenic hosts including reptiles, birds, and mammals including humans. The principal mode of human infection by
Gnathostoma is consumption of raw or undercooked flesh of second intermediate hosts containing 3rd-stage larvae [
4].
Human gnathostomiasis has been known to be prevalent in Thailand and Japan [
1]. However, in Myanmar, a neighboring country of Thailand, only a few reports have been available [
8-
10]. It is of particular note that recently there was an outbreak of human gnathostomiasis due to
G. spinigerum around Yangon area, the former capital of Myanmar, among 60 Korean immigrants who consumed raw freshwater fish [
8]. Human subcutaneous tissue infections possibly by
Gnathostoma malaysiae were also reported in 2 Japanese men who visited Myanmar and had eaten raw freshwater shrimps [
11]. However, studies on the occurrence of larval infections in fish or amphibian hosts are scarce. Thus, the present study was carried out to determine the presence of
Gnathostoma larvae in a species of snakehead fish purchased in a central part of Myanmar.
A total of 15 snakeheads,
Channa striatus (
Fig. 1), 15-20 cm in length, were purchased from a local market in a suburban area of Naypyidaw, the new capital located in the central part of Myanmar. To examine
Gnathostoma larvae, the flesh of snakeheads was ground in a meat grinder, and incubated with an artificial digestive juice containing 0.6% pepsin and 0.08% HCl at 37℃ for 2-3 hr. The larvae were collected from the digested material, washed twice with 0.85% saline, and examined using a stereomicroscope [
8]. In order to identify the species, one partially broken larva with an intact head part was washed 3 times with physiological saline (pH 7.2) and fixed in 2.5% glutaraldehyde at 4℃. After 3 washings with this buffer, the larva was dehydrated in a graded ethanol series (50%, 70%, 80%, 90%, 95%, and absolute alcohol), dried in a critical point dryer, coated with gold, and observed using a scanning electron microscope (SEM) (ISI DS-130C, Akashi Co., Tokyo, Japan) with an accelerating voltage of 10 kV.
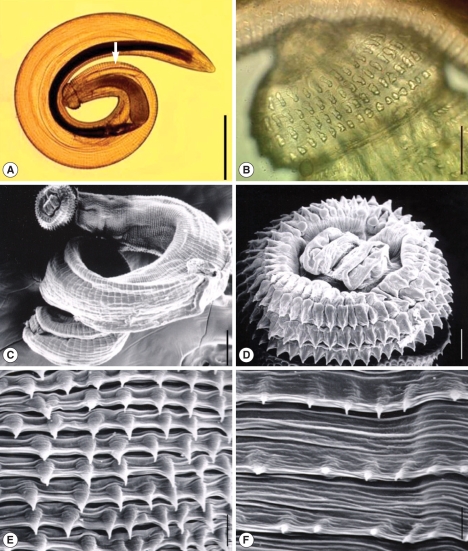
Two larval gnathostomes were harvested from 2 (13.3%) of 15 snakeheads examined (
Table 1). One larva was morphologically intact, and the other was destroyed a little. The body of the intact larva was short, thick, coiled, and 2.65 mm long and 0.32 mm wide (
Fig. 2A). It had a characteristic head bulb with 4 rows of hooklets (
Fig. 2A-D). The number of hooklets in the 1st, 2nd, 3rd, and 4th row was 45, 48, 50, and 52, respectively. A pair of lips was located at the anterior end of the body and 4 ballonets around them (
Fig. 2D). The spines were densely distributed on the body surface of the anterior part and gradually decreased in the size and number toward the middle portion and then increased at the posterior end portion, as revealed by SEM (
Fig. 2C, E, F). The muscular esophagus was large, long (0.80 mm long), and connected to the intestine (
Fig. 2A). Two pairs of cervical sacs (0.43 mm long) were observed around the esophagus (
Fig. 2A).
Different species of larval gnathostomes can be discriminated by the morphology of the head bulb [
1,
2]. The larvae of
G. nipponicum have a head bulb with 3 rows of hooklets, whereas the larvae of
G. spinigerum have 4 rows of hooklets, to which our specimens are compatible. The larvae of
G. hispidum also have 4 rows of hooklets [
12]; however, the number of hooklets on each row is smaller than that in
G. spinigerum. Based on the above morphological characters, we identified our larvae as the advanced L3 of
G. spinigerum.
The second intermediate hosts of
G. spinigerum are known to include various species of freshwater fish and amphibia [
4]. Species of freshwater fish reported in Japan include
Channa argus argus (snakehead),
Channa maculate (blotched snakehead),
Parasilurus asotus (=
Silurus asotus; amur catfish),
Misgurnus anguillicaudatus (loach),
Acanthogobius hasta (goby), and
Cyprinus auratus (=
Carassius auratus; carp) [
1]. Fish hosts reported from Thailand include
Anabas testudineus (climbing perch),
Channa striata (snakehead murrel),
Channa micropeltes (giant snakehead),
Channa lucius,
Clarias batrachus (walking catfish), and
Monopterus albus (swamp eel) [
13]. In the Republic of Korea, 2 third-stage larvae of
G. spinigerum were detected in the abdominal muscle of a snakehead,
Channa argus argus [
14]. The outbreak of human gnathostomiasis among Korean immigrants in Yangon area, Myanmar, was suggested to be due to consumption of raw catfish or snakehead, containing 3rd-stage larvae of
G. spinigerum [
8].
The present study first confirmed the presence of a G. spinigerum life cycle by detecting the advanced L3 in freshwater fish in the central part of Myanmar. Based on our results there is a potential risk of human gnathostomiasis in the central part of Myanmar if improperly cooked snakeheads are consumed.
References
Fig. 1The snakehead fish (15-20 cm long), Channa striatus, purchased from a central part of Myanmar.

Fig. 2An advanced 3rd-stage larva of G. spinigerum recovered from the muscle of a snakehead fish. Light microscopic (LM) (A, B) and scanning electron microscopic (SEM) (C-F) views. (A) LM view of a whole larva showing the head bulb, esophagus, and cervical sacs (arrow) (Bar = 500 µm). (B) The head bulb showing 4 rows of hooklets, 45, 48, 50, and 52, from the anterior to posterior rows (Bar = 100 µm). (C) SEM view of a whole larva (Bar = 50 µm). (D) SEM view of the head bulb showing 4 rows of hooklets and a pair of lips protruded on the anterior end of the body (Bar = 20 µm). (E, F) SEM views of the body surface of the anterior end part (E) and middle part (F) of the larva (Bar = 5 µm).
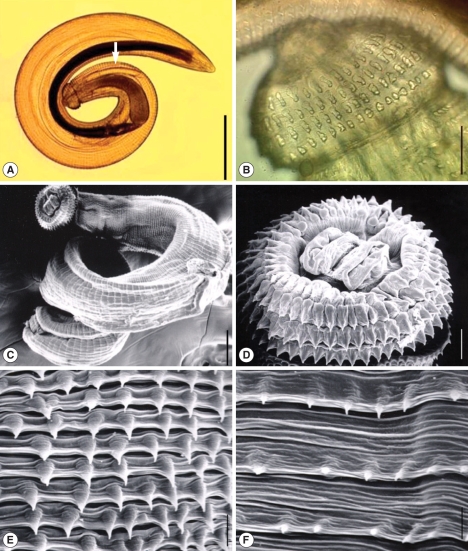
Table 1.Infection status of snakeheads with advanced L3 of Gnathostoma spinigerum
Table 1.
|
Species of snakehead |
No. of fish examined |
No. (%) of fish infected |
No. of larvae detected
|
|
Total |
Average per infected fish |
|
Channa striatus
|
15 |
2 (13.3) |
2 |
1.0 |
Citations
Citations to this article as recorded by

- The occurrence and clinical importance of infectious stage of Echinocephalus (Nematoda: Gnathostomidae) larvae in selected Australian edible fish
Shokoofeh Shamsi, Eleanor Steller, Xiaocheng Zhu
Parasitology International.2021; 83: 102333. CrossRef - Detection of Gnathostoma spinigerum Advanced 3rd-Stage Larvae in the Chinese Edible Frog, Hoplobatrachus rugulosus, from Local Markets in Phnom Penh, Cambodia
Woon-Mok Sohn, Bong-Kwang Jung, Sooji Hong, Seungwan Ryoo, Keon Hoon Lee, Virak Khieu, Jong-Yil Chai
The Korean Journal of Parasitology.2021; 59(5): 519. CrossRef - Larval Gnathostomes and Spargana in Chinese Edible Frogs, Hoplobatrachus rugulosus, from Myanmar: Potential Risk of Human Infection
Jong-Yil Chai, Bong-Kwang Jung, Jin-Youp Ryu, Hyun-Seung Kim, Sung-Jong Hong, Thi Thi Htoon, Htay Htay Tin, Byoung-Kuk Na, Woon-Mok Sohn
The Korean Journal of Parasitology.2020; 58(4): 467. CrossRef - Larval Gnathostomes and Zoonotic Trematode Metacercariae in Fish from a Local Market in Yangon City, Myanmar
Jong-Yil Chai, Bong-Kwang Jung, Keon Hoon Lee, Jin-Youp Ryu, Hyeon-Seung Kim, Sung-Jong Hong, Thi Thi Htoon, Htay Htay Tin, Byoung-Kuk Na, Woon-Mok Sohn
The Korean Journal of Parasitology.2020; 58(6): 701. CrossRef - Molecular identification and genetic diversity of Gnathostoma spinigerum larvae in freshwater fishes in southern Lao PDR, Cambodia, and Myanmar
Patcharaporn Boonroumkaew, Oranuch Sanpool, Rutchanee Rodpai, Lakkhana Sadaow, Chalermchai Somboonpatarakun, Sakhone Laymanivong, Win Pa Pa Aung, Mesa Un, Porntip Laummaunwai, Pewpan M. Intapan, Wanchai Maleewong
Parasitology Research.2019; 118(5): 1465. CrossRef - Gnathostomatidae nematode parasite of Colomesus psittacus (Osteichthyes, Tetraodontiformes) in the Ilha de Marajó, Brazilian Amazon
Raul Henrique da Silva Pinheiro, Ricardo Luís Sousa Santana, Francisco Tiago Vasconcelos Melo, Jeannie Nascimento dos Santos, Elane Guerreiro Giese
Revista Brasileira de Parasitologia Veterinária.2017; 26(3): 340. CrossRef - Larval Gnathostoma spinigerum Detected in Asian Swamp Eels, Monopterus albus, Purchased from a Local Market in Yangon, Myanmar
Jong-Yil Chai, Woon-Mok Sohn, Byoung-Kuk Na, Jong-Bok Park, Hoo-Gn Jeoung, Eui-Hyug Hoang, Thi Thi Htoon, Htay Htay Tin
The Korean Journal of Parasitology.2015; 53(5): 619. CrossRef - Zoonoses in South-East Asia: a regional burden, a global threat
Marion Bordier, François Roger
Animal Health Research Reviews.2013; 14(1): 40. CrossRef - Gnathostoma spinigerum: Immunodepression in experimental infected mice
Wilai Saksirisampant, Sunida Thaisom, Mai Ratanavararak, Benjamas Wongsatayanon Thanomsub
Experimental Parasitology.2012; 132(3): 320. CrossRef