Abstract
To determine alteration of immune responses during visceral larva migrans (VLM) caused by Toxascaris leonina at several time points, we experimentally infected mice with embryonated eggs of T. leonina and measured T-helper (Th) cell-related serial cytokine production after infection. At day 5 post infection (PI), most larvae were detected from the lungs, spleen, intestine, and muscle. Expression of thymic stromal lymphopoietin (TSLP) and CCL11 (eotaxin) showed a significant increase in most infected organs, except the intestine. However, expression of the CXCL1 (Gro-α) gene was most highly enhanced in the intestine at day 14 PI. Th1-related cytokine secretion of splenocytes showed increases at day 28 PI, and the level showed a decrease at day 42 PI. Th2-related cytokine secretion of splenocytes also showed an increase after infection; in particular, IL-5 level showed a significant increase at day 14 PI, and the level showed a decrease at day 28 PI. However, levels of Th17-related cytokines, IL-6 and IL-17A, showed gradual increases until day 42 PI. In conclusion, Th1, Th2, and Th17-related cytokine production might be important in immune responses against T. leonina VLM in experimental mice.
-
Key words: Toxascaris leonina, visceral larva migrans (VLM), Th1, Th2, Th17, cytokine
Most roundworms, which infect only 1 or 2 specific hosts, live in the intestine of their definitive host, and are generally well adapted to the immune system of their host. Although they can sometimes cause mild allergic responses in their hosts during the larval stage (migration period), after growing into the adult worm they are not likely to cause severe pathological changes in their definitive host [
1]. However, when juveniles of several species of nematodes gain entry to an improper host, they begin a typical tissue migration called visceral larva migrans (VLM). They do not complete the normal migration but undergo developmental arrest and begin an extended and random wandering through various organs and tissues of the body [
2].
Most studies on VLM-specific immune responses have reported on
Toxocara canis larva infection. In human toxocariasis, elevation of T-helper type-2 (Th2) cell-related cytokine production has been reported [
3,
4]. In mice, various cytokines were produced during toxocariasis. Those included Th2 cytokines (IL-4 and IL-5), Th1 cytokines (IFN-γ, IL-12, and TNF-α), and regulatory T (T
reg) cell related cytokines (IL-10 and TGF-β), which were expressed during experimental toxocariasis in mice [
5,
6]. However, in most investigations, a specific time point was used, and alteration of immune responses using serial time points has not been investigated. In addition, there are a few studies about immune responses against VLM cause by
Toxascaris leonina. In this study, to determine alteration of immune responses during VLM caused by
T. leonina at several time points, we experimentally infected mice with the embryonated eggs of
T. leonina and measured Th cell-related serial cytokine production after infection.
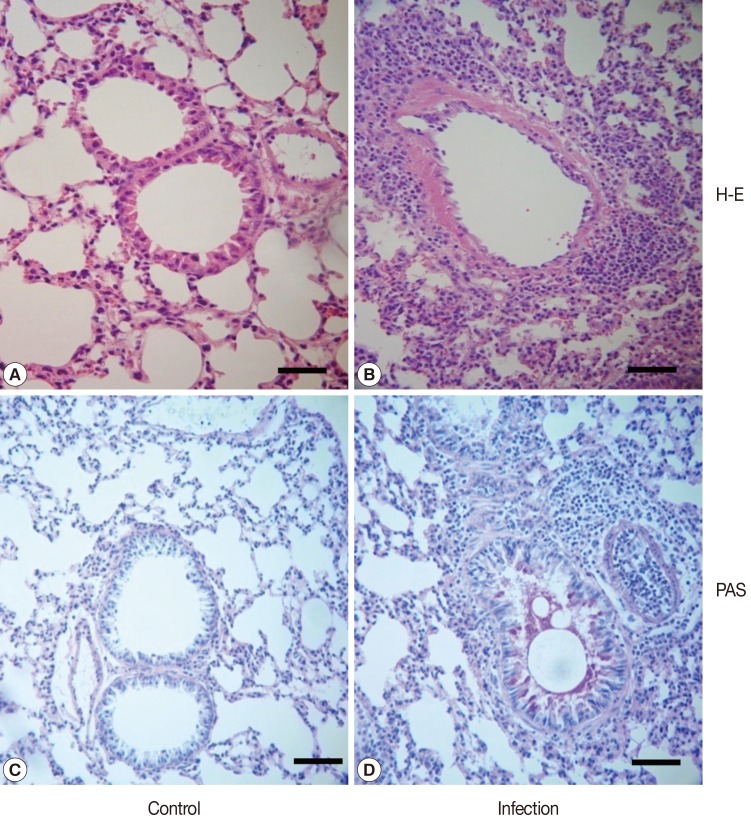
Adult T. leonina females were obtained from naturally infected dogs. Eggs were obtained from the uteri under microscopy and washed 6 times with sterile PBS. After collection, in order to prevent contamination with bacteria, the eggs were thoroughly and carefully washed several times over a 3-hr period in PBS containing antibiotics. The eggs were then incubated at 25℃ until they contained embryonated larva (second stage larva). C57BL/10 female mice, 6 weeks of age, were purchased from Samtako Co. (Suwon, Gyeonggi-do, Korea). Total 25 mice (5 per each group) were orally infected with a single dose of 500 embryonated T. leonina eggs. The mice were sacrificed on days 5, 14, 28, and 42 post-infection (PI), and sera and various organs (brain, heart, intestine, liver, lung, muscle, and spleen) were obtained from mice at each time point. Sera were stored at -20℃ before use. Each organ was weighed and digested in artificial digestive juice (0.2% pepsin, pH 2.0) at 37℃ during a period of 4 hr, and the number of released larvae was counted. In addition, lung tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin blocks. Transverse sections (5 µm) of each organ tissue were stained with hematoxylin and eosin (H&E) and PAS. All animal studies were approved by the Pusan National University Animal Care and Use Committee.
Total RNA was extracted from the brain, heart, intestine, liver, lung, muscle, and spleen in infected (on 14 days PI) and uninfected mice using 1 ml of QIAzol (Qiagen Science, Valencia, California, USA). The RNA extraction was performed in accordance with the manufacturer's protocols. The CCL11 (eotaxin for eosinophil recruitment), thymus, and activation-regulated chemokine thymic stromal lymphopoietin (TSLP for Th2 cell activation) and CXCL1 (Gro-α for neutrophil recruitment). RNA levels were determined via real-time PCR using iCyclerTM (Bio-Rad, Richmond, California, USA) real-time PCR machines. GAPDH was utilized as the reference gene. The primer sequences were designed as described previously [
7]. To investigate changes in the cytokine level by
T. leonina larvae experimental infection, the culture supernatants of splenocytes were measured for determination of IFN-γ (Th1 cytokine), IL-4, IL-5 (Th2 cytokines), IL-6, IL-17A (Th17 cytokines), IL-10, and TGF-β (regulatory T-cell cytokines) levels using an ELISA kit (eBioscience, San Diego, California, USA). The splenocytes were isolated according to the previous reports [
8]. The splenocytes were plated in 48-well plates at 5×10
6 cells/ml in RPMI 1640 with 10% fetal bovine serum (FBS) and penicillin/streptomycin. For CD3 stimulation experiments, 0.5 µg/ml CD3 antibody (eBioscience) was added to cell-plated wells. The plated cells were incubated for 72 hr at 37℃ containing 5% CO
2. After incubation, the culture media was harvested and stored at -20℃. ELISA was performed 3 times. The assay was performed according to the manufacturer's recommended protocols (eBioscience). The mean±SD was calculated from data collected from individual mice. The Student's
t-test or ANOVA was used for determination of significant differences. Statistical analysis was performed using the GraphPad Prism 4.0 software.
T. leonina larvae were found in various organs; their distribution differed according to the infection period. The number of larvae per gram of tissues in various organs is shown in
Table 1. At day 5 PI, most larvae were detected from the lungs, spleen, intestine, and muscle. Although,
T. leonina larvae were isolated from most organs at week 2 PI, the number of larvae in organs was lower than those in infected organs at day 5 PI. However, the number of larvae in the lungs at day 14 PI was not reduced from the one at day 5 PI. These results suggested that the lung is the organ most easily infected by
T. leonina larvae. The airway tract was infiltrated with immune cells, and goblet cells showed greater proliferation than those of control mice. Mucus production in the lungs was enhanced after infection with
T. leonina larvae (
Fig. 1). After day 28 PI, the number of larvae in the muscle increased, compared to those in infected organs at days 5 and 14 PI. However, we did not find any larvae in any organ after day 42 PI. Although
T. leonina infection in dogs and cats, as well as
T. canis and
T. cati, has been frequently reported to date [
9,
10], few cases of VLM due to
T. leonina in other hosts have been reported. According to Prokopic and Figallova [
11], who studied migration of
T. leonina larvae in white mice, larvae were found in lungs of 96% of infected mice at days 4-135, and also in genital organs (84%), intestinal mucosa (81%), and skeletal muscles (100%) at day 10 PI. In this study, although larvae were found in various organs, including the brain, we did not find any larvae at day 42 PI, which differed from other studies. Perhaps
T. leonina larvae infection and the survival rates might be different according to mouse strains.
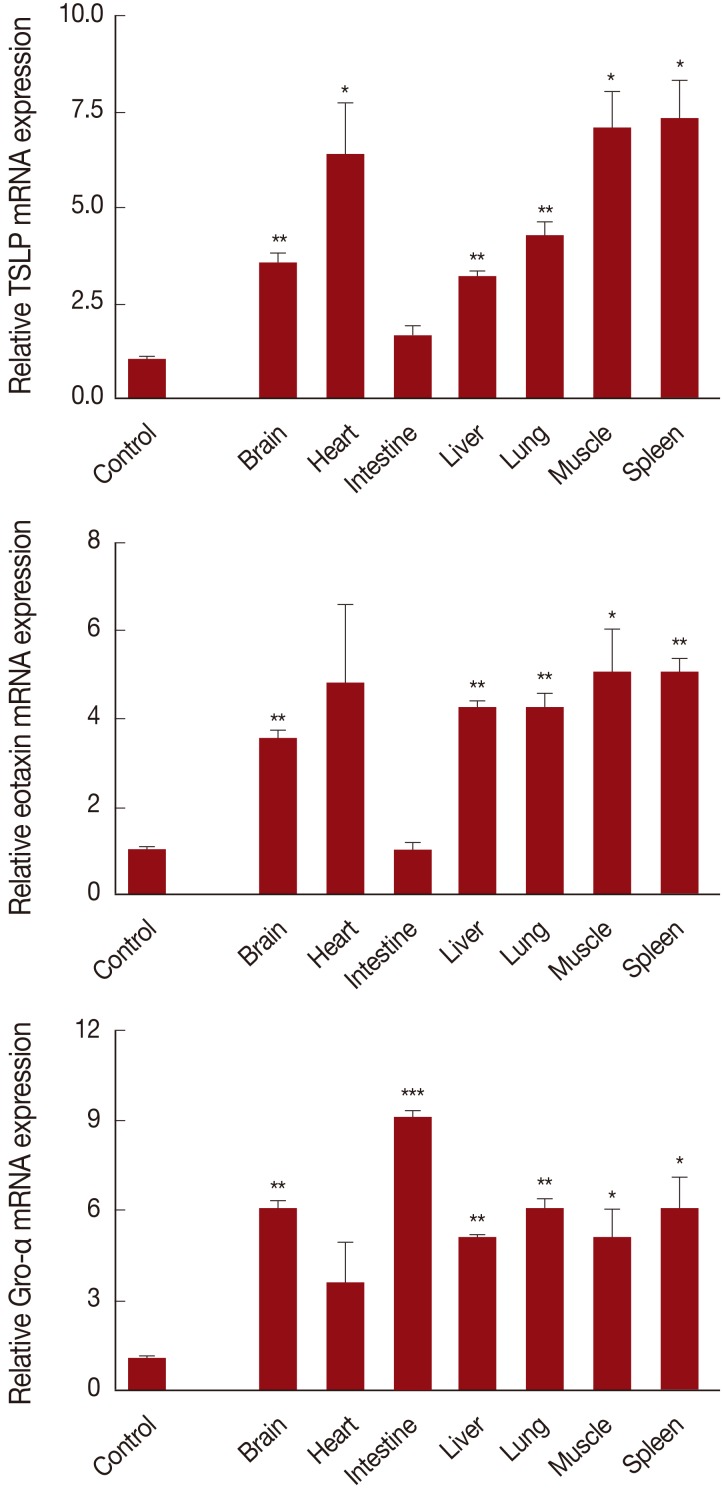
In order to determine whether several chemokine gene expression increased by
T. leonina larvae infection, we evaluated the gene expression levels of CXCL1, CCL11, and TSLP in organs at day 14 PI. Expression of TSLP, an IL-7-like cytokine, is associated with skin or epithelial cells. In parasite infections, TSLP, known as an alarmin cytokine, along with IL-25 and IL-33, induces rapid initiation of Th2 responses [
12]. In addition, Th2 cells preferentially express several receptors, including CCR3 (receptor of CCL11, CCL13, and CCL28), CCR4 (receptor of TARC and MDC), and CCR8 (receptor of CCL1), and active chemokines on these receptors are increased in inflammation, making them attractive candidates for mediation of Th2 cell-specific recruitment [
13,
14]. Expression of TSLP and CCL11 showed a significant increase (3- to>6-fold) in most internal organs, except the intestine at day 14 PI. However, expression of the CXCL1 gene was most highly enhanced (over 9-fold) in the intestine (
Fig. 2). At that time, we found that most larvae migrate to other organs from the intestine (
Table 1). These results suggested that
T. leonina larva and/or their products stimulated tissue epithelial cells and/or dendritic cells to express chemokines for activation and differentiation of Th2 cells.
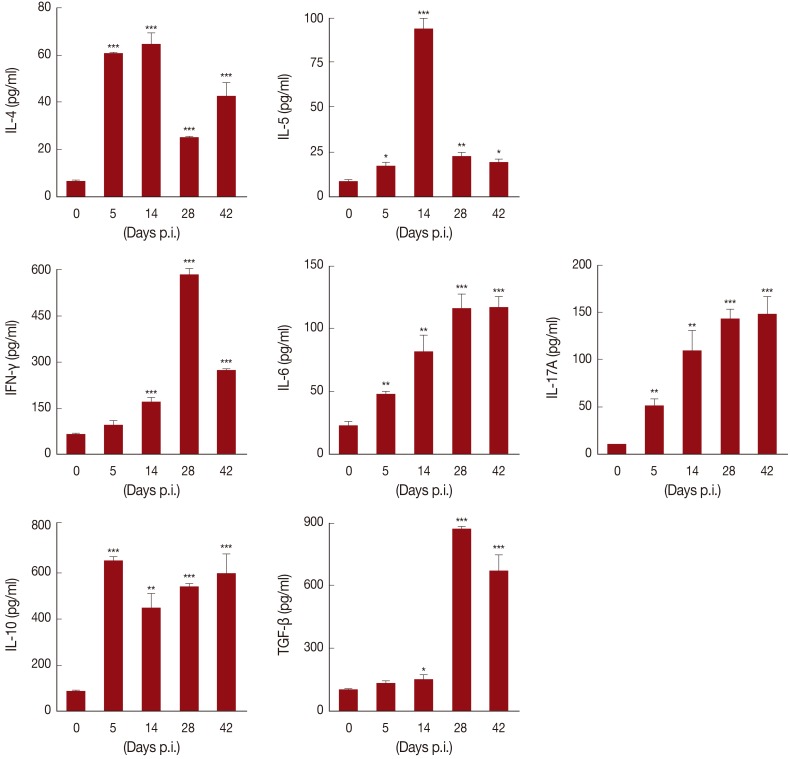
In order to determine host immune responses against
T. leonina infection, Th1 (IFN-γ), Th2 (IL-4 and IL-5), Th17 (IL-6 and IL-17), and T
reg cell (IL-10 and TGF-β) related cytokine production in supernatants from CD3 stimulated splenocytes was evaluated at several time points after infection (
Fig. 3). The levels of IFN-γ showed a graduate increase after infection and reached a peak at day 28 PI. Th2-related cytokine levels showed an increase after infection; in particular, IL-5 was significantly increased at day 14 PI and then decreased at day 28 PI. IL-6 levels showed a gradual increase until day 42 PI. In addition, IL-17A levels showed a significant and continual increase until day 42 PI. Of particular interest was that IL-10 levels were increased as early as day 5 PI, whereas TGF-β levels were slightly decreased at day 14 PI and then showed a rapid increase on day 28 PI. Although some studies on the immune responses of the host against VLM have demonstrated VLM due to
T. leonina in mice by experimental infection, they did not show any specific immune responses [
15]. Th17 is a pro-inflammatory Th cell subset, which plays important roles in the host defense and is involved in various autoimmune and inflammatory diseases, mainly by secretion of IL-17A and other cytokines, like IL-21 and IL-22 [
16]. Th17 cells are induced from naive CD4
+ T cells, mainly by IL-6 and TGF-β [
17]. Recent studies suggested that Th17 responses against nematode infection should be one of the most important host protective mechanisms [
18-
20]. Although we do not know the exact role of Th17 cells in
T. leonina larval infection, Th17 cells might be involved in induction of immune cell recruitment in order to kill the larvae. CXCL1 was highly expressed in the tissue of infected mice (
Fig. 2). CXCL1 is one of the main chemokines expressed by eosinophils. Eosinophils were found to constitutively express receptors for IL-17A, IL-17F, and IL-23 (Th17 cytokines). IL-17A, IL-17F, and IL-23 could induce release of chemokines CXCL1, IL-8/CXCL8, and MIP-1beta/CCL4 from eosinophils [
21]. These results suggested that eosinophils, neutrophils, and Th17 cells might be important in development of protective mechanisms against
T. leonina larvae infection. In order to demonstrate this hypothesis, we need more information about the role of Th17 cells in infected tissues.
IL-10, which is referred to as the cytokine synthesis inhibitory factor, is an anti-inflammatory cytokine capable of inhibiting synthesis of proinflammatory cytokines [
22]. Helminth parasites stimulate production of immunoregulatory mediators, which likely perform a function in the maintenance of the chronicity of infection, without any marked induction of pathology. IL-10 was generated principally by CD4
+CD25
+Foxp3
+ T (T
reg) cells, and IL-10 induced differentiation of T
reg cells [
23]. Although
T. leonina larvae infection also induced production of IL-10 and TGF-β in this study (
Fig. 3), we did not check alteration of the T
reg cell population.
In conclusion, we demonstrated that T. leonina larva first activated Th2-related chemokine gene expression and cytokine production. Then, the larvae activated Th1- and Th17-related chemokine gene expression and cytokine production. Since there were no larvae detected in tissues on day 42 PI, Th1 and Th17 immune responses might be as important as Th2 responses in T. leonina VLM.
Pusan National UniversityBrain Busan 21 Project
Notes
-
We have no conflict of interest related with this study.
ACKNOWLEDGMENTS
This work was supported by a 2-year Research Grant of Pusan National University and Brain Busan 21 Project in 2013.
References
- 1. Lee KH, Park HK, Jeong HJ, Park SK, Lee SJ, Choi SH, Cho MK, Ock MS, Hong YC, Yu HS. Immunization of proteins from Toxascaris leonina adult worm inhibits allergic specific Th2 response. Vet Parasitol 2008;156:216-225.
- 2. Roberts LS, Schmidt GD, Janovy J. Foundations of Parasitology. New York, USA. McGraw-Hill; 2009.
- 3. Nagy D, Bede O, Danka J, Szenasi Z, Sipka S. Analysis of serum cytokine levels in children with chronic cough associated with Toxocara canis infection. Parasite Immunol 2012;34:581-588.
- 4. Nagy D, Bede O, Danka J, Szenasi Z, Sipka S. Analysis of serum cytokine levels in children with chronic cough associated with Toxocara canis infection. Parasite Immunol 2012;34:581-588.
- 5. Takamoto M, Wang ZX, Watanabe N, Matsuzawa A, Nariuchi H, Sugane K. Eosinophilia, IgE production, and cytokine production by lung T cells in surface CD4-deficient mutant mice infected with Toxocara canis. Immunology 1998;95:97-104.
- 6. Torina A, Caracappa S, Barera A, Dieli F, Sireci G, Genchi C, Deplazes P, Salerno A. Toxocara canis infection induces antigen-specific IL-10 and IFN-gamma production in pregnant dogs and their puppies. Vet Immunol Immunopathol 2005;108:247-251.
- 7. Park HK, Cho MK, Park MK, Kang SA, Kim YS, Kim KU, Lee MK, Ock MS, Cha HJ, Yu HS. A 24 kDa excretory-secretory protein of Anisakis simplex larvae could elicit allergic airway inflammation in mice. Korean J Parasitol 2011;49:373-380.
- 8. Park MK, Cho MK, Kang SA, Park HK, Kim YS, Kim KU, Ahn SC, Kim DH, Yu HS. Protease-activated receptor 2 is involved in Th2 responses against Trichinella spiralis infection. Korean J Parasitol 2011;49:235-243.
- 9. Canto GJ, Garcia MP, Garcia A, Guerrero MJ, Mosqueda J. The prevalence and abundance of helminth parasites in stray dogs from the city of Queretaro in central Mexico. J Helminthol 2011;85:263-269.
- 10. Kim YH, Huh S. Prevalence of Toxocara canis, Toxascaris leonina, and Dirofilaria immitis in dogs in Chuncheon, Korea (2004). Korean J Parasitol 2005;43:65-67.
- 11. Prokopic J, Figallova V. The migration of larvae of Toxascaris leonina (Linstow, 1909) in experimentally infected white mice. Folia Parasitol (Praha) 1982;29:233-238.
- 12. Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol 2012;24:459-466.
- 13. Mathew A, MacLean JA, DeHaan E, Tager AM, Green FH, Luster AD. Signal transducer and activator of transcription 6 controls chemokine production and T helper cell type 2 cell trafficking in allergic pulmonary inflammation. J Exp Med 2001;193:1087-1096.
- 14. Sallusto F. The role of chemokines and chemokine receptors in T cell priming and Th1/Th2-mediated responses. Haematologica 1999;84(suppl EHA-4):28-31.
- 15. Okoshi S, Usui M. Experimental studies on Toxascaris leonina. VI. Experimental infection of mice, chickens and earthworms with Toxascaris leonina, Toxocara canis and Toxocara cati. Nippon Juigaku Zasshi 1968;30:151-166.
- 16. Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature 2008;453:1051-1057.
- 17. Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, Liao YH. MicroRNA-155 modulates Treg and Th17 cell differentiation and Th17 cell function by targeting SOCS1. PLoS One 2012;7:e46082.
- 18. Kang SA, Cho MK, Park MK, Kim DH, Hong YC, Lee YS, Cha HJ, Ock MS, Yu HS. Alteration of helper T-cell related cytokine production in splenocytes during Trichinella spiralis infection. Vet Parasitol 2012;186:319-327.
- 19. Bazzone LE, Smith PM, Rutitzky LI, Shainheit MG, Urban JF, Setiawan T, Blum AM, Weinstock JV, Stadecker MJ. Coinfection with the intestinal nematode Heligmosomoides polygyrus markedly reduces hepatic egg-induced immunopathology and proinflammatory cytokines in mouse models of severe schistosomiasis. Infect Immun 2008;76:5164-5172.
- 20. Reece JJ, Siracusa MC, Southard TL, Brayton CF, Urban JF Jr, Scott AL. Hookworm-induced persistent changes to the immunological environment of the lung. Infect Immun 2008;76:3511-3524.
- 21. Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol 2008;180:5625-5635.
- 22. Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001;2:725-731.
- 23. Presser K, Schwinge D, Wegmann M, Huber S, Schmitt S, Quaas A, Maxeiner JH, Finotto S, Lohse AW, Blessing M, Schramm C. Coexpression of TGF-beta1 and IL-10 enables regulatory T cells to completely suppress airway hyperreactivity. J Immunol 2008;181:7751-7758.
Fig. 1Representative pathological changes in the lung of mice infected with T. leonina on day 14 PI. Pathological changes were observed in T. leonina infected mice (B, D), compared with non-infected mice (A, C). After histological processing, sections of the lung tissue were stained with H-E (A, B) and PAS stain (B, D). Bar=100 µm.

Fig. 2Chemokine gene expression of T. leonina infected mouse organs at day 14 PI. After sacrifice, RNA was extracted from 1 g of each organ tissue from infected and uninfected mice. The expression level was compared with that of the GAPDH gene (*P<0.05, **P<0.01, ***P<0.001, compared with controls, in 3 independent experiments).

Fig. 3Production of cytokines in splenocytes of T. leonina infected mice. Wells were incubated with 0.5 µg/ml (final concentration) of anti-CD3 antibody for 16 hr at 4℃, and splenocytes were added to the well, and incubated for 3 days. After activation, the levels of cytokines were measured in the supernatant using ELISA kits (*P<0.05, **P<0.01, ***P<0.001, compared with controls, in 3 independent experiments).

Table 1.Number of Toxascaris leonina larvae per gram of various mouse organs after infection
Table 1.
|
Organs |
No. of larvae recovered at day post-infection
|
|
5 |
14 |
28 |
42 |
|
Brain |
0 |
0.6 ± 0.31 |
1.3 ± 1.90 |
0 |
|
Heart |
0 |
5.7 ± 2.1 |
0 |
0 |
|
Intestine |
109.1 ± 12.0 |
33.4 ± 1.8 |
27.1 ± 0.33 |
0 |
|
Liver |
0 |
5.8 ± 3.2 |
4.1 ± 1.8 |
0 |
|
Lung |
145.1 ± 20.0 |
151.5 ± 24.3 |
7.1 ± 3.0 |
0 |
|
Muscle |
12.3 ± 5.0 |
9.3 ± 3.1 |
26.1 ± 9.1 |
0 |
|
Spleen |
111.2 ± 2.1 |
12.8 ± 3.6 |
0 |
0 |