Abstract
The weight gain phenomenon associated with sparganosis has been well documented and was first recognized in the 1960s. Many studies have been conducted regarding the plerocercoid growth factor in the larva of Spirometra mansoni. In the present study, we hypothesized that the weight gain may be affected by the adipocyte secreted hormones, i.e., adiponectin, which is secreted from the adipose tissues in case of tissue migrating parasitic infections. Specifically, we attempted to ascertain whether the serum levels of adiponectin change in murine sparganosis. However, serum adiponectin levels assayed by ELISA evidenced no significant changes after an experimental infection (P > 0.05). Finally, the weight gain phenomenon in mouse sparganosis is not associated with changes in adiponectin levels, and further investigations involving parasitic infection-induced weight gain remain necessary.
-
Key words: Spirometra mansoni, sparganum, sparganosis, adipocyte, adiponectin
Human sparganosis is a disease caused by the ingestion of snake meat containing the plerocercoid of
Spirometra mansoni in the snake [
1]. The weight gain phenomenon characteristic of sparganosis has been well documented since the 1960s [
2]. Several papers have been published regarding the substances-binding of growth hormone receptors, which results in the growth of the infected host. Finally, Phares and Kubik [
3] purified a growth factor from the plerocercoid of
Spirometra mansonoides plerocercoid growth factor (PGF). However, the exact mechanisms responsible for parasitic-infection induced weight gain remain a matter of some controversy.
After infection in the host, the sparganum can evade the host's immune responses during the migration period of worms primarily using excretory-secretory proteases [
4]. Also, the sparganum surface and excretory-secretory products have been recognized as the principal modulators of the host immune system [
5]. Coupled with the main function of adipose tissue as an energy storage reservoir, it has been suggested that adipokines including adiponectin, tumor necrosis factor-α(TNF-α) and interleukin-6 (IL-6) secreted in the adipose tissues will be altered due to the active migratory activity of sparganum. One of these adipokines, i.e., adiponectin, is a protein which is secreted exclusively by adipocytes, and is the most abundant adipose tissue-derived protein [
6]. In the present study, we hypothesized that the sparganum-induced body weight changes may be influenced by the adiponectin secreted from the adipocyte, which is stimulated by excretory-secretory proteases from the sparganum. Therefore, we determined the serum levels of adiponectin, in order to ascertain whether the observed changes occurred during the infection.
Larval spargana were collected from the snakes caught in the Republic of Korea. A total of 10 BALB/c mice (Daehan Biolink, Daejon, Korea) were purchased and grouped into a non-infected normal control group and an infected mice group. One sparganum was infected into each mouse and the blood was collected serially from the tail until week 13 after infection. Adiponectin levels in the mouse sera were assessed in duplicate by an adiponectin ELISA kit (AdipoGen, Seoul, Korea). In brief, the blood was centrifuged for 10 min at 1,000 g, and the serum was collected. The adiponectin levels in the serum were determined in accordance with the manufacturer's recommendation. A standard curve was obtained in a range of adiponectin between 0.125 and 2 ng/ml. The adiponectin concentration was calculated from the standard curve and the data are expressed as the means. Statistical significance was assessed via Student's t-test. A P-value of < 0.05 was considered significant.
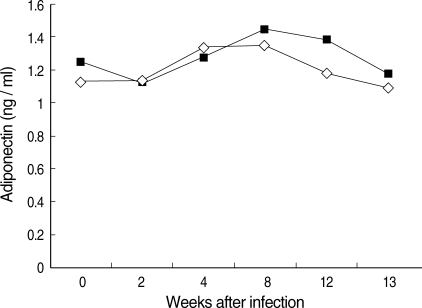
No significant changes in the serum level of adiponectin were observed in the infected mice (
P > 0.05;
Fig. 1). However, body weight increased significantly in the infected mice (
P < 0.05, data not shown).
Adiponectin, an adipocyte-derived hormone, is the most abundant adipokine from the adipose tissue. Serum adiponectin concentration and mRNA expression have been shown to be reduced in obese humans, despite their increased adiposity [
7]. Furthermore, the administration of adiponectin to obese mice causes weight loss and reduces circulating free fatty acid levels via enhanced muscle fat oxidation [
8]. In addition, adipokines exert significant effects on metabolism and lipogenesis, as well as the regulation of human inflammatory responses [
9]. Thus, we surmised that adiponectin levels should have been reduced after infection. However, we noted no significant changes in the serum levels of adiponectin.
Recent advances in our understanding of obesity may be understood as a state of chronic low-grade inflammation [
10,
11]. Also, increased serum levels of several markers of inflammation, both pro-inflammatory cytokines (IL-6 and TNF-α) and acute-phase proteins (C-reactive protein) have been previously noted to exist in the experimental obesity group [
12,
13]. In addition, sparganosis elicits subcutaneous migration and chronically forms a subcutaneous mass.
In a recent study concerning obesity, the adipocyte was determined to be the infection site for the development of atherosclerosis and diabetes coupled with abdominal obesity. However, adiponectin levels were not altered in the production of pre-adipocytes infected with several microorganisms [
14]. Thus, the infection-induced inflammation is not associated with changes in the level of adipocyte-secreting hormones, but may possibly be associated with other inflammatory cytokines, including TNF-α and IL-6.
Murine sparganosis may constitute a good model for the study of immuno-endocrinological functions of adipocytes due to the active migratory activity of the sparganum. Also, the excretory-secretory proteases of spargana may exert immuno-endocrinological effects on the adipose tissues of the host. Examples of reciprocal endocrine regulation between the parasite and the host, including humans, have been previously described [
15,
16]. In the present study, we hypothesized that serum adiponectin levels might be altered in the cases of murine sparganosis. However, we observed no positive results with regard to the modulation of adipokines in murine sparganosis. The weight gain phenomenon associated with murine sparganosis should be investigated in future studies.
ACKNOWLEDGEMENTS
The authors wish to thank Je-Young Ryu for his technical assistance. This work was supported by grants from the Tropical and Endemic Parasitic Diseases Control Programme of the National Institute of Health (NIH 347-6111-211), Ministry of Health and Welfare, Republic of Korea
References
Fig. 1Serum adiponectin levels in murine experimental sparganosis. No significant changes between the normal and sparganum-infected groups were observed (P > 0.05). ◇, normal ■, sparganum-infected group.
Citations
Citations to this article as recorded by

- A Protein Microarray for the Rapid Screening of Patients Suspected of Infection with Various Food-Borne Helminthiases
Jia-Xu Chen, Mu-Xin Chen, Lin Ai, Jun-Hu Chen, Shao-Hong Chen, Yong-Nian Zhang, Yu-Chun Cai, Xing-Quan Zhu, Xiao-Nong Zhou, Patrick J. Lammie
PLoS Neglected Tropical Diseases.2012; 6(11): e1899. CrossRef
