Abstract
Lymphatic filariasis is a common parasitic disease of cats in tropical regions including Thailand. The objective of this study was to determine the efficacy of ivermectin against microfilariae of Brugia pahangi in naturally infected cats. Eight cats naturally infected with B. pahangi were divided into control (untreated) and treated groups. Cats in the latter group were given ivermectin injection at 400 µg/kg weekly for 2 months. Microfilariae were counted every week until 48 weeks. Microfilaremia was significantly decreased in the treated group 4 weeks after starting the treatment and become zero at week 9 and afterwards. On the other hand, cats in the control group had high microfilaremia throughout the study. It was successful to treat and control B. pahangi infection in naturally infected cats using ivermectin.
-
Key words: Brugia pahangi, treatment, cat
Lymphatic filariasis is a major problem in tropical and sub-tropical countries including Thailand. Lymphatic filariasis in cats is caused by
Brugia spp., such as
B. malayi and
B. pahangi. The former species is a significant cause of human lymphatic filariasis which is also endemic in southern parts of Thailand [
1]. However,
B. pahangi is commonly found in cats and dogs in Bangkok area [
2]. Both
Brugia species are transmitted by mosquitoes especially
Armigeres spp. and
Mansonia spp. [
3]. Pathology is progressed when lymphatic vessels are obstructed. The severity of disease depends on the number of worms present [
2].
Macrocyclic lactones are broad-spectum anthelmintics that are used to treat a variety of nematode infections. Ivermectin is the only macrocyclic lactone that is approved for use in human [
4]. In control program for filariasis, ivermectin is the drug of choice, especially in areas with onchocercosis [
5]. There are several studies about the efficacy of ivermectin against filarial worms [
1,
6-
8]. For
Onchocerca volvulus, treatment with ivermectin reduces microfilarial motility in vitro [
9], and embryogenesis is impaired in adult female worms [
6]. Moreover, a single dose of ivermectin was effective to cure cats naturally infected with
B. malayi, although microfilariae could not be completely cleared in cats [
10]. However, treatment of
B. pahangi infection with ivermectin in cats has never been reported. Thus, the objective of this study was to determine the efficacy of multiple doses of ivermectin against microfilariae of
B. pahangi in naturally infected cats.
This study was conducted as a blinded, randomized study following the standard protocol accepted by the Laboratory Animal Ethics Committee of the Faculty of Veterinary Science, Chulalongkorn University (Approval No. 0831006), Bangkok, Thailand.
Eight cats were naturally infected with
B. pahangi. The species of worms was confirmed by staining microfilariae using the acid phosphatase technique [
11]. All cats were kept individually in the Parasitology Unit, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand.
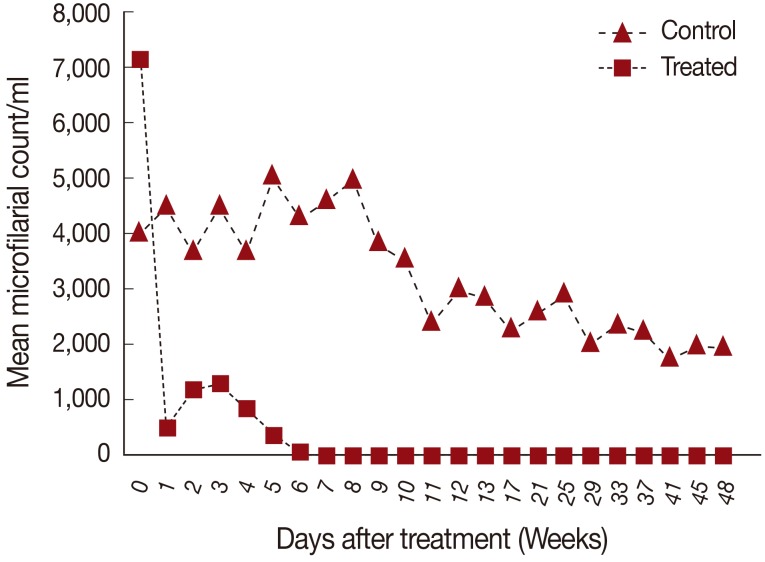
Eight cats were divided into 2 groups (n=4 each), an untreated control group and a group given subcutaneous ivermectin injections at 400 µg/kg every week for 2 months. After treatment (day 0;
Fig. 1), 60 µl blood smears from ear pricks were examined for microfilariae every week until 48 weeks. Smears were stained with 2.5% Giemsa to estimate the numbers of microfilariae (mf) per ml blood.
The efficacy of ivermectin treatment was calculated based on the arithmetic mean of microfilarial counts from the untreated control group and treated group. Microfilarial counts were summarized and compared at multiple time points using the powerful multivariate Wilcoxon-Mann-Whitney test with the simultaneous application of a 1-sided Wei-Lachin procedure [
12,
13], accepting a significance level of alpha=0.025.
At day 0, the mean microfilarial count was 4,070 mf/ml in the control group (n=4) and 7,130 mf/ml in the treated group (n=4). The mean microfilarial count decreased significantly in the treated group after 4 weeks from the start of the treatment. From week 9 and onwards, no microfilariae were found in any of the treated cats. The difference between the groups was statistically significant (
P<0.0001) from week 5 after treatment until the end of the experiment at week 48. By contrast, the microfilarial counts remained high (1,990±1,059.53 mf/ml) in the untreated control group for the rest of the study period (
Fig. 1).
Ivermectin is known to reduce the motility of microfilariae of filarial nematodes and inhibit molting of the third-stage larvae (L3) [
14]. Its efficacy against various stages of filarial parasites such as
Dirofilaria immitis in dogs,
Setaria equina in horses, and
Onchocerca spp. in cattle was observed [
15]. In
B. malayi, ivermectin induces muscular passivity in microfilariae and impairs exsheathing [
4]. In this study, we demonstrated a significant decrease in microfilaremia after 4 weekly treatments with ivermectin injection via the subcutaneous route at the dose of 400 µg/kg. The cat received 400 µg/kg ivermectin injections weekly for 2 months. The reason for this long-term treatment was because 1 of the cats in the treated group which had high parasitemia from the beginning (at day 0; 26,550 mf/ml) remained microfilaremia until week 7 post-treatment. However, microfilaremia did not appear in the blood circulation after week 8.
In our study, cats naturally infected with
B. pahangi were successfully treated using multiple ivermectin injections. Clinical side effects such as salivation and ataxia after treatment were not observed in these cats. The results of the present study indicated that adult worms were possibly affected by ivermectin by sterilization of female worms resulting in a decrease of microfilaremia. A similar effect was reported for
Wuchereria bancrofti [
8]. Therefore, it seems that the main target of ivermectin is reproduction of filarial worms, as the drug impairs embryogenesis and decrease microfilaremia [
4,
7,
16].
In conclusion, ivermectin can be used for treating B. pahangi in cats. This host-parasite system can therefore be used as a model to develop control and treatment protocols for B. malayi in cats which is a reservoir host for human lymphatic filariasis.
Chulalongkorn UniversityHigher Education Research PromotionNational Research University Project of ThailandHR1160A-56
Thailand Research FundRTA558004
Khon Kaen UniversityACKNOWLEDGMENTS
This study was supported by Faculty of Veterinary Science Research fund, Chulalongkorn University and Higher Education Research Promotion and National Research University Project of Thailand (HR1160A-56). This research was also supported in part by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA558004 and Research and Diagnostic Center for Emerging Infectious Disease, Faculty of Medicine, Khon Kaen University, Thailand.
References
- 1. Phantana S, Sarataphan N, Chansiri G, Chansiri K. Microfilaricidal efficacy of ivermectin on zoonotic Brugia malayi in naturally infected cats. J Trop Med Parasitol 2002;25:11-16.
- 2. Junhom W, Chungpivat S, Visseshakul N. The observation of microfilaria rate and density in cats inoculated with increasing numbers of Brugia pahangi infective larvae. Southeast Asian J Trop Med Public Health 2006;37(suppl 3):40-42.
- 3. Chungpivat S, Sucharit S. Microfilariae in cats in Bangkok. Thai J Vet Med 1993;23:75-87.
- 4. Tompkins JB, Stitt LE, Ardelli BF. Brugia malayi: in vitro effects of ivermectin and moxidectin on adults and microfilariae. Exp Parasitol 2010;124:394-402.
- 5. Speare R, Durrheim D. Mass treatment with ivermectin: an underutilized public health strategy. Bull World Health Organ 2004;82:562.
- 6. Bottomley C, Isham V, Collins RC, Basanez MG. Rates of microfilarial production by Onchocerca volvulus are not cumulatively reduced by multiple ivermectin treatments. Parasitology 2008;135:1571-1581.
- 7. McCall JW, Genchi C, Kramer L, Guerrero J, Dzimianski MT, Supakorndej P, Mansour AM, McCall SD, Supakorndej N, Grandi G, Carson B. Heartworm and Wolbachia: therapeutic implications. Vet Parasitol 2008;158:204-214.
- 8. Stolk WA, Van Ootmarssen GJ, Pani SP, De Vlas SJ, Subramanian S, Das PK, Habbema JD. Effects of ivermectin and diethylcabamazine on microfilariae and overall microfilaria production in bancroftian filariasis. Am J Trop Med Hyg 2005;73:881-887.
- 9. Tagboto SK, Townson S. Onchocerca volvulus and O. lienalis: the microfilaricidal activity of moxidectin compared with that of ivermectin in vitro and in vivo. Ann Trop Med Parasitol 1996;90:497-505.
- 10. Chansiri G, Khawsak P, Phantana S, Sarataphan N, Chansiri K. The efficacy of a single-oral-dose administration of ivermectin and diethylcarbamazine on the treatment of feline Brugia malayi. Southeast Asian J Trop Med Public Health 2005;36:1105-1109.
- 11. Chungpivat S, Taweethavonsawat P. The differentiation of microfilariae in dogs and cats using Giemsa's staining and the detection of acid phosphatase activity. J Thai Vet Prac 2008;20:47-55.
- 12. Lachin JM. Some large-sample distribution-free estimators and tests for multivariate partially incomplete data from two populations. Stat Med 1992;11:1151-1170.
- 13. Thall PF, Lachin JM. Analysis of recurrent events: nonparametric methods for random interval count data. J Am Stat Assoc 1988;83:339-347.
- 14. Baird JK, Riberu W, Masbar S, Purnomo . Ivermectin inhibits molting of Wuchereria bancrofti third stage larvae in vitro. J Parasitol 1991;77:162-163.
- 15. Campbell WC. Efficacy of the avermectins against filarial parasites: a short review. Vet Res Commun 1982;5:251-262.
- 16. Mancebo OA, Verdi JH, Bulman GM. Comparative efficacy of moxidectin 2% equine oral gel and ivermectin 2% equine oral paste against Onchocerca cervicalis (Railiet and Henry. 1910) microfilariae in horses with naturally acquired infections in Formosa (Argentina). Vet Parasitol 1997;73:243-248.
Fig. 1Mean values of microfilaremia at pre- (day 0) and post-treatment days (weeks) with ivermectin compared with untreated control group.

Citations
Citations to this article as recorded by

- Filariasis of the breast caused by Brugia pahangi: A concomitant finding with invasive ductal carcinoma
Jerapas Thongpiya, Doonyapat Sa-nguanraksa, Norasate Samarnthai, Patsharaporn T. Sarasombath
Parasitology International.2021; 80: 102203. CrossRef - Comparison of efficacy of ivermectin and diethylcarbamazine against naturally infected Brugia malayi microfilaria in dogs
Poojary Vineeta Sadarama, Deepa Chirayath, Usha Narayana Pillai, Bindu Lakshmanan
Journal of Parasitic Diseases.2019; 43(4): 554. CrossRef - Therapeutic trial of doxycyclin plus ivermectin for the treatment of Brugia malayi naturally infected cats
Ladawan Khowawisetsut, Patsharaporn T. Sarasombath, Suwich Thammapalo, Sumart Loymek, Therayot Korbarsa, Hathai Nochote, Achinya Phuakrod, Wej Choochote, Sirichit Wongkamchai
Veterinary Parasitology.2017; 245: 42. CrossRef