Abstract
IL-23 and IL-12 are structurally similar and critical for the generation of efficient cellular immune responses. Toxoplasma gondii induces a strong cell-mediated immune response. However, little is known about IL-23 secretion profiles in T. gondii-infected immune cells in connection with IL-12. We compared the patterns of IL-23 and IL-12 production by THP-1 human monocytic cells in response to stimulation with live or heat-killed T. gondii tachyzoites, or with equivalent quantities of either T. gondii excretory/secretory proteins (ESP) or soluble tachyzoite antigen (STAg). IL-23 and IL-12 were significantly increased from 6 hr after stimulation with T. gondii antigens, and their secretions were increased with parasite dose-dependent manner. IL-23 concentrations were significantly higher than those of IL-12 at the same multiplicity of infection. IL-23 secretion induced by live parasites was significantly higher than that by heat-killed parasites, ESP, or STAg, whereas IL-12 secretion by live parasite was similar to those of ESP or STAg. However, the lowest levels of both cytokines were at stimulation with heat-killed parasites. These data indicate that IL-23 secretion patterns by stimulation with various kinds of T. gondii antigens at THP-1 monocytic cells are similar to those of IL-12, even though the levels of IL-23 induction were significantly higher than those of IL-12. The detailed kinetics induced by each T. gondii antigen were different from each other.
-
Key words: Toxoplasma gondii, IL-23, IL-12, THP-1 monocytic cell
INTRODUCTION
Toxoplasma gondii is a widespread apicomplexan parasite of warm-blooded vertebrates. It normally produces asymptomatic infection in its vertebrate intermediate hosts, including humans [
1]. However, in immunocompromised hosts, the parasite may emerge as a life-threatening pathogen causing severe toxoplasmosis. The course of infection in healthy individuals is characterized by an acute phase that is associated with dissemination of rapidly dividing tachyzoites capable of invading virtually all nucleated cells. This is followed by a long-term chronic phase, which correlates with the rise in host adaptive immunity [
2]. Thus, the host immune status is critical to the pathophysiology of toxoplasmosis.
IL-12 family cytokines, i.e., IL-12, IL-23, and IL-27, have been shown to play crucial roles in the regulation of innate and adaptive immune responses. Although IL-12 is widely studied, little is known about IL-23 and IL-27. Structurally, IL-23 and IL-12 are heterodimers and they share the p40 subunit, but they have unique second subunits, IL-23p19 and IL-12p35. Also, their heterodimeric receptors share the IL-12Rβ1 chain [
3]. When stimulated by
T. gondii, macrophages secrete several cytokines such as tumor necrosis factor-α (TNF-α), IL-1, IL-12, and IL-23 [
4-
7]. IL-12 is required for resistance to acute and chronic toxoplasmosis due to its essential role in stimulating the production of IFN-γ. Early production of IL-12 by dendritic cells (DCs), neutrophils, and macrophages occurs in response to
T. gondii antigens [
4-
7]. IL-23 was discovered only recently and is structurally similar to IL-12. Lieberman et al. [
6] reported that IL-12 plays the dominant role in resistance to toxoplasmosis, but in the absence of IL-12, IL-23 can provide a limited mechanism of resistance to this infection. Thus, IL-23 and IL-12 play separate but complementary roles in innate immunity.
IL-23 and IL-12 have been shown to be similar in terms of their activity, and both can be produced by DCs, monocytes, and macrophages [
3]. The balance and timing of the production of these 2 regulatory cytokines are important for generation of effective cellular immune responses. Even though
T. gondii induces strong cell-mediated immune responses, different types of
T. gondii antigens may have distinct types of epitopes, thus different antigen can manipulate the peculiar signaling pathways due to different cellular and molecular interactions [
3,
7]. A few studies have indicated a role of IL-23 and IL-12 in
T. gondii-infected hosts; however, little is known about IL-23 production profiles in
T. gondii-infected immune cells in connection with IL-12. To compare the production patterns of both IL-23 and IL-12 in response to various
T. gondii antigens, THP-1 human monocytic cells were stimulated with live or heat-killed tachyzoites, and either
T. gondii excretory/secretory proteins (ESPs) or soluble tachyzoite antigen (STAg), both at a multiplicity of infection (MOI) of 1 parasite-equivalent, and then the IL-23 and IL-12 levels generated were quantified by ELISA.
MATERIALS AND METHODS
Purification of T. gondii proteins
Tachyzoites of T. gondii RH strain were maintained via intraperitoneal passages in BALB/c mice, and then harvested from infected mice at 3-4 days post-infection. Mice were intraperitoneally injected with PBS, and peritoneal fluids were collected. Tachyzoites were purified by centrifugation over 40% Percoll (Pharmacia Biotech, Uppsala, Sweden) in PBS and passed through a 3 µm pore-size polycarbonate membrane. Then, they were washed twice with PBS and kept at -70℃ until use. Heat-killed tachyzoites were obtained after incubation at 56℃ for 50 min. The viability of tachyzoites was examined using a trypan blue dye-exclusion assay.
Preparation of T. gondii ESP and STAg
ESPs were produced as described previously [
8], with slight modifications. Briefly, freshly purified tachyzoites (1×10
8) from the peritoneal cavity were incubated at 37℃ for 3 hr under mild agitation in test tubes containing 1.0 ml Hank's balanced salt solution (Gibco BRL, Rockville, Maryland, USA). After centrifugation for 5 min at 6,000 g, the ESP-containing supernatant was aliquoted and stored at -70℃ until used.
STAg was prepared from RH tachyzoites, as described previously [
5]. Briefly, freshly purified parasites were counted and washed twice in PBS, centrifuged at 5,000 g for 3 min, and disrupted by 3 cycles of freezing at -70℃ and thawing at 15℃. Finally, the lysate was sonicated 4 times for 20 sec. The protein concentration was determined by the Bradford method using bovine serum albumin (BSA) as the standard, and the samples were stored at -70℃ until use.
To verify the presence of T. gondii antigens, proteins were resolved by SDS-PAGE and subjected to Western blotting using a monoclonal antibody against T. gondii dense granule protein 6 (GRA6, kindly provided by Prof. Ho-Woo Nam, Catholic University of Korea, Seoul, Korea).
In vitro culture of THP-1 human monocytic cells
THP-1 is a promonocytic cell line derived from acute lymphocytic leukemia (ATCC, Manassas, Virginia, USA). THP-1 cells were cultured in RPMI 1640 medium (Gibco-Invitrogen, Carlsbad, California, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-Invitrogen), 2 mM L-glutamine, 25 mM HEPES, and antibiotics-antimycotics. Cell cultures were maintained at 37℃ and 5% CO2, and cell viability was determined using a trypan blue dye-exclusion assay.
Stimulation of THP-1 cells with T. gondii antigens and quantification of cytokine secretion
THP-1 cells were stimulated by adding a MOI of 1 fresh live T. gondii RH tachyzoite or heat-killed tachyzoite, or either ESP or STAg at a MOI 1 parasite-equivalent. Cell culture supernatants were collected at 0 (before treatment), 3, 6, 18, 24, or 48 hr post-stimulation, and stored at -20℃. LPS (1 µg/ml) and 10 ng/ml recombinant human TNF-α (R&D Systems Europe, Lille, France) were used as controls of THP-1 cell activation. All assays were performed in triplicate.
To quantify the cytokine secretion at culture supernatants, levels of both IL-23 and IL-12 were measured in triplicate using commercially available ELISA kits, according to the manufacturer's instructions (BD Biosciences, Pont de Claix, France). The minimum detectable concentration of both cytokines was 2.7 pg/ml, as determined by the manufacturer.
RNA extraction and RT-PCR
THP-1 cells were infected or stimulated as described previously, and 6 hr later, total cellular RNA was extracted using the TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, California, USA) and amplified by RT-PCR. RNA (1 µg) was reverse transcribed in a final volume of 20 µl using Superscript II reverse transcriptase (Invitrogen Life Technologies), as described by the manufacturer. PCR reactions were carried out in a total volume of 25 µl. Each PCR mixture contained 0.125 µl Takara Ex Taq (5 U/µl), 2.5 µl 10× Ex Taq Buffer, 2 µl dNTP mixture (2.5 mM each), 1.5 µl each primer (10 pmol/µl), and 1 µl cDNA. All PCR reactions were performed using a MyCycler instrument (Bio-Rad, Richmond, California, USA). Denaturation of DNA (94℃ for 5 min) was followed by 30 cycles of amplification (98℃ for 10 sec, 60℃ for 30 sec, and 72℃ for 30 sec), and a final extension for 7 min at 72℃. PCR primer sequences were as follows: p19-F (5'-GAGCAGCAACCCTGAGTCCCTA-3'), p19-R (5'-CAAATTTCCCTTCCCATCTAATAA-3'), p40-F (5'-GCTATGGTGAGCCGTGAT-3'), p40-R (5'-CATGCTAATGAGAAAGGGATT-3'), p35-F (5'-AGGCCCTGAATTTCAACA-3'), p35-R (5'-GATGTAATAGTCCCATCCTTCTTT-3'), IL-12Rb1-F (5'-CCTCCTCTGGGAGACATCAA-3'), IL-12Rβ1-R (5'-GGACAGCATGTGGAGCTGTA-3'), IL-23R-F (5'-GAGACTACCCGCAAAACTCG-3'), IL-23R-R (5'-TAGCGAGTTTTGCGGGTAGT-3'), IL-12Rβ2-F (5'-ACAGAGGAAAAGGGGAGCAT-3'), IL-12Rβ2-R (5'-CAAAAGCATGGTGGTTTCCT-3'), HPRT-F (5'-TGTGTGCTCAAGGGGGGC-3'), and HPRT-R (5'-CGTGGGGTCCTTTTCACC-3'). Amplified products were resolved in 2% agarose gels and visualized with ethidium bromide. To determine the necessary number of amplification cycles, we checked the amounts of amplification products of each gene at various numbers of PCR cycles. The PCR products were increased linearly within the number of PCR cycles of each gene.
Statistical analyses
Data are expressed as the mean±SD of at least 3 independent determinations, unless otherwise indicated. The statistical significance of the data was determined using an unpaired Student's t-test. Values of P<0.05 were considered significant.
RESULTS
Preparation of 4 types of T. gondii
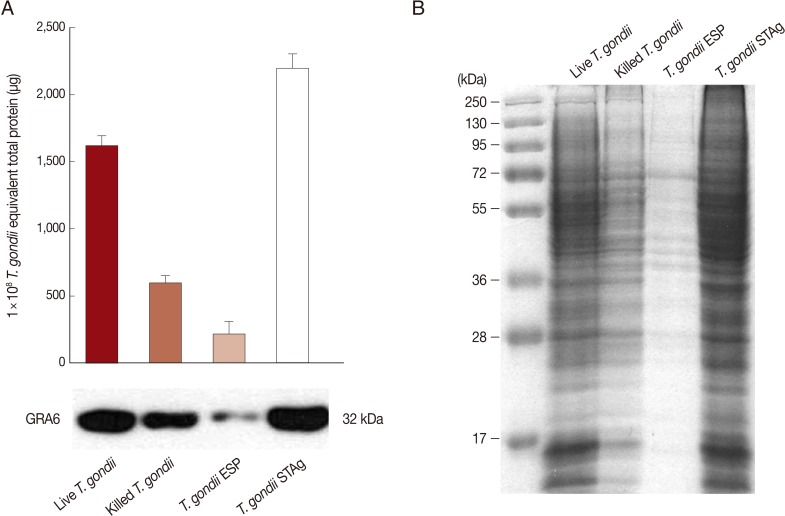
To investigate IL-23 and IL-12 secretion in THP-1 monocytic cells in response to stimulation with 4 types of T. gondii, we purified fresh RH tachyzoites and then prepared live and heat-killed tachyzoites, T. gondii ESP, and STAg using an equivalent number of live parasites.
The protein concentrations of 1×10
8 live or heat-killed
T. gondii and 1×10
8 equivalent
T. gondii ESP or STAg were measured by the Bradford assay. The total protein level in heat-killed
T. gondii was lower than that in live
T. gondii, but higher than that of ESP. The level of
T. gondii-specific GRA6 was greatest in STAg, followed by live
T. gondii, heat-killed
T. gondii, and ESP (
Fig. 1A). Equal quantities of each were stained with Coomassie blue. STAg yield from 1×10
8 tachyzoites was greater than that of ESP (
Fig. 1B).
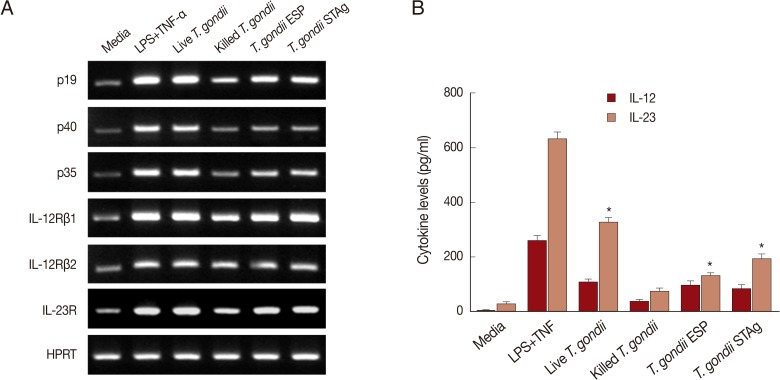
To assess whether stimulation with the 4
T. gondii preparations would induce expression of IL-23, IL-12, and their receptors, THP-1 cells were stimulated for 6 hr by adding a MOI of 1 live or heat-killed tachyzoite or quantities of ESP or STAg corresponding to MOI 1 parasite. As shown in
Fig. 2A, stimulation with live
T. gondii significantly enhanced expression of p19, p40, p35, IL-12Rβ1, IL-23R, and IL-12Rβ2 transcripts. Stimulation with
T. gondii ESP or STAg resulted in mRNA levels higher than those generated by heat-killed
T. gondii-stimulated cells, but lower than live
T. gondii-infected cells. However, significant IL-23, IL-23R, IL-12, and IL-12R mRNA increments were detected in the LPS and TNF-α co-treated cells used as positive controls. These data demonstrate that THP-1 cells stimulated with live
T. gondii, heat-killed
T. gondii, or
T. gondii ESP or STAg express mRNAs coding for IL-12 (p40 and p35 subunits), IL-23 (p40 and p19 subunits), IL-12R, and IL-23R.
To examine the capacity of human monocytic cells to produce IL-23 or IL-12 in response to
T. gondii, THP-1 cells were cultured with either MOI 1 live or heat-killed tachyzoite, or MOI 1-equivalent quantities of either ESP or STAg. At 18 hr post-stimulation, IL-12 and IL-23 levels in culture supernatants were quantified. As shown in
Fig. 2B, THP-1 cells stimulated with the 4
T. gondii preparations secreted both IL-23 and IL-12. The levels of IL-23 and IL-12 were significantly increased by all 4
T. gondii preparations compared to non-stimulated (medium-treated) cell culture supernatants. IL-23 levels in the supernatants of cells infected with live parasites were significantly higher than those of cells exposed to heat-killed tachyzoites, ESP, or STAg (
P<0.01). However, IL-12 secretion in the live- and ESP-treated groups did not differ significantly. Stimulation of THP-1 cells with heat-killed parasites induced the lowest levels of IL-23 and IL-12 secretion. IL-23 production by THP-1 cells stimulated with the 4
T. gondii preparations were significantly higher than that of IL-12 at the same MOI (
P<0.05).
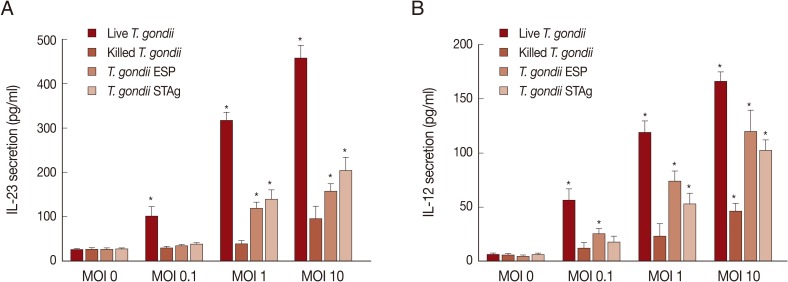
IL-23 is an essential cytokine involved in the expansion of Th17 cells, and it mediates
T. gondii-induced immunopathology [
9]. To identify the effects of the parasite on secretion of IL-23 and IL-12, THP-1 cells were cultured with various MOIs of live or heat-killed tachyzoites, or MOI-equivalent
T. gondii ESP or STAg, for 18 hr. As shown in
Fig. 3, IL-23 and IL-12 secretion increased with
T. gondii exposure in a dose-dependent manner, and IL-23 secretion was always higher than that of IL-12 at the same MOI. After stimulation with a dose equivalent to MOI 0.1 parasites, IL-23 and IL-12 secretion was induced significantly only by live parasites or ESP. High levels of IL-23 and IL-12 were also measured in the supernatant of THP-1 cells after stimulation with MOI 1 or 10 live or heat-killed
T. gondii or the equivalent ESP and STAg dose.
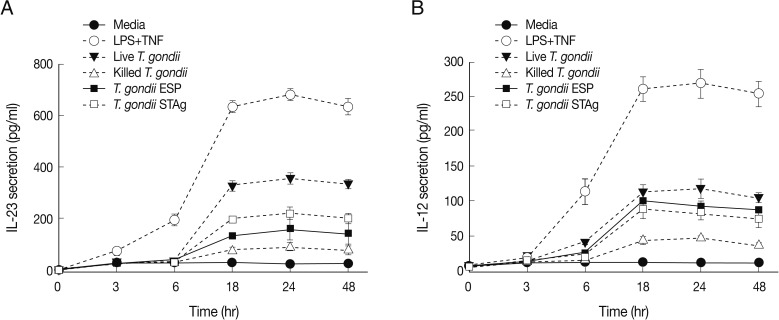
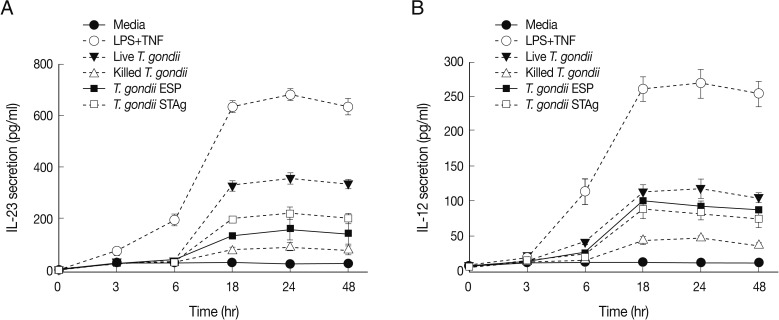
We performed time-course studies of IL-23 secretion after stimulation with the 4
T. gondii preparations in connection with IL-12 secretion. IL-23 was secreted 6-48 hr after stimulation with MOI 1 live or heat-killed parasite or quantities of either ESP or STAg corresponding to MOI 1 parasite (
Fig. 4). The general patterns of secretion of both IL-23 and IL-12 after stimulation with
T. gondii antigens were similar to those measured post-stimulation of THP-1 cells by LPS+TNF-α, but IL-12 was produced at lower levels than IL-23. In live
T. gondii-infected THP-1 cells, IL-23 production was markedly increased after 6 hr and peaked at 24 hr post-infection, remaining at a high level up to 48 hr. IL-23 production by both ESP- and STAg-stimulated cells peaked at 24 hr post-stimulation and then gradually decreased. Moreover, IL-23 and IL-12 production by heat-killed
T. gondii-stimulated cells was detected at low levels and peaked at 24 hr after stimulation (
Fig. 4A). IL-12 production by live
T. gondii-infected THP-1 cells significantly increased from 6 to 24 hr post-infection, and then decreased. In both ESP- and STAg-stimulated cells, IL-12 levels peaked at 18 hr and then gradually decreased (
Fig. 4B).
DISCUSSION
In the present study, we investigated the secretion profiles of IL-23 and IL-12 in THP-1 monocytic cells after stimulation with 4 T. gondii preparations. Live T. gondii, heat-killed tachyzoites, and T. gondii ESP and STAg triggered both IL-23 and IL-12 production by THP-1 cells in a parasite dose-dependent manner. The secretion patterns of IL-23 were similar to those of IL-12, even though IL-23 levels were significantly higher than those of IL-12 at the same MOI. Live T. gondii induced the highest levels of IL-23 and IL-12, whereas heat-killed T. gondii tachyzoites had the least effect. These data suggest that various types of T. gondii molecules could induce IL-23 and IL-12 secretions in monocytic cells. However, the kinetics of both cytokine productions were different from types of T. gondii antigens.
IL-23 is structurally similar to IL-12 and is likely to be important for the recruitment and activation of the range of inflammatory cells required for induction of chronic inflammation and granuloma formation [
10]. In the present study, we prepared 4 different types of
T. gondii antigens using the same number of parasites (
Fig. 1A, B). The amounts of IL-23 and IL-12 secretions of each type of antigen were detected at mRNA and protein levels. THP-1 cells stimulated with live
T. gondii, heat-killed tachyzoites, or
T. gondii ESP or STAg expressed transcripts coding for IL-23 and IL-23R, and IL-23 secretion was increased in a
T. gondii dose-dependent manner (
Fig. 2A). These results were similar to a previous report that IL-23/IL-23R expression was upregulated following infection with
T. gondii [
6]. Interestingly, IL-23 secretion was always much higher than IL-12 secretion at the same MOI (
Fig. 2B). Similar phenomena were also found after treatment with serum amyloid A [
11] and prostaglandin E
2 [
12]. He et al. [
11] reported that serum amyloid A-induced IL-12p40 production was accompanied by sustained expression of IL-23p19, but not IL-12p35, resulting in a preferential secretion of IL-23, but not IL-12. Also, Khayrullina et al. [
12] indicated that low IL-12 and high IL-23 production in bone marrow-derived DC generated in the presence of PGE
2 was associated with downregulation of p35 and upregulation of p19 expression, strongly favoring IL-23 secretion. Our data also presented that IL-23p19 mRNA expression levels after stimulation with 4 types of
T. gondii antigens were higher than those of IL-12p35 mRNA (
Fig. 1A). However, it was reported that IL-23 functionally had limited protective effects in the absence of IL-12 in
T. gondii-infected host cells, even though IL-23 secretion was higher than that of IL-12 [
5].
IL-12 and IL-23 are critical for generation of an effective cellular immune response. IL-12 may also contribute to maintaining immunity for IFN-γ induction [
13] and the activation of natural killer (NK) cells to produce IFN-γ as well as driving the proliferation of type 1 CD4
+ and CD8
+ T cells [
14]. Thus, Th1 responses rapidly wane in the absence of IL-12, leading to a loss in protective immunity against intracellular pathogens, such as
Leishmania and
Toxoplasma [
15]. IL-23 exerts its biological activities through the interaction with a heterodimeric receptor complex composed of IL-12Rb1 and IL-23R, and IL-23R is mainly expressed by T cells, NK cells, and to a lower extent by monocytes and DC populations [
16]. Increased IL-23 levels were associated with several inflammatory diseases, including inflammatory bowel disease and toxoplasmosis [
6,
17]. IL-23 mediates
T. gondii-induced immunopathology in small intestinal inflammation via matrix metalloproteinase-2 and IL-22, but independent of IL-17 [
9]. Moreover, increased IL-23 levels were detected in mice infected with
T. gondii, and in vitro stimulation of DCs with
T. gondii resulted in increased IL-23 mRNA levels [
6].
In this study, we examined the effects of various types of
T. gondii antigens to stimulate IL-23 and IL-12 secretions from THP-1 cells. As shown in
Fig. 3, IL-23 and IL-12 secretions were increased with
T. gondii exposure in a dose-dependent manner, and both cytokines were induced maximally by live parasites. Heat-killed
T. gondii induced low-level IL-23 and IL-12 secretions; however, increasing the dose of heat-killed
T. gondii led to increase levels of IL-23 and IL-12. These data imply that active invasion of cells is the most potent condition to produce both cytokines and some
T. gondii proteins were heat-stable at 55℃ for 50 min. However, Subauste et al. [
10] reported that IL-12 production by human monocyte-derived DCs was induced by viable
T. gondii, but not killed tachyzoites, suggesting that the immune system is capable of distinguishing between viable and killed
T. gondii tachyzoites through modulation of CD28 and CD40L signaling. The different results in IL-12 secretion by killed tachyzoites according to authors may be a result of different experimental systems; namely, we used the THP-1 monocytic cell line whereas Subauste et al. [
10] used human monocyte-derived DCs. Next, we checked the kinetics of IL-23 and IL-12 secretions from THP-1 cells at stimulation with various types of
T. gondii antigens. Both IL-23 and IL-12 productions by THP-1 cells were significantly increased from 6 hr after stimulation with all 4
T. gondii preparations. The general patterns of secretion of both IL-23 and IL-12 after stimulation with
T. gondii antigens were similar to those of LPS+TNF-α, but IL-12 was produced at lower levels than IL-23 (
Fig. 4). Like the same as
Fig. 3, live
T. gondii-infected THP-1 cells produced the highest levels of IL-23 and IL-12 secretion, whereas heat-killed
T. gondii led to the lowest levels of both cytokines. The time-course patterns of IL-12 secretion after stimulation with the 4
T. gondii preparations were similar to the results of Aldebert et al. [
17]. However, our study is the first on the kinetics of IL-23 secretion with stimulation by various types of
T. gondii antigens at THP-1 monocytic cells.
IL-23 and IL-12 work in concert to regulate cellular immune responses critical for host defense and tumor suppression. The secretion profiles of these interleukins by T. gondii-infected THP-1 monocytic cells have not been reported until now. Here, we showed that the secretion patterns of IL-23 and IL-12 were similar each other; however, the kinetics of both cytokine productions were different by types of T. gondii antigens. Further investigations are needed to clarify the regulation and signaling pathways involved in IL-23 production by human monocytes stimulated with T. gondii antigens and the interaction between IL-23 and IL-17 in T. gondii-infected host cells.
ACKNOWLEDGMENTS
This research was supported by Infection Signaling Network Research Center at Chungnam National University (2007-0054932), Basic Science Research Program (2011-0023501), and through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science & Technology. The authors have no commercial interest related with this study.
References
- 1. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000;30:1217-1258.
- 2. Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol 2009;39:23-39.
- 3. Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004;202:96-105.
- 4. Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1α and -1β in response to Toxoplasma gondii antigens. J Immunol 1999;162:7369-7375.
- 5. Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J Immunol 2004;172:3686-3694.
- 6. Lieberman LA, Cardillo F, Owyang AM, Rennick DM, Cua DJ, Kastelein RA, Hunter CA. IL-23 provides a limited mechanism of resistance to acute toxoplasmosis in the absence of IL-12. J Immunol 2004;173:1887-1893.
- 7. Aldebert D, Durand F, Mercier C, Brenier-Pinchart MP, Cesbron-Delauw MF, Pelloux H. Toxoplasma gondii triggers secretion of interleukin-12 but low level of interleukin-10 from the THP-1 human monocytic cell line. Cytokine 2007;37:206-211.
- 8. Son ES, Nam HW. Detection and characterization of excretory/secretory proteins from Toxoplasma gondii by monoclonal antibodies. Korean J Parasitol 2001;39:49-56.
- 9. Muñoz M, Heimesaat MM, Danker K, Struck D, Lohmann U, Plickert R, Bereswill S, Fischer A, Dunay IR, Wolk K, Loddenkemper C, Krell HW, Libert C, Lund LR, Frey O, Hölscher C, Iwakura Y, Ghilardi N, Ouyang W, Kamradt T, Sabat R, Liesenfeld O. Interleukin (IL)-23 mediates Toxoplasma gondii-induced immunopathology in the gut via matrix metalloproteinase-2 and IL-22 but independent of IL-17. J Exp Med 2009;206:3047-3059.
- 10. Subauste CS, Wessendarp M. Human dendritic cells discriminate between viable and killed Toxoplasma gondii tachyzoites: dendritic cell activation after infection with viable parasites results in CD28 and CD40 ligand signaling that controls IL-12-dependent and -independent T cell production of IFN-gamma. J Immunol 2000;165:1498-1505.
- 11. He R, Shepard LW, Chen J, Pan ZK, Ye RD. Serum amyloid A is an endogenous ligand that differentially induces IL-12 and IL-23. J Immunol 2006;177:4072-4079.
- 12. Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol 2008;181:721-735.
- 13. Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol 2006;177:31-35.
- 14. Reis e Sousa C, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med 1997;186:1819-1829.
- 15. Park AY, Scott P. IL-12: keeping cell-mediated immunity alive. Scand J Immunol 2001;53:529-532.
- 16. Boniface K, Blom B, Liu YJ, Waal Malefyt R. From interleukin-23 to T-helper 17 cells: human T-helper cell differentiation revisited. Immunol Rev 2008;226:132-146.
- 17. Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev 2008;226:147-159.
Fig. 1Protein levels of Toxoplasma gondii antigens. (A) Total protein from 1×108 live or heat-killed T. gondii tachyzoites, or 1×108 equivalent T. gondii excreted/secreted proteins (ESP) or soluble tachyzoite lysate antigens (STAg). Protein concentration was determined by the Bradford assay using bovine serum albumin (BSA) as the standard (upper). Live T. gondii, heat-killed T. gondii, and T. gondii ESP or STAg were analyzed by Western blotting using a T. gondii-specific anti-GRA6 mAb (bottom). (B) Coomassie blue staining of an SDS/PAGE gel showing protein bands in the lanes containing live T. gondii, heat-killed T. gondii, and T. gondii ESP and STAg. Data are representative of 2 independent replicates.
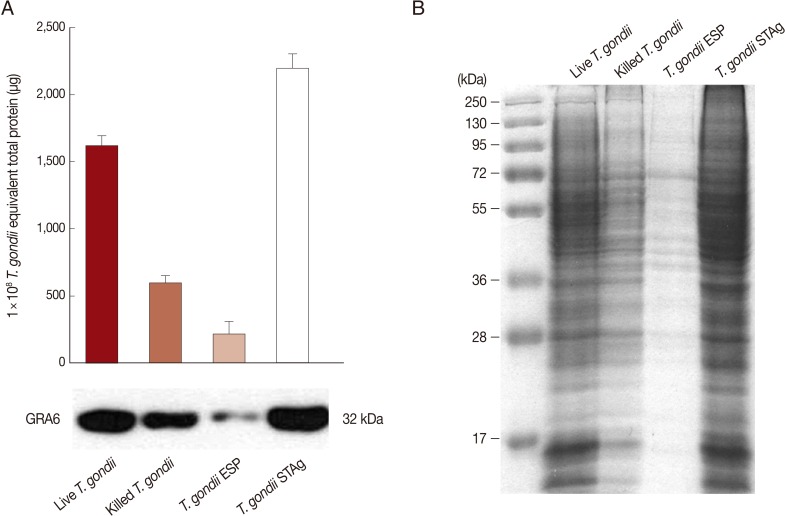
Fig. 2
T. gondii triggers secretion and expression of IL-23, IL-12, and their receptors. (A) THP-1 human monocytic cells were incubated in complete media alone, media with LPS+TNF-α, or stimulated with live T. gondii, heat-killed T. gondii, or T. gondii ESP or STAg. Cells were harvested 6 hr post-infection or post-stimulation, RNA was extracted, and IL-23, IL-23R, IL-12, IL-12R, and HPRT transcripts were amplified by RT-PCR and visualized on a 2% agarose gel. HPRT was used as a loading control. A representative of 3 independent replicates with similar results is shown. (B) THP-1 cells were incubated in complete media alone, media with LPS+TNF-α, or stimulated with live or killed T. gondii at a multiplicity of infection (MOI) 1 or MOI 1-equivalent T. gondii ESP or STAg. Culture supernatants were collected at 18 hr post-stimulation, and levels of both IL-23 and IL-12 were measured using specific ELISAs. Results represent the mean±SD from a representative of 3 independent experiments. LPS+TNF-α was used as a positive control (*P<0.05, compared to media-treated control). *Denotes P<0.05, as compared with media alone.

Fig. 3Effects of T. gondii antigen dose on IL-23 and IL-12 production. THP-1 cells were stimulated with either live or killed T. gondii at an MOI of 0, 0.1, 1, 10, or the MOI-equivalent of T. gondii ESP or STAg. Culture supernatants were collected at 18 hr post-stimulation and IL-23 (A) and IL-12 (B) levels were measured using specific ELISAs. Values represent the mean±SD of triplicate determinations. Results are 1 representative of 3 independent experiments. *Denotes P<0.05, as compared with non-stimulated MOI 0.
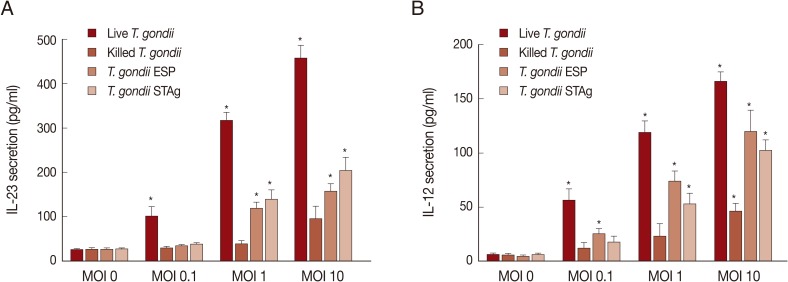
Fig. 4IL-23 and IL-12 secretion kinetics after stimulation with T. gondii antigens. One representative of 3 independent determinations of IL-23 (A) or IL-12 (B) secretion kinetics is shown. THP-1 cells were stimulated with live or killed T. gondii at an MOI 1 or MOI 1-equivalent of T. gondii ESP or STAg. Culture supernatants were collected at 0, 3, 6, 18, 24, and 48 hr post-stimulation. IL-12 and IL-23 levels were measured using specific ELISAs. Controls were complete culture media or culture media with LPS+TNF-α.