Abstract
The aim of this cross sectional case control study was to examine the serofrequency and serointensity of Toxoplasma gondii (Tg) IgG, IgM, and DNA among patients with schizophrenia. A total of 101 patients with schizophrenia and 55 healthy controls from Sungai Buloh Hospital, Selangor, Malaysia and University Malaya Medical Center (UMMC) were included in this study. The diagnosis of schizophrenia was made based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). The presence of Tg infection was examined using both indirect (ELISA) and direct (quantitative real-time PCR) detection methods by measuring Tg IgG and IgM and DNA, respectively. The serofrequency of Tg IgG antibodies (51.5%, 52/101) and DNA (32.67%, 33/101) among patients with schizophrenia was significantly higher than IgG (18.2%, 10/55) and DNA (3.64%, 2/55) of the controls (IgG, P=0.000, OD=4.8, CI=2.2-10.5; DNA, P=0.000, OD=12.9, CI=2.17-10.51). However, the Tg IgM antibody between patients with schizophrenia and controls was not significant (P>0.005). There was no significant difference (P>0.005) in both serointensity of Tg IgG and DNA between patients with schizophrenia and controls. These findings have further demonstrated the strong association between the active Tg infection and schizophrenia.
-
Key words: Toxoplasma gondii, schizophrenia, serofrequency, serointensity
INTRODUCTION
Schizophrenia is a chronic debilitating psychiatric disorder. Despite many studies examining the etiology of schizophrenia the exact cause remains unknown. The evidence of infection as a cause of schizophrenia was supported by the brain histopathological changes found in patients with schizophrenia [
1-
3]. Studies had documented that astrocytes and neurons of the brain could be infected by
Toxoplasma gondii (
Tg). The infection then stimulated the production of a variety of cytokines by microglia, astrocytes, and neurons which in turn initiated inflammatory responses [
4,
5]. The parasites formed cysts within the brain and produce an enzyme called tyrosine hydroxylase, which was needed for dopamine production [
4-
6]. Dopamine’s role in schizophrenia is well documented. The tyrosine hydroxylase converted L-Dopa to dopamine. An excess of dopamine as a pathological basis for schizophrenia was substantiated by the fact that antipsychotic drugs decreased the brain dopamine thus reducing the symptoms of schizophrenia [
7-
9]. In addition, antipsychotics inhibited the replication of
Tg [
10,
11].
Tg infection of the brain increased levels of dopamine [
12-
16] and caused psychotic symptoms resembling schizophrenia [
17,
18]. Artemether, an antiparasitic agent, significantly reduced negative symptoms of schizophrenia compared to controls [
19].
Almost all previous studies concerning the association of
Tg infection and schizophrenia were based on indirect measurement by examining antibodies, i.e., IgG and IgM [
20-
23], and the results were inconclusive [
24]. Whilst some studies [
24-
27] demonstrated positive associations, others found the contrary [
28,
29]. The purpose of this study was, therefore, to specifically appraise the relationship between schizophrenia and
Tg infection using both indirect and direct methods by measuring
Tg antibodies and DNA, respectively, in comparison to the controls. The findings would further help in understanding the etiology of schizophrenia which in turn would contribute to the preventive measures and development of a new pharmacological treatment approach for schizophrenia.
MATERIALS AND METHODS
Subjects
This was a cross-sectional case control study examining the serofrequency and serointensity of
Tg among patients with schizophrenia and controls, assessed through indirect and direct methods by measuring
Tg IgG and IgM antibodies and DNA, respectively. A total of 101 patients with schizophrenia and healthy individuals as controls (n=55) attending Sungai Buloh Hospital, Selangor, Malaysia and University Malaya Medical Center (UMMC) who fulfilled the criteria were recruited in this study. Controls were recruited from all consecutive patients attending medical out-patient clinic comprised of patients with chronic hypertension and diabetic with no psychiatry illness. The diagnosis of schizophrenia was made by psychiatrists using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), American Psychiatric Association, 1994 [
30]. The ethical approvals were obtained from Universiti Teknologi Mara (UiTM), [UiTM 600-RMI(5/1/6)], UMMC (UMMC 932.46), and National Medical Register Research (NMRR), (NMRR-10-852-6764) Ethics Committees. The purpose of the study was explained and a written consent was obtained from patients or the legal guardians. Their demographic data were also recorded.
Five ml of blood samples were collected, centrifuged at 1,500 rpm in 15 min at 4˚C, and stored at -20˚C. Tg IgG and IgM antibodies and DNA were measured using ELISA (IBL Company, Hamburg, Germany) and quantitative real-time PCR (qPCR), respectively. The extraction of DNA was performed using QIAamp® Blood Mini Kit from Qiagen (Hilden, Germany). The tests were all conducted according to the instructions from the manufacturers. Tg IgG and IgM antibodies were measured from serum using the commercial ELISA kit according to the manufacturer’s instructions. Samples absorbance were read using the microtiter plate reader at absorbance of 450/620 nm. Patients and control sera were obtained from blood at the same time as the interviews. Each sample was done triplicate to ensure the reliability of results and the experiments were done in sterile conditions. Positive results were recorded when the quantity of antibodies of IgG and IgM were more than 35 IU/ml and 11 IU/ml, respectively.
The forward primer for qPCR was (5´-TCCCCTCTGCTGGCGAAAAGT-3´), whilst (5´-AGCGTTCGTGGTCAACTATCGATTG-3´) was the reverse primer and (5´6FAM-TCTGTGCAACTTTGGTGTATTCGCA-BHQ1-3´) was the probe primer. A 8-μl of template DNA was added to the final volume of 25 μl reaction mixture, which consists of 6.5 μl of iTAQ Universal Probes Supermix, 0.25 μl (20 μM) Taqman probe, 0.625 μl (20 μM) of each primer, and distilled water. The amplification processes were performed using the Bio-Rad CFX96 machine (Bio-Rad, Hercules, California, USA). The PCR cycling condition was done at 95˚C for 10 min (initial denaturation), followed by 40 cycles at 95˚C for 15 sec (further denaturation), 60˚C for 1 min (annealing), they were then hold for 10˚C. Several sets of PCR amplification using 6 different concentrations of positive samples were performed to calibrate the real-time PCR condition in order to obtain a standard curve with R2 more than 85% and Cq standard deviation of less than 0.5. The quantity of the gene was determined using the cycle threshold value (CT value) by BIORAD CFX Software Version 2.1 (Bio-Rad).
Statistical analysis
The results were analyzed using Statistical Package for Social Sciences (SPSS) version 20 (Chicago, Illinois, USA). The serofrequency of Tg IgG and IgM antibodies were calculated by descriptive statistics (frequencies), and the differences between the groups were calculated using the chi-square or Fisher’s exact test. The differences in medians were compared using the Mann-Whitney U test. The odd ratio (OR) and its 95% Confident Interval (CI) were used to estimate the strength of the association between Tg infection and schizophrenia.
RESULTS
The demographic profiles of the patients with schizopherenia (n=101) and controls (n=55) were comparable in terms of age, gender, and ethnicity (
Table 1). The mean ages of controls and patients with schizophrenia were 45.3±14.5 years (range; 21-63 years) and 41.1±10.9 years (18-65 years), respectively. The duration of illness for patients with schizophrenia was 6.5±4.9 years (1.0-13.5 years). Unemployment was significantly (
P=0.01) higher among patients with schizophrenia (63.2%) compared to controls (50.9%). The majority of patients with schizophrenia were single (56.1%) compared to controls (27.3%) (
P=0.0005). Patients with schizophrenia had a lower level of education (
P=0.001) and income (
P=0.0005) than controls. The seropositivity and seronegativity of
Tg IgG, IgM, and DNA are shown in
Table 2.
Tg IgG antibody was positive in 52 (51.5%) and 10 (18.2%) of patients with schizophrenia and controls, respectively (
P=0.0005, OD=4.78, 95% CI=2.17-10.51). Meanwhile, the IgM antibody was found in 4 (7.7%) of patients with schizophrenia and none (0%) in controls (OR=3.97, P=0.34, CI= 0.20-78.25). DNA for
Tg was positive in 33 (32.67%) of patients with schizophrenia and 2 (3.64%) of controls (
P= 0.0005, OD=12.86, CI=2.95-56.03).
Table 3 shows the serointensity of
Tg IgG, IgM, and DNA among patients and controls. The levels of
Tg IgG antibody were not significantly different (
P>0.005) in patients with schizophrenia (214.6±163.1 IU/ml) compared to controls (138.4±83.6 IU/ml,
P=0.06). There was no significant difference between
Tg DNA levels of patients with schizophrenia (0.09 ng/ml) vs controls (0.03± 0.65 ng/ml) (
P=0.23).
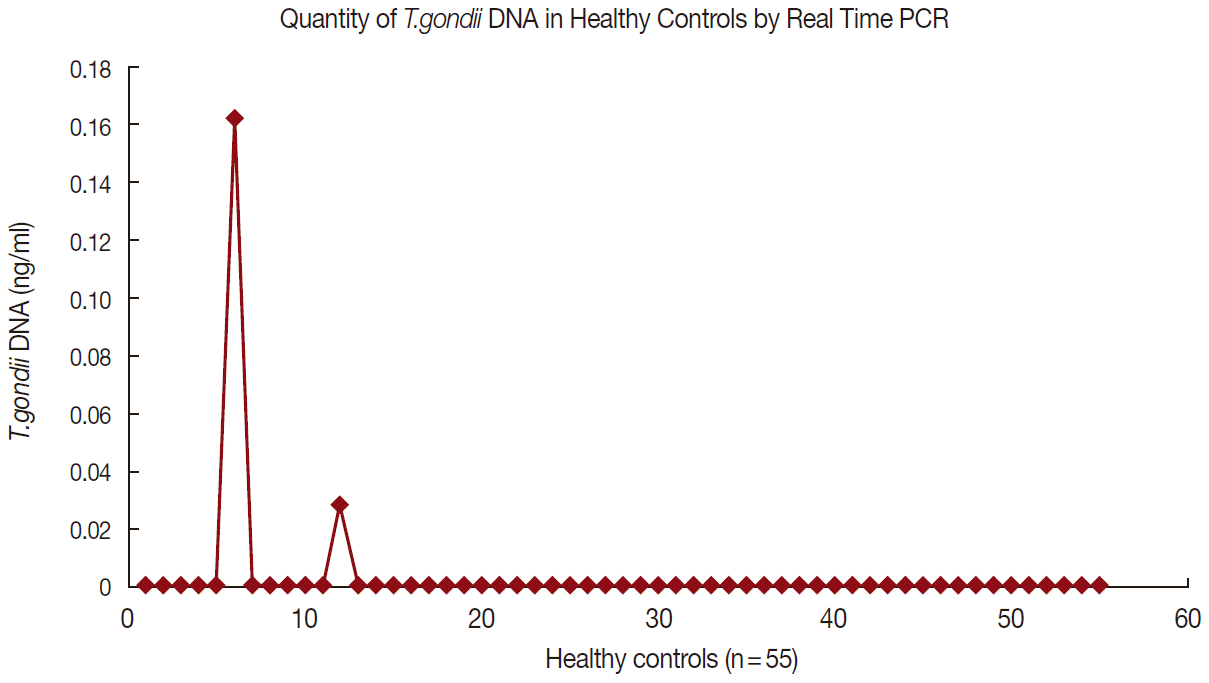
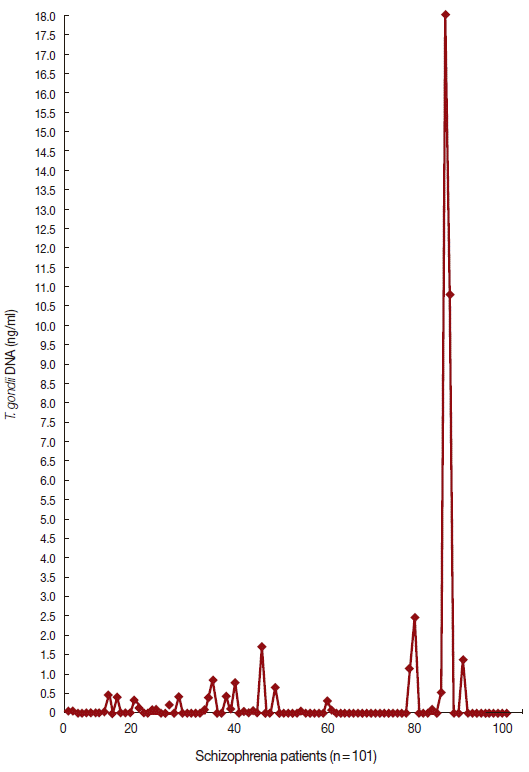
Figs. 1 and
2 show the data of qPCR in controls and patients with schizophrenia, respectively.
DISCUSSION
Our findings showed that there were significant differences between seropositivity of
Tg IgG antibody and DNA among patients with schizophrenia compared to controls, suggesting the important role of
Tg infection in schizophrenia. These results were consistent with previous studies which found a positive association between
Tg IgG antibody and schizophrenia [
20,
23-
25,
31,
32]. The majority of previous studies determined the presence of
Tg infection through an indirect method (using ELISA) by measuring
Tg IgG and IgM antibodies. There was, however, no study using real-time qPCR to examine the association between schizophrenia and
Tg infection. The strength of this study was the use of both indirect and direct methods for examining the presence of
Tg antibodies and DNA, respectively.
The positivity of
Tg DNA was significantly higher in patients with schizophrenia than controls with the OR of 12.9. The average OR of previous studies (based on positivity of antibodies) over 5 decades in 17 countries demonstrated an average OR of 2.54 [
20,
33]. In our study, the OR of having schizophrenia among those with positive
Tg DNA (OR=12.9) was much higher than those with positive
Tg IgG antibody (OR=4.8). The high OR of
Tg infection in schizophrenia measured by
Tg DNA in our study indicated that the risk of having schizophrenia among those infected with
Tg was much higher than previously considered, signifying that there was a strong relationship between
Tg and schizophrenia. The finding also suggested that acute infections of
Tg could specifically play an important role in schizophrenia. The detection of
Tg infection through direct measurement of DNA was more reliable because it signified the active
Tg infection, whereas the seropositivity of
Tg IgG would merely indicate the presence of antibodies (as a result of exposure to
Tg).
There was no significant difference between
Tg IgM antibody in patients with schizophrenia and controls. This finding was consistent with previous studies [
34-
36]. This could probably be due to the fact that patients often present late to the hospital. The presence of IgM antibodies is time-dependent, and IgM is secreted immediately following infection and would remain positive for 1 or 2 weeks indicating a recent infection. This issue was addressed in this study by examining
Tg DNA which reflected the presence of an acute
Tg infection.
Although there was significant difference in the seropositivity of
Tg IgG and
Tg DNA between the 2 groups, however, there was no significant difference between the serointensity of
Tg IgG antibody and DNA between schizophrenia patients and controls. These could possibly be due to the effects of antipsychotic medications which caused the reduction in the level of
Tg IgG antibody and DNA by inhibiting the replication of
Tg [
10,
11]. The antibody levels for untreated patients with schizophrenia were found to be the highest followed by the treated and controls [
34]. The effects of antipsychotic drugs on
Tg IgG and DNA levels could be addressed by examining patients with schizophrenia at acute stage, those with the first episode or drug naïve. Secondly, the level of IgG was related to timing of infection and individual body response.
Although our study had strongly demonstrated the association between Tg infection and schizophrenia, a more comprehensive research is required to consolidate these findings. A cohort study involving drug naïve schizophrenia patients would be critical in demonstrating the specificity of the association and would further enlighten the etiological role of Tg in schizophrenia. The clinical factors such as the stages of illness, presentation of symptom, sub-types of schizophrenia as well as treated and non-treated cases should also be considered and addressed.
There were some limitations in this study namely it was a cross-sectional study and there was no comparison to other types of psychosis. A prospective case control study involving a larger sample would be of great help in examining this association. Nevertheless, our study showed that there was a highly significant association between Tg infection and schizophrenia. The strength of the association between Tg infection and schizophrenia was much higher than previously considered. Identifying Tg roles in schizophrenia would help in the preventive measures and pharmacological approaches in the treatment of schizophrenia.
Notes
-
We have no conflict of interest related to this study.
We would like to express our deepest gratitude to the Director, Head of Department of Psychiatry, staff of Sungai Buloh Hospital, and Head of Department and staff of University Malaya Medical Centre. This work was financially supported by the Fundamental Research Grant Scheme (FRGS), grant no. FRGS/1/10/SP/UiTM/01/9.
Fig. 1.Quantity of T. gondii DNA in healthy controls by real-time PCR.
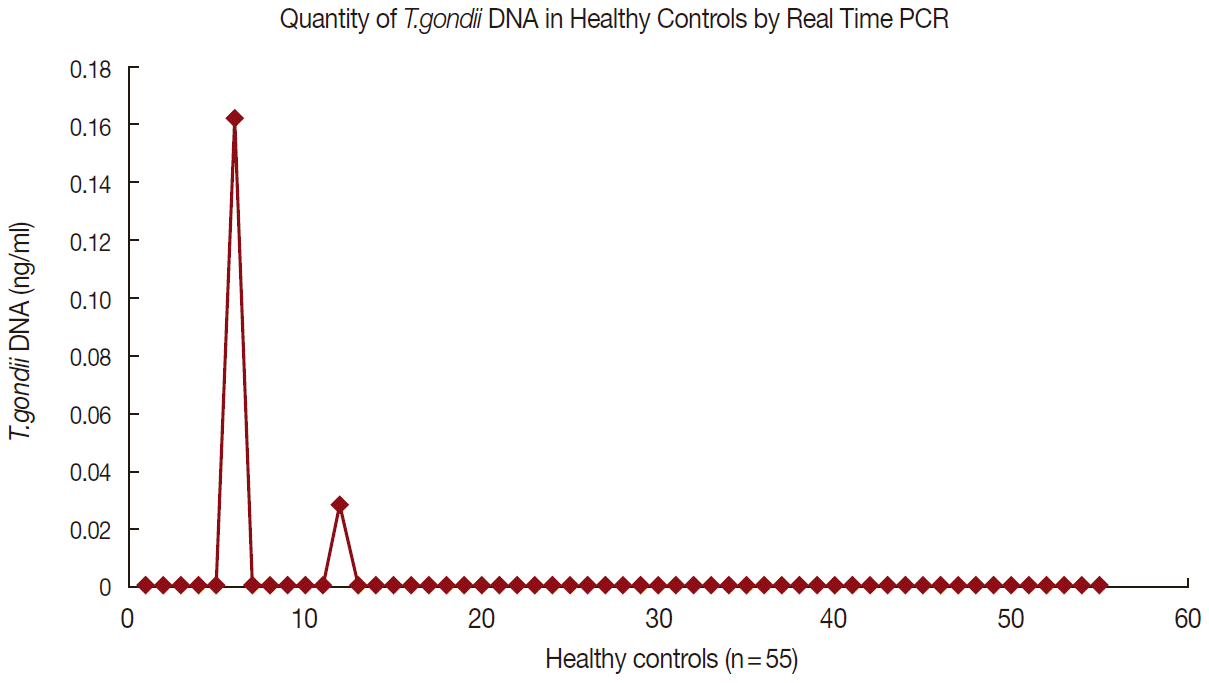
Fig. 2.Quantity of T. gondii DNA in schizophrenia patients by realtime PCR.
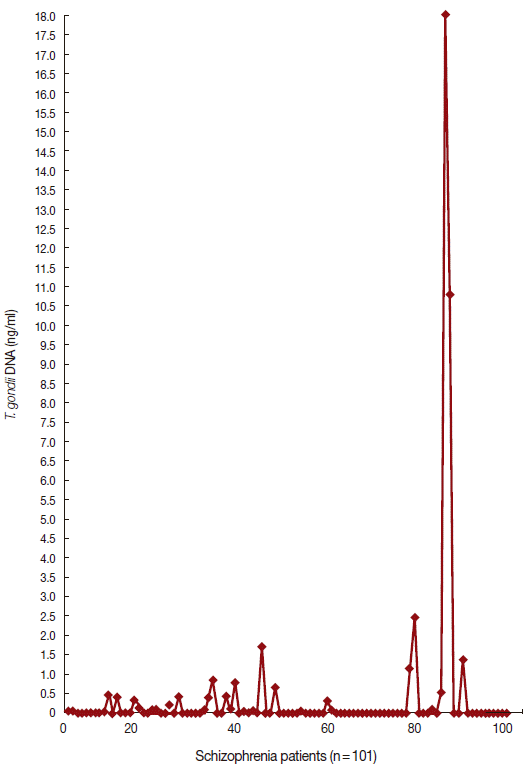
Table 1.Demographic profiles of patients with schizophrenia and control
Table 1.
|
Demographic profile |
|
Control (n=55) |
Schizophrenia (n=101) |
Chi square value |
P-value |
|
Age |
Mean (SD) |
45.3 ± 14.5 |
41.1 ± 10.9 |
1.86 |
0.07 |
|
Range |
21-63 years |
|
|
18-65 years |
|
|
|
|
Duration of Illness |
Mean (SD) |
|
|
|
6.5 ± 4.9 |
|
|
|
|
Range |
|
|
|
1-13.5 years |
|
|
|
|
|
Control (n=55)
|
Schizophrenia (n=101)
|
Chi square value
|
P-value
|
|
|
n
|
(%)
|
n
|
(%)
|
|
|
Occupation |
Employed |
27 |
|
49.1 |
28 |
36.8 |
7.12 |
0.01 |
|
Unemployed |
28 |
|
50.9 |
73 |
63.2 |
|
|
|
Gender |
Male |
24 |
43.6 |
|
58 |
52.6 |
2.72 |
0.10 |
|
Female |
31 |
56.4 |
|
43 |
47.4 |
|
|
|
Ethnicity |
Malay |
22 |
40.0 |
|
45 |
40.4 |
0.33 |
0.85 |
|
Chinese |
20 |
36.4 |
|
35 |
42.1 |
|
|
|
Indian |
13 |
23.6 |
|
21 |
17.5 |
|
|
|
Marital status |
Married |
38 |
69.1 |
|
30 |
35.1 |
22.47 |
0.0005 |
|
Single/Widowed/divorced |
17 |
27.3 |
|
71 |
56.1 |
|
|
|
Education level |
Upper Secondary School and above |
39 |
70.9 |
|
44 |
43.9 |
10.69 |
0.001 |
|
Lower Secondary School and below |
16 |
29.1 |
|
57 |
56.1 |
|
|
|
Family Income per month |
< RM2000 |
25 |
|
25.5 |
92 |
64.9 |
39.55 |
0.0005 |
|
RM2000 and above |
30 |
|
74.5 |
9 |
35.1 |
|
|
Table 2.Seropositivity and seronegativity of T. gondii IgG and IgM antibodies and DNA among patients with schizophrenia and control
Table 2.
|
Immunoglobulin and DNA |
Control (n=55) |
Schizophrenia (n=101) |
P-value |
Chi-square |
OR (95% CI) |
|
IgG positive |
10 |
52 |
0.0005 |
16.43 |
4.78 (2.17-10.51) |
|
IgG negative |
45 |
49 |
|
|
|
|
IgM positive |
0 |
4 |
0.34 |
0.93 |
3.97 (0.20-78.25) |
|
IgM negative |
55 |
97 |
|
|
|
|
DNA positive |
2 |
33 |
0.0005 |
17.25 |
12.86 (2.95-56.03) |
|
DNA negative |
53 |
68 |
|
|
|
Table 3.Serointensity of Toxoplasma gondii IgG and IgM antibodies and DNA among patients with schizophrenia and control
Table 3.
|
Serointensity of antibodies/DNA |
Control (n=55)
|
Schizophrenia patient (n=101)
|
P-value |
Test value |
|
Median |
Range |
IQR |
Median |
Range |
IQR |
|
IgG IU/ml |
138.38 |
214.58 |
83.64 |
214.58 |
441.53 |
163.07 |
0.063 |
1.86 |
|
IgM IU/ml |
NA |
NA |
NA |
42.03 |
71.43 |
65.18 |
NA |
NA |
|
DNA ng/ml |
0.09 |
0.13 |
NA |
0.30 |
21.68 |
0.65 |
0.227 |
1.208 |
References
- 1. Cotter DR, Pariante CM, Everall IP. Glial cell abnormalities in major psychiatric disorders: the evidence and implications. Brain Res Bull 2001;55:585-595.
- 2. Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol 2002;22:25-33.
- 3. Horacek J, Flegr J, Tintera J, Verebova K, Spaniel F, Novak T, Brunovsky M, Bubenikova-Valesova V, Holub D, Palenicek T, Höschi C. Latent toxoplasmosis reduces gray matter density in schizophrenia but not in controls: voxel-based-morphometry (VBM) study. World J Biol Psychiatry 2012;13:501-509.
- 4. Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophrenia Bull 2007;33:745-751.
- 5. Prandovszky E, Gaskell E, Martin H, Dubey JP, Webster JP, McConkey GA. The neurotrophic parasite Toxoplasma gondii increases dopamine metabolism. PLoS One 2011;6:e23866.
- 6. McConkey GA, Martin HL, Bristow GC, Webster JP. Toxoplasma gondii infection and behaviour-location, location, location? J Exp Biol 2013;216:113-119.
- 7. Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. J Neuropsychiatry Clin Neurosci 1996;8:223-226.
- 8. Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse 1987;1:133-152.
- 9. Richtand NM, Welge JA, Loque AD, Keck PE Jr, Strakowski SM, McNamara RK. Dopamine and serotonin receptor binding and antipsychotic efficacy. Neuropsychopharmacology 2007;32:1715-1726.
- 10. Jones-Brando L, Torrey EF, Yolken R. Drugs used in the treatment of schizophrenia and bipolar disorder inhibit the replication of Toxoplasma gondii. Schizophr Res 2003;62:237-244.
- 11. Webster JP, Lamberton PHL, Donnelly CA, Torrey EF. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviour. Proc Biol Soc 2006;273:1023-1030.
- 12. Stibbs HH. Changes in brain concentrations of catecholamines and indolamines in Toxoplasma gondii infected mice. Ann Trop Med Parasitol 1985;79:153-157.
- 13. Petitto JM, McCarthy DB, Rinker CM, Huang Z, Getty T. Modulation of behavioral and neurochemical measures of forebrain dopamine function in mice by species-specific interleukin-2. J Neuroimmunol 1997;73:183-190.
- 14. Gatkowska J, Wieczorek M, Dziadek B, Dzitko K, Dlugonska H. Sex-dependent neurotransmitter level changes in brains of Toxoplasma gondii infected mice. Exp Parasitol 2013;133:1-7.
- 15. Flegr J. How and why Toxoplasma makes us crazy. Trends Parasitol 2013;29:156-163.
- 16. Xiao J, Li Y, Prandovszky E, Karuppagounder SS, Talbot CC Jr, Dawson VL, Dawson TM, Yolken RH. MicroRNA-132 dysregulation in Toxoplasma gondii infection has implications for dopamine signaling pathway. Neuroscience 2014;268:128-138.
- 17. Torrey EF, Yolken RH. Toxoplasma gondii and schizophrenia. Emerg Infect Dis 2003;9:1375-1380.
- 18. Wang T, Tang ZH, Li JF, Li XN, Wang X, Zhoa ZJ. A potential association between Toxoplasma gondii infection and schizophrenia in mouse models. Exp Parasitol 2013;135:497-502.
- 19. Wang HL, Xiang YT, Li QY, Wang XP, Liu ZC, Hao SS, Liu X, Liu LL, Wang GH, Wang DG, Zhang PA, Bao AY, Chiu HF, Ungvari GS, Lai KY, Buchanan RW. The effect of artemether on psychotic symptoms and cognitive impairment in first-episode, antipsychotic drug-naïve persons with schizophrenia seropositive to Toxoplasma gondii. J Psychiatr Res 2014;53:119-124.
- 20. Torrey EF, Bartko JJ, Lun ZR, Yolken RH. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 2007;33:729-736.
- 21. Ahmad D, Mehdi S, Sayed HH, Sayed AK, Shizad G. Serological survey of Toxoplasma gondii in schizophrenia patients referred to Psychiatric Hospital, Sari City, Iran. Trop Biomed 2010;27:476-482.
- 22. Tedla Y, Shibre T, Ali O, Tadele G, Woldeamanuel Y, Asrat D, Aseffa A, Mihret W, Abebe M, Alem A, Medhin G, Habte A. Serum antibodies to Toxoplasma gondii and Herpesvidae family viruses in individuals with schizophrenia and bipolar disorder: a case-control study. Ethiop Med J 2011;49:211-220.
- 23. Arias I, Sorlozano A, Villegas E, de Dios Luna J, McKenney K, Cervilla J, Gutierrez B, Gutierrez J. Infectious agents associated with schizophrenia: a meta-analysis. Schizophr Res 2012;136:128-136.
- 24. Alvarado-Esquivel C, Urbina-Álvarez JB, Estrada-Martinez S, Toress-Castorena A, Molotla-de-León G, Liesenfeld O, Dubey JP. Toxoplasma gondii infection and schizophrenia: a case control study in a low Toxoplasma seroprevalence Mexican population. Parasitol Int 2011;60:151-155.
- 25. Cetinkaya ZS, Yazar S, Gecici O, Namli MN. Anti-Toxoplasma gondii antibodies in patients with schizophrenia- preliminary findings in a Turkish sample. Schizophr Bull 2007;33:789-791.
- 26. Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among U.S. military personnel. Am J Psychiatry 2008;165:99-106.
- 27. Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull 2012;38:642-647.
- 28. Thomas HV, Thomas DR, Salmon RL, Lewis G, Smith AP. Toxoplasma and Coxiella infection and psychiatric morbidity: a retrospective cohort analysis. BMC Psychiatry 2004;4:32.
- 29. Xiao Y, Yin J, Jiang N, Xiang M, Hao L, Lu H, Sang H, Liu X, Ankarklev J, Lindh J, Chen Q. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis 2010;10:4.
- 30. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-4. 4th ed. Washington DC, USA. American Psychiatric Press; ISBN: 0-89042-062-9. 1994, p 886.
- 31. Yolken RH, Dickerson FB, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol 2009;31:706-715.
- 32. Hamidinejat H, Ghorbanpoor M, Hosseini M, Alavi SM, Nabavi L, Jalali MHR, Borojeni MP, Jafari H, Mohammadaligol S. Toxoplasma gondii infection in first-episode and inpatient individuals with schizophrenia. Int J Infect Dis 2010;14:e978-e981.
- 33. Pearce BD, Hubbard S, Rivera HN, Wilkins PP, Fisch MC, Hopkins MH, Hasenkamp W, Gross R, Bilwise N, Jones JL, Duncan E. Toxoplasma gondii exposure affects neural processing speed as measured by acoustic startle latency in schizophrenia and controls. Schizophr Res 2013;150:258-261.
- 34. Leweke FM, Gerth CW, Koethe D, Klosterkötter J, Ruslanova I, Krivogorsky B, Torrey EF, Yolken RH. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci 2004;254:4-8.
- 35. Tamer GS, Dundar D, Yalug I, Caliskan S, Yazar S, Aker A. The schizophrenia and Toxoplasma gondii connection: infectious, immune or both? Adv Ther 2008;25:703-709.
- 36. Juanah LY, Jalaludin J, Osman M, Osman ZJ. Seroprevalence of Toxoplasma gondii among schizophrenics at Hospital Kajang. Am J Infect Dis 2013;9:11-16.