Abstract
Serologic tests are widely accepted for diagnosing Toxoplasma gondii but purification and standardization of antigen needs to be improved. Recently, surface tachyzoite and bradyzoite antigens have become more attractive for this purpose. In this study, diagnostic usefulness of 3 recombinant antigens (SAG1, SAG2, and SAG3) were evaluated, and their efficacy was compared with the available commercial ELISA. The recombinant plasmids were transformed to JM109 strain of Escherichia coli, and the recombinants were expressed and purified. Recombinant SAG1, SAG2, and SAG3 antigens were evaluated using different groups of sera in an ELISA system, and the results were compared to those of a commercial IgG and IgM ELISA kit. The sensitivity and specificity of recombinant surface antigens for detection of anti-Toxoplasma IgG in comparison with commercially available ELISA were as follows: SAG1 (93.6% and 92.9%), SAG2 (100.0% and 89.4%), and SAG3 (95.4% and 91.2%), respectively. A high degree of agreement (96.9%) was observed between recombinant SAG2 and commercial ELISA in terms of detecting IgG anti-Toxoplasma antibodies. P22 had the best performance in detecting anti-Toxoplasma IgM in comparison with the other 2 recombinant antigens. Recombinant SAG1, SAG2, and SAG3 could all be used for diagnosis of IgG-specific antibodies against T. gondii.
-
Key words: Toxoplasma gondii, recombinant antigen, toxoplasmosis, SAG1, SAG2, SAG3
INTRODUCTION
Toxoplasma gondii is an important human pathogen with worldwide distribution. Diagnosis of this infection in humans is very crucial, especially in immunosuppressed patients [
1] and pregnant women, since this parasite can be transmitted from a recently infected mother to her fetus [
2,
3].
Immunodominant surface antigen of
T. gondii; SAG1 (P30), is considered an important antigen for development of effective diagnostic tests and subunit vaccines [
4,
5]. SAG1 is stage-specific and only detectable in tachyzoite stage of the parasite [
6]. The surface antigen 2, SAG2 (P22), is another protein that has been shown to be an attachment ligand and also to have good immunogenicity [
7,
8]. Monoclonal antibodies raised against these 2 major tachyzoite antigens (SAG1, SAG2) are 100-fold more reactive with the tachyzoite stage than with oocyst (sporozoite) and bradyzoite stages [
9]. Recombinant surface antigens like SAG1 or SAG2 are useful in serodiagnosis of toxoplasmosis in cats [
10,
11] and humans [
1,
12]. The surface antigen 3, SAG3 (P43), is found in both tachyzoites and bradyzoites, and like SAG1, it is anchored to the membrane via glycosylphosphatidylinositol groups. It is very similar to SAG1 in structure and function, and SAG1 and SAG3 proteins participate in cellular invasion and attachment [
13,
14,
15]. It has been shown that mice immunized with recombinant SAG3 produce significantly more specific IgG2a antibodies [
16].
The diagnosis of acute infection depends on detection of
Toxoplasma-specific IgG and IgM antibodies [
17]. IgG avidity test and IgM analysis are considered to be suitable methods for determining acute infections. Recently, recombinant fusion proteins have been used in an attempt to improve serologic tests for diagnosis of
T. gondii [
18,
19,
20].
In this study, we used 3 recombinant antigens of T. gondii (SAG1, SAG2, and SAG3) to evaluate their diagnostic usefulness and efficacy compared with the commercially available ELISA for diagnosis of toxoplasmosis and improvement of identification methods.
MATERIALS AND METHODS
Gene confirmation
SAG1, SAG2, and SAG3 genes were cloned in pQE30, pGMEX1, and pGMEX1 vectors, respectively, and sequenced by Kazemi and Arabpour method [
21,
22,
23]. pGEM43, pGEM22, and pQE30 containing P43, P22, and P30 genes were confirmed by PCR using Tox43, Tox22, and Tox30 primers [
21,
22,
23]. PCR products were electrophoresed on 1.5% agarose gel, stained with ethidium bromide, and visualized under ultraviolet. PCR products with 1,158, 957, and 560 bp bands confirmed the insertion genes, respectively.
The recombinant plasmids pGEM43, pGEM22, and pQE30 were transformed into
Escherichia coli JM109 competent cells using Hanahan method [
24]. The reaction was spread on LB agar plate containing 50 µg/ml ampicillin.
A single colony was cultured in LB medium (Merck, Darmstadt, Germany) containing 100 µg/ml ampicillin and was incubated overnight at 37℃. It was then sub-cultured using 10-fold fresh LB medium containing ampicillin at 37℃. The cells were grown to OD
600=0.6 at 37℃ with 200 rpm shaking. The plasmid promoter was induced by 1 mM iso-propyl-D-thiogalactopyranoside (IPTG) for 5 hr and mass cultivated in LB medium. The bacterial cells were harvested by 20,000 g centrifugation for 15 min, and expressed recombinant P43 and P22 proteins were purified using T7-affinity chromatography (Novagen, Foster City, California, USA) according to the manufacturers' instruction with some modifications. The cell pellet was extracted from 25 ml of liquid culture and resuspended in 5 ml of equilibrating buffer with 10 mM of PMSF (Sigma, St. Louis, Missouri, USA) on ice for 2 hr. The suspension was then sonicated and centrifuged (700 g for 15 min at 4℃). The supernatant was transferred to a column containing 2 ml of equilibrated resin. The column was incubated overnight at 4℃ and then washed with 10 ml washing buffer (KH
2PO
4, Na
2HPO
4, KCl, NaCl, and Tween 20). Finally, elution buffer was added (citric acid, 1 mol/L) and incubated for 2 hr, the recombinant P43 and P22 proteins were eluted and neutralized with Tris-HCl 1 mol/L), and the protein concentration was measured by a biophotometer (Eppendorf, Hamburg, Germany). The P30 recombinant protein was purified using Ni-affinity chromatography (Novagen, Madison, Wisconsin, USA) according to the manufacturer's instruction with some modifications [
25]. The recombinant proteins were analyzed by SDS-PAGE and western blotting as in previous studies [
22,
25,
26].
Serum samples were collected from different laboratories around Shiraz and Tehran using commercial IgG and IgM ELISA kits (Euroimmun, Lubeck, Germany). For evaluation of recombinant IgG ELISA, 27 cases with infections other than toxoplasmosis, 30 sera from healthy individuals, and 110 anti-T. gondii IgG positive cases were used. The cut-off point was set at mean±2SD of 20 anti-Toxoplasma IgG negative serum samples. For determination of anti-T. gondii IgM, 30 negative and 28 positive samples were applied. The cut-off point was determined by calculating mean and SDs for 30 IgM and IgG negative serum samples.
Flat-bottomed 96-well microtiter plates (Greiner Bio One, Frickenhausen, Germany) were coated with 3 µg/ml of each of P22, P30, and P43 antigens in 50 mM carbonate bicarbonate buffer (Na2Co3: 15 mM, NaHCo3: 35 mM) (pH=9.6) and incubated overnight at 4℃. The plate was washed 3 times with PBS-T. After blocking with 300 µl of 1% bovine serum albumin in PBS-T for 2 hr, the plates were washed as before and 100 µl of sera (1:200 dilution in PBS-T with 1% bovine serum albumin) was added to each well. The plates were incubated for 1.5 hr and washed as before.
The plates were incubated with 100 µl of peroxidase-conjugated goat anti-human IgG and IgM (1:20,000 and 1:10,000 dilutions, respectively) and were incubated for 1 hr, washed, and then 100 µl of TMB (Sigma) was added. After 15 min of incubation at room temperature, the reaction was stopped using 50 µl of 2N H2SO4 as a stopping solution. Finally, the optical density was measured by an ELISA reader (Sunrise-can, Mannedorf, Switzerland).
Statistical analysis
The sensitivity and specificity were calculated from TP/(TP+FN)×100, and TN/(TN+FP)×100, respectively. Positive predictive and negative predictive values were achieved from TP/(TP+FP) and TN/(TN+FN), respectively. Agreement was calculated as follows: (TP+TN)/(TP+TN+FP+FN)×100 (TP, TN, FP, and FN represent the number of true positive, true negative, false positive, false negative, respectively). All results were assessed by software program of the website URL (
http://vassarstats.net/clin1.html).
RESULTS
Recombinant plasmids containing P22, P30, and P43 genes were confirmed by PCR and the corresponding bands of 958, 560, and 1,158 bp PCR products were visualized on 1.5% of agarose gel, respectively.
IgG ELISA with recombinant SAG1
The first set of samples consisting of 110 IgG positive, 30 IgG negative, and 27 non-relative serum samples were evaluated by SAG1 antigen. The cut-off point was 0.31. Out of 110 positive serum samples, 103 were found to be positive by recombinant SAG1. Four out of 30 healthy controls were found positive, and no cross reactivity was found in non-relative cases. The results of commercial and recombinant ELISA are summarized in
Table 1. The assay showed sensitivity of 93.6% (95% CI, 86.8-97.1%) and specificity of 92.9% (95% CI, 82.1-97.7%) for recombinant SAG1 in comparison with commercial ELISA (
Table 2).
The set of IgG positive and negative sera were tested by recombinant SAG2 in the ELISA system with the same protocol used for evaluating SAG1. The cut-off point was 0.13. As shown in
Table 2, all the 110 positive sera were positive by this antigen, although 5 out of 30 healthy controls were found positive and 1 cross reactivity was found with diseases other than toxoplasmosis. As shown in
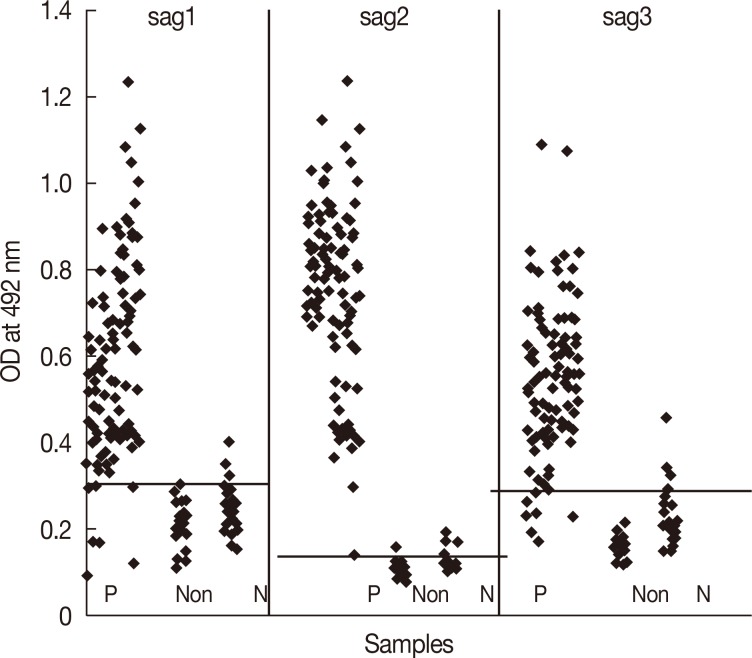
Table 3, the sensitivity and specificity of this antigen were 100.0% and 89.4%, respectively and a high degree of agreement (96.9%) was observed. There was marked difference in the absorbance values between sera from IgG positive and IgG negative groups with rSAG2 ELISA in comparison with 2 other recombinant antigens (
Fig. 1).
Using SAG3 in the ELISA system and the cut-off point of 0.28, 105 out of 110 positive sera were detected as positive, and 5 out of 30 health individuals were determined as positive. No cross reactivity was found with other diseases. According to the results, the sensitivity of 95.4% and the specificity of 91.2% were calculated for the assay. A fair agreement (94%) was found between this antigen and commercial ELISA.
IgM ELISA with recombinant SAG1
Twenty-eight IgM positive and 30 IgM negative sera were tested in the ELISA system for detecting
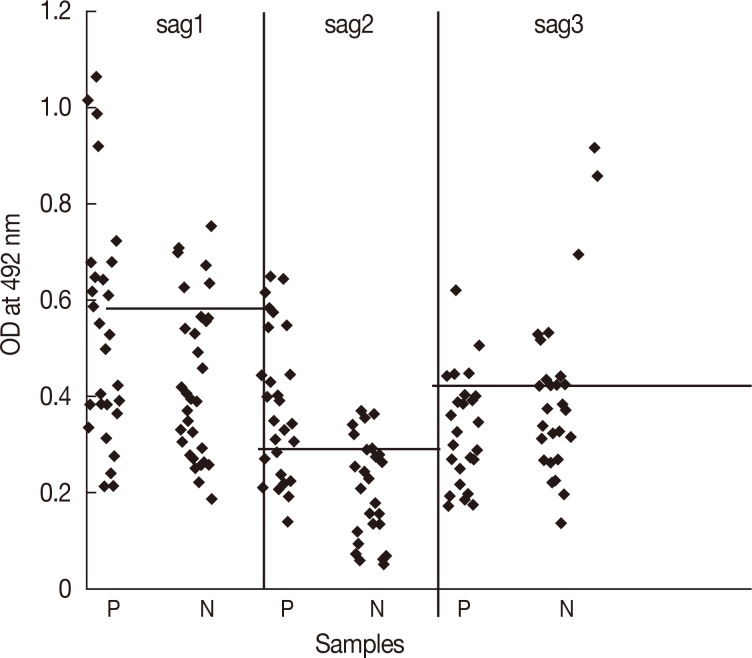
Toxoplasma-specific IgM. The cut-off (0.6) was determined by calculating mean and SD for 30 IgM and IgG negative serum samples. The assay showed a positive reaction in 11 out of 28 IgM positive and 6 out of 30 IgM negative samples (
Fig. 2). Accordingly, the sensitivity and specificity of 39.3% (95% CI, 22.1-59.2%) and 80.0% (95% CI, 60.8-91.5%) were calculated for the assay. The sensitivity and specificity of IgM rec-ELISA and commercial ELISA are shown in
Table 3.
The cut-off point of 0.31 was calculated for SAG2-ELISA. Positive reaction was noticed in 18 out of 28 IgM positive and 5 out of 30 IgM negative sera samples (
Fig. 2). All 18 IgM positive and 5 IgM negative sera that were detected by SAG1 and SAG3 recombinant antigens were also detected by SAG2 recombinant antigen. Accordingly, the sensitivity of 64.3% (95% CI, 44.1-80.6%) and the specificity of 83.3% (95% CI, 64.5-93.6%) were measured for the test. The best agreement (74.1%) was observed between recombinant SAG2 and commercial ELISA (
Table 3).
When the serum samples were tested by SAG3-ELISA, positive reactions were noticed in 5 out of 28 IgM positive and 7 out of 30 IgM negative sera samples (considering the cut-off point of 0.44). The sensitivity of 17.9% (95% CI, 6.7-37.5%) and the specificity of 76.7% (95% CI, 57.2-89.3%) were found for the assay (
Table 3).
DISCUSSION
Although serodiagnostic tools such as ELISA and immunoblots are effective for diagnosing T. gondii infection, advent of purified recombinant antigens obtained via molecular biology is an attractive alternative for detection of serum antibodies. In our study, 3 recombinant antigens of T. gondii were expressed and purified, and their performance was evaluated in the diagnosis of toxoplasmosis. These antigens were selected to determine diagnostic potential of recombinant fusion proteins carrying antigenic portions of this protein.
The aim of our study was to use the 3 recombinant antigens (SAG1, SAG2, and SAG3) to evaluate their diagnostic usefulness and efficacy compared with the available commercial ELISA for diagnosing toxoplasmosis and improving identification methods.
The findings of our study showed the sensitivity and specificity of 93.6% and 92.9% for SAG1 in comparison with the commercially available ELISA, respectively. In a previous study conducted in the same laboratory, recombinant SAG1 showed sensitivity and specificity of 88.4% and 88%, respectively [
25]. Beghetto et al. [
27] reported that SAG1 reacted with 92% of IgG positive sera of toxoplasmosis cases, which was consistent with our findings. In a study by Bufalono et al. [
5], 75% of sera from congenitally infected infants reacted with GST-SAG1 and the sensitivity and specificity of rSAG1 for diagnosis of IgM ELISA were 65.7% and 95.8%, respectively [
5]. In another work by Selseleh et al. [
28], an ELISA was described using recombinant SAG1 for detecting IgM and IgG antibodies. The sensitivity and specificity were 87% and 95% for IgM and 93% and 95% for IgG [
28]. Their results with IgM were different from ours, showing the sensitivity of 39.3% and specificity of 80%. It seems that a robust immunological response to the surface antigen SAG1 is associated with chronic
Toxoplasma invasion as mentioned in some previous studies [
25,
29].
The surface antigen 2 (P22) of
T. gondii is a major surface protein known as an attachment ligand [
7] which has good antigenicity and immunogenicity [
1,
8,
14]. Recombinant SAG2 expressed in
E. coli is effective in detecting IgG antibody against
T. gondii in patients with acute toxoplasmosis [
1,
14]. The recombinant SAG2 has been used for detecting
Toxoplasma antibodies in cats, where antibodies were detected in 100% of IgG positive animals and only 2% of IgG negative animals [
10].
Parmley et al. [
1] reported that recombinant SAG2 detected all toxoplasmosis patients in the acute phase (IgM, IgG positive) and 11 out of 13 (84%) of the cases in chronic phase (IgG positive) with IgG-ELISA. Significant differences in the absorbance values were reported between the 2 groups in their study. The assay also detected 6 out of 13 (46%) of IgM positive sera [
1]. Our findings were consistent with their results, as we found the sensitivity and specificity of rSAG2 for IgG-ELISA and IgM-ELISA of 100.0% and 89.4%, and 64.3% and 83.3%, respectively. In some studies, rSAG2 expressed in
E. coli was shown to be effective in detecting IgG antibodies against
T. gondii in human patients with acute toxoplasmosis [
1,
14]. In contrast, in other studies, rSAG2 was detected in both acute and chronic infections [
30,
31]. Further improvement of the assay might be achieved using anti-SAG2 antibodies to trap rSAG2 on ELISA plates instead of coating the wells directly with rSAG2. Furthermore, rSAG2 might be used for detecting IgG antibodies in acute and chronic serum samples to differentiate these 2 important stages of the disease.
SAG3 is a surface antigen of tachyzoites and bradyzoites of
Toxoplasma. Vaccination with rSAG3 in mice can induce partial immunity against
T. gondii infection through inducing a Th1 type immune response and producing IgG2a [
16]. The present results about rSAG3 showed the sensitivity and specificity of 95.4% and 91.2% for IgG and 17.9% and 76.7% for IgM, respectively. This indicates efficacy of this antigen for detecting specific IgG antibodies and low reactivity of this antigen with IgM anti-
Toxoplasma antibodies as was expected for this common tachyzoite and bradyzoite antigen. In a study conducted by Dai et al. [
32], the sensitivity of rSAG1, rSAG2, and rSAG3 in diagnosis of IgM antibodies were 68.6%, 56.3%, and 46.8%, respectively [
32], which were consistent with the present results with sensitivities of 39.3%, 64.3%, and 17.9% for rSAG1, rSAG2, and rSAG3 antigens, respectively. In another study, they used a pooled antigen and claimed that it could differentiate acute toxoplasmosis from the chronic phase [
32,
33]. It seems that pooled recombinant antigens are better for the diagnosis but need more evaluation in the future.
The best outcome, in terms of the sensitivity, specificity, positive predictive value, negative predictive value, and agreement was found with recombinant SAG2 in diagnosing IgM antibodies compared with recombinant SAG1 and SAG3. This indicates the usefulness of this antigen in the diagnosis of acute infection as shown in another study [
14].
In conclusion, although recombinant SAG1, SAG2, and SAG3 produced in E. coli are suitable antigens which can be used in the diagnosis of IgG anti-Toxoplasma antibodies, recombinant SAG3 is more sensitive and specific. SAG2 showed the best performance in the diagnosis of IgM-specific antibody against T. gondii.
Office of Vice-Chancellor for Research, Shiraz University of Medical Sciences89-5229
Notes
-
We have no conflict of interest related to this study.
ACKNOWLEDGMENTS
Authors would like to thank the Office of Vice-Chancellor for Research, Shiraz University of Medical Sciences for the financial support. The study was funded by Office of Vice-Chancellor for Research, Shiraz University of Medical Sciences (Grant no. 89-5229). Authors also would like to thank Dr. Motahareh Motazedian for her final English revised.
References
- 1. Parmley SF, Sgarlato GD, Mark J, Prince JB, Remington JS. Expression, characterization, and serologic reactivity of recombinant surface antigen P22 of Toxoplasma gondii. J Clin Microbiol 1992;30:1127-1133.
- 2. Remington JS, McLeod R, Desmonts G. Toxoplasmosis. In Remington JS, Klein JO eds, Infectious diseases of the fetus and newborn infant. Philadelphia, Pennsylvania, USA. Saunders WB; 1995, pp 140-267.
- 3. Wong SY, Remington JS. Toxoplasma in pregnancy. Clin Infect Dis 1994;18:727-729.
- 4. Handman E, Remington JS. Antibody responses to Toxoplasma antigens in mice infected with strains of different virulence. Infect Immun 1980;29:215-220.
- 5. Buffolano W, Beghetto E, Del Pezzo M, Spadoni A, Di Cristina M, Petersen E, Gargano N. Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J Clin Microbiol 2005;43:5916-5924.
- 6. Kasper LH, Bradley MS, Pfefferkorn ER. Identification of stage specific sporozoite antigens of Toxoplasma gondii by monoclonal antibodies. J Immunol 1984;132:443-449.
- 7. Grimwood J, Smith JE. Toxoplasma gondii: the role of parasite surface and secreted proteins in host cell invasion. Int J Parasitol 1996;26:169-173.
- 8. Aubert D, Maine GT, Villena I, Hunt JC, Howard L, Sheu M, Brojanac S, Chovan LE, Nowlan SF, Pinon JM. Recombinant antigens to detect Toxoplasma gondii-specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol 2000;38:1144-1150.
- 9. Kasper LH. Identification of stage-specific antigens of Toxoplasma gondii. Infect Immun 1989;57:668-672.
- 10. Huang X, Xuan X, Kimbita EN, Battur B, Miyazawa T, Fukumoto S, Mishima M, Makala LH, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Mikami T, Igarashi I. Development and evaluation of an enzyme linked immunosorbent assay with recombinant SAG2 for the diagnosis of Toxoplasma gondii infection in cats. J Parasitol 2002;88:804-807.
- 11. Kimbita EN, Xuan X, Huang X, Miyazawa T, Fukumoto S, Mishima M, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Suzuki N, Mikami T, Igarashi I. Serodiagnosis of Toxoplasma gondii infection in cats by enzyme-linked immunosorbent assay using recombinant SAG1. Vet Parasitol 2001;102:35-44.
- 12. Pietkiewicz H, Hiszczyńska-Sawicka E, Kur J, Petersen E, Nielsen HV, Stankiewicz M, Andrzejewska I, Myjak P. Usefulness of Toxoplasma gondii-specific recombinant antigens in serodiagnosis of human toxoplasmosis. J Clin Microbiol 2004;42:1779-1781.
- 13. Burg JL, Perelman D, Kasper LH, Ware PL, Boothroyd JC. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol 1988;141:3584-3591.
- 14. Prince JB, Auer KL, Huskinson J, Parmley SF, Araujo FG, Remington JS. Cloning, expression, and cDNA sequence of surface antigen P22 from Toxoplasma gondii. Mol Biochem Parasitol 1990;43:97-106.
- 15. Jacquet A, Coulon L, De Nève J, Daminet V, Haumont M, Garcia L, Bollen A, Jurado M, Biemans R. The surface antigen SAG3 mediates the attachment of Toxoplasma gondii to cell-surface proteoglycans. Mol Biochem Parasitol 2001;116:35-44.
- 16. Lee YH, Shin DW, Lee JH, Nam HW, Ahn MH. Vaccination against murine toxoplasmosis using recombinant Toxoplasma gondii SAG3 antigen alone or in combination with Quil A. Yonsei Med J 2007;48:396-404.
- 17. Beaman MH, McCabe RE, Wong SY, Remington JS. Toxoplasma gondii. In Mandel GL, Bennett JE, Dolin R eds, Principles and Practices of Infectious Diseases. 4th ed. New York, USA. Churchill and Livingstone, Inc; 1995, pp 2455-2475.
- 18. Johnson AM, Illana S. Cloning of T. gondii gene fragments encoding diagnostic antigens. Gene 1991;99:127-132.
- 19. Tenter AM, Johnson AM. Human antibody response to the nucleoside triphosphate hydrolase of T. gondii. J Immunoassay 1990;11:579-590.
- 20. Tenter AM, Johnson AM. Recognition of recombinant T. gondii antigens by human sera in an ELISA. Parasitol Res 1991;77:197-203.
- 21. Kazemi B, Bandehpour M, Maghen L, Solgi GH. Gene cloning of 30 kDa Toxoplasma gondii tachyzoites surface antigen (SAG). Iran J Parasitol 2007;2:1-8.
- 22. Arabpour M, Bandehpour M, Niyyati M, Abdollahi SH, Kochaki A, Kazemi B. Cloning and expression of Toxoplasma gondii tachyzoite P22 protein. Afr J Biotechnol 2011;10:7746-7750.
- 23. Kazemi B, Maghen L, Bandehpour M, Shahabi S, Haghighi A. Gene cloning of p43 surface protein of Toxoplasma gondii tachyzoite and bradyzoite (SAG3). GTMB 2007;11:113-116.
- 24. Hanahan D. Studies on transformation on E. coli with plasmids. J Mol Biol 1983;166:557-580.
- 25. Jalallou N, Bandehpour M, Khazan M, Haghighi A, Abdollahi SH, Kazemi B. Recombinant SAG1 antigen to detect Toxoplasma gondii specific immunoglobulin G in human sera by ELISA test. Iran J Parasitol 2010;5:1-9.
- 26. Khanaliha K, Motazedian MH, Sarkari B, Bandehpour M, Sharifnia Z, Kazemi B. Expression and purification of P43 Toxoplasma gondii surface antigen. Iran J Parasitol 2012;7:48-53.
- 27. Beghetto E, Buffolano W, Spadoni A, Del Pezzo M, Cristina MD, Minenkova O, Petersen E, Felici F, Gargano N. Use of an immunoglobulin G avidity assay based on recombinant antigens for diagnosis of primary Toxoplasma gondii infection infection during pregnancy. J Clin Microbiol 2003;41:5414-5418.
- 28. Selseleh MM, Keshavarz H, Mohebali M, Shojaee S, Modarressi M, Eshragian M. Production and evaluation of Toxoplasma gondii recombinant surface antigen 1 (SAG1) for serodiagnosis of acute and chronic Toxoplasma infection in human sera. Iran J Parasitol 2012;7:1-9.
- 29. Gatkowska J, Hiszczynska-Sawicka E, Kur J, Holec L, Dlugonska H. Toxoplasma gondii: an evaluation of diagnostic value of recombinant antigens in a murine model. Exp Parasitol 2006;114:220-227.
- 30. Lau YL, Fong MY. Toxoplasma gondii: serological characterization and immunogenicity of recombinant surface antigen 2 (SAG2) expressed in the yeast Pichia pastoris. Exp Parasitol 2008;119:373-378.
- 31. Kotresha D, Noordin R. Recombinant proteins in the diagnosis of toxoplasmosis. APMIS 2010;118:529-542.
- 32. Dai J, Jiang M, Wang Y, Qu L, Gong R, Si J. Evaluation of a recombinant multiepitope peptide for serodiagnosis of Toxoplasma gondii infection. Clin Vaccine Immunol 2012;19:338-342.
- 33. Dai JF, Jiang M, Qu LL, Sun L, Wang YY, Gong LL, Gong RJ, Si J. Toxoplasma gondii: enzyme-linked immunosorbent assay based on a recombinant multiepitope peptide for distinguishing recent from past infection in human sera. Exp Parasitol 2013;133:95-100.
Fig. 1Results of IgG ELISA with recombinant SAG1, SAG2, and SAG3 in human sera. P, IgG positive; Non, non-relative (cases with different infectious diseases but not toxoplasmosis); N, negative reactions (Epstein-Barr virus, Varicella zoster virus, Herpes simplex virus, rubella, measles, Helicobacter pylori, cytomegalovirus, malaria, Echinococcus granulosus, Hymenolepis nana, Leishmania, and rheumatoid factor).

Fig. 2Results of IgM ELISA with recombinant SAG1, SAG2, and SAG3 in human sera. P, IgM positive; N, IgM negative. Cut-off value; 0.60 (SAG1), 0.31 (SAG2), and 0.44 (SAG3), respectively.

Table 1.Comparison of commercial ELISA and IgG rec-ELISA using SAG1, SAG2, SAG3 for detection of IgG in toxoplasmosis patients
Table 1.
|
Commercial ELISA
|
|
Antigen |
Positivea (%) |
Negativeb (%) |
Total |
|
Rec-ELISA |
SAG1 |
|
|
|
|
Positive |
103 (93.6) |
4 (7.0) |
107 |
|
Negative |
7 (6.3) |
53 (92.9) |
60 |
|
SAG2 |
|
|
|
|
Positive |
110 (100.0) |
6 (10.5) |
116 |
|
Negative |
0 (0.0) |
51 (89.4) |
51 |
|
SAG3 |
|
|
|
|
Positive |
105 (95.4) |
5 (8.7) |
110 |
|
Negative |
5 (4.5) |
52 (91.2) |
57 |
|
Total |
110 |
57 |
167 |
Table 2.Sensitivity and specificity of the IgG rec-ELISA and commercial ELISA for diagnosis of toxoplasmosis
Table 2.
|
Test and antigen IgG-ELISA |
Sensitivity (%) |
Specificity (%) |
Positive predictive value (%) |
Negative predictive value (%) |
Agreement (%) |
|
SAG1 |
93.6 |
92.9 |
96.2 |
88.3 |
93.4 |
|
SAG2 |
100.0 |
89.4 |
94.8 |
100.0 |
96.9 |
|
SAG3 |
95.4 |
91.2 |
95.4 |
91.2 |
94.0 |
Table 3.Sensitivity and specificity of the IgM rec-ELISA and commercial ELISA for diagnosis of toxoplasmosis
Table 3.
|
Test and antigen IgM-ELISA |
Sensitivity (%) |
Specificity (%) |
Positive predictive value (%) |
Negative predictive value (%) |
Agreement (%) |
|
SAG1 |
39.3 |
80 |
64.7 |
58.5 |
60.3 |
|
SAG2 |
64.3 |
83.3 |
78.3 |
71.4 |
74.1 |
|
SAG3 |
17.9 |
76.7 |
41.7 |
50 |
48.2 |