Abstract
To evaluate the seroprevalence against circumsporozoite protein (CSP) of Plasmodium vivax in sera of Korean patients, the central repeating domain (CRD) of CSP was cloned and analyzed. From the genomic DNA of patient's blood, 2 kinds of CSPs were identified to belong to a VK210 type, which is the dominant repeating of GDRA(D/A)GQPA, and named as PvCSPA and PvCSPB. Recombinantly expressed his-tagged PvCSPA or PvCSPB in Escherichia coli reacted well against sera of patients in western blot, with the detecting rate of 47.9% (58/121), which included 15 cases positive for PvCSPA, 6 cases positive for PvCSPB, and 37 cases for both. The mixture of PvCSPA and PvCSPB was loaded to a rapid diagnostic test kit (RDT) and applied with the same set of patient sera, which resulted in detection rates of 57.0% (69/121). When the protein sequences of PvCSPA were compared with those of P. vivax in endemic regions of India and Uganda, they were compatibly homologous to PvCSPA with minor mutations. These results suggested that the recombinant PvCSPA and PvCSPB loaded RDT may be a milestone in latent diagnosis which has been a hot issue of domestic malaria and important for radical therapy in overlapped infections with P. falciparum in tropical and subtropical areas. During the biological process of malarial infection, exposure of CSP to antigen-antibody reaction up to 57.0% is the first report in Korea.
-
Key words: Plasmodium vivax, central repeating domain, circumsporozoite protein, variant VK210, rapid diagnostic test
INTRODUCTION
Plasmodium vivax is the world-widely distributed malaria parasite of humans and is the primary agent of malaria in Korea. Reemergence of vivax malaria in the Republic of Korea (= South Korea) in 1993 was due to expansion of malaria endemic areas in North Korea [
1,
2]. Malaria cases in South Korea rapidly increased till 2000 and decreased during 2001 and 2004, after then rapid re-increasing patterns were shown [
3,
4,
5,
6,
7]. For this reason, detection of seroprevalence and rapid diagnosis of acute
P. vivax malaria were urgently needed in Korea.
In the life cycle of malaria, sporozoites are injected to the host by infected mosquitoes during the blood meal. After then, sporozoites invade hepatocytes and proliferate into merozoites. Therefore, circumsporozoite protein (CSP) is expressed during the pre-hepatic sporozoite stage till merozoites are emerged to the blood stream [
8]. Clinically,
P. vivax has irregular incubation period and discriminative characteristics with direct division of merozoites or re-division from hypnozoites. For this reason, diagnosis of vivax malaria is highly important in asymptomatic, latent stage patients or relapsed infections. It is necessary to detect antibodies against CSP for early stage infections and following a complete chemotherapy. To date, diagnostic tools for vivax malaria are microscopic examinations for erythrocytic stage malaria, nested PCR, and rapid diagnostic tests (RDTs) which use recombinant antigens such as LDH or aldolase. CSP contains a central tandem-repeat amino acid sequences region which is species-specific and contains B-cell immunodominant epitopes [
9].
P. vivax has 3 different serotypes of CSP gene such as VK210, VK247, and
P. vivax-like. For diagnosis of malaria in South Korea, the knowledge on the type of
P. vivax CSP is important. In the study of Korean strains, VK210 type sequences (GDRAD/AGQPA) are dominantly identified [
10,
11].
For evaluation of the seroprevalence of CSP antibodies in malaria patients, we cloned the VK210 gene from the genomic DNA of P. vivax patient's blood and characterized its antigenicity by using western blot and recombinant PvCSP loaded RDT kit for its usefulness in diagnosis of asymptomatic P. vivax patients, latent-stage or relapsed infections in South Korea.
MATERIALS AND METHODS
Molecular cloning of P. vivax CSP and MSP genes
A total of 121
P. vivax patients were diagnosed from the endemic regions of South Korea during 1998 to 2001 previously used in [
14] and 5 additional cases from India or Uganda, which were confirmed by microscopic diagnosis with thin blood films of Diff-Quick staining. They were evaluated for CSP gene cloning and seroprevalences of
P. vivax. Genomic DNA of
P. vivax patients (Korea, India, and Uganda) were extracted by using the QIAamp DNA mini kit (Qiagen, Valencia, California, USA) according to the manufacturer's protocol. Primers were designed as follows: CSP-forward: 5'-TGC GTT TCC TCC TGC TGC CTG-3', CSP-reverse: 5'-CGC ATT TCC TCC TGC TGC CTG-3' depending on the immunodominant central repeated domain (CRD) of
P. vivax circumsporozoite protein gene (CSP-1: GenBank accession no. M34697) and MSP-forward: 5'-CTA CTA CTT GAT GGT CCT CAA-3', MSP-reverse: 5'-TTG TGA CAT GCG TAA GCG GAT-3' depending on the merozoite surface protein (MSP-1: GenBank accession no. M60807). PCR amplified CSP or MSP gene fragments were cloned into pET-28a plasmid (Novagen, Hilden, Germany) and sequenced using ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit FS (Perkin Elmer, Cambridge, Massachusetts, USA). The sequence data were analyzed with BLAST search of the National Center for Biotechnology information (NCBI). The hydrophobicity profile of the deduced linear polypeptide sequence of PvCSPs was analyzed using the ProtScale tool (
http://web.expasy.org/protscale/) based on the Kyte and Doolittle hydrophobic scale.
Two types of central repeating domain (CRD) genes were found in CSP region both belonging to VK210 variant, which were named as PvCSPA and PvCSPB, respectively. These and MSP-1 proteins were expressed as his-tagged forms in E. coli BL21(DE3). His-tagged recombinant proteins were purified with Ni-NTA metal ion affinity chromatography (Qiagen). Purified recombinant proteins were analyzed in 12% SDS-PAGE and western blotting using anti-his-antibody or patient's serum. The immune complexes were detected with enhanced chemiluminescence (ECL, Luminata Classico, Millipore Corp., Billerica, Massachusetts, USA) and analyzed with Luminant Image Analysis System (LAS-3000, Fuji Film, Tokyo, Japan).
Malaria RDT test
Colloidal gold particles (40 nm mean diameter) were prepared and conjugated with recombinant PvCSPA and PvCSPB mixture or MSP-1 antigens according to the previously described procedure [
12]. The assay procedure was as follows. The first step is dropping 10 µl sera drop onto the hole in assemble plastic cassettes and then dropping 100 µl of buffer A (0.1% casein and 1% Tween-20 in 0.1 M Tris-Cl buffer, pH 8.0) to the same hole in the sample pad. After 5 min of buffer treatment, the results were interpreted. Control line (C) should appear in all tests as a red-colored band. After then, if the red band appears in the T line (under the control band), it means the presence of anti-
P. vivax CSP or MSP-1 antibodies in the sample sera, which is decided as the positive.
RESULTS
Molecular cloning and characterization of CSP variants among Korean malaria patients
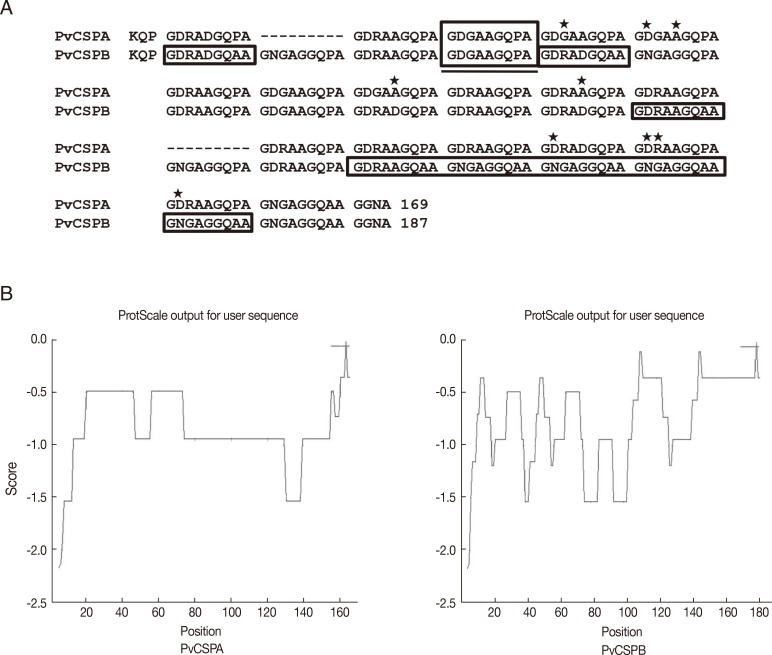
Two types of CRD genes in CSP were amplified by PCR from the genomic DNA of malaria patients. After ligated to pET-28a plasmid vector, the plasmid containing the PCR product was sequenced, identified with the number of repeats, and named as PvCSPA (507 bp) and PvCSPB (561 bp) belonging to a VK210 variant. In PvCSPA type, deduced amino acid sequence of GDRADGQPA which is common repeating unit of VK210 variants was repeated 18 times and consisted of total 169 amino acids. On the while, in PvCSPB type, the GDRADGQA (P)A repeats 20 times and consisted of 187 amino acids, of which the proline residue was substituted to the alanine which resulted in less hydrophobic structural change (
Fig. 1B).
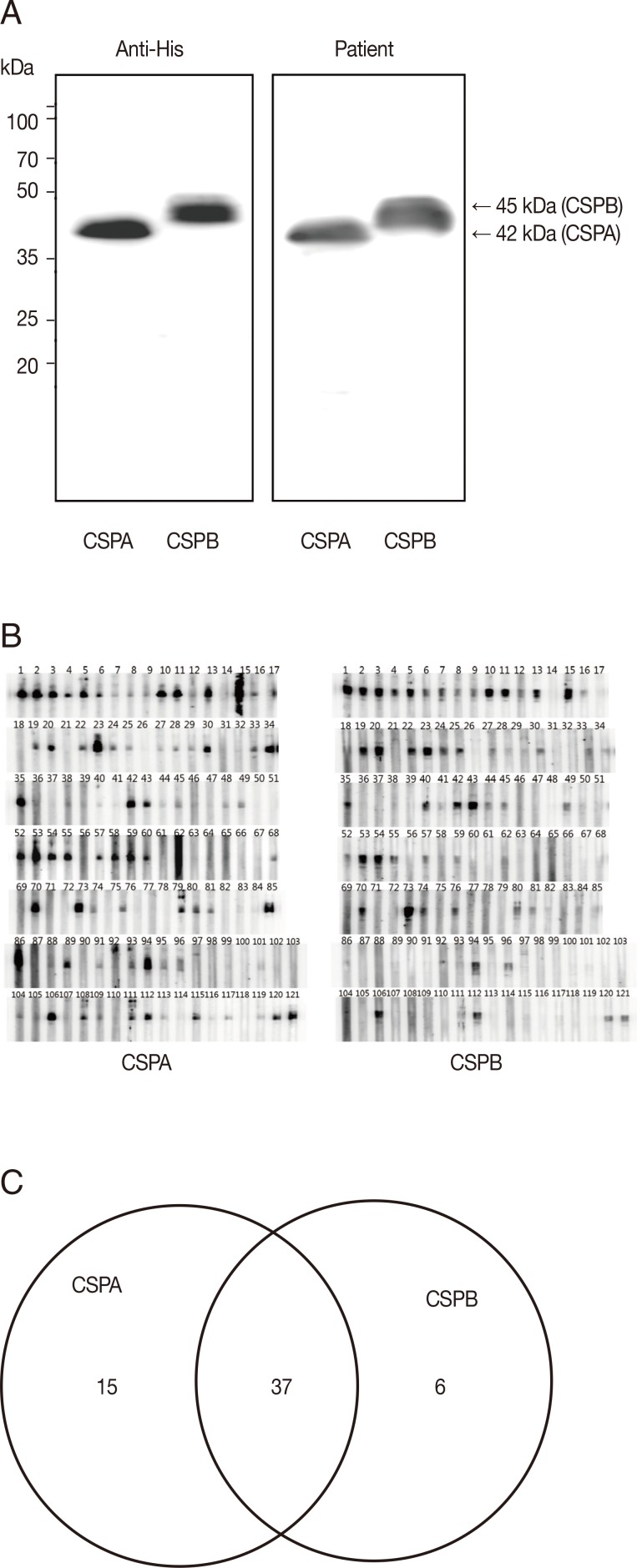
To evaluate the antigenicity of PvCSPA or PvCSPB protein, each was expressed in
E. coli as a his-tagged recombinant protein and then was purified through the Ni-NTA column as 42 kDa (PvCSPA) or 45 kDa (PvCSPB). When blotted with anti-his antibody and sera of malaria patients, significant reactive bands were detected as the same patterns (
Fig. 2A). Furthermore, 58 of 121 malaria patients (47.9%) were positive by western blot. Among them, 15 cases reacted only with PvCSPA, 6 cases only with PvCSPB, and 37 cases reacted with both antigens (
Fig. 2B).
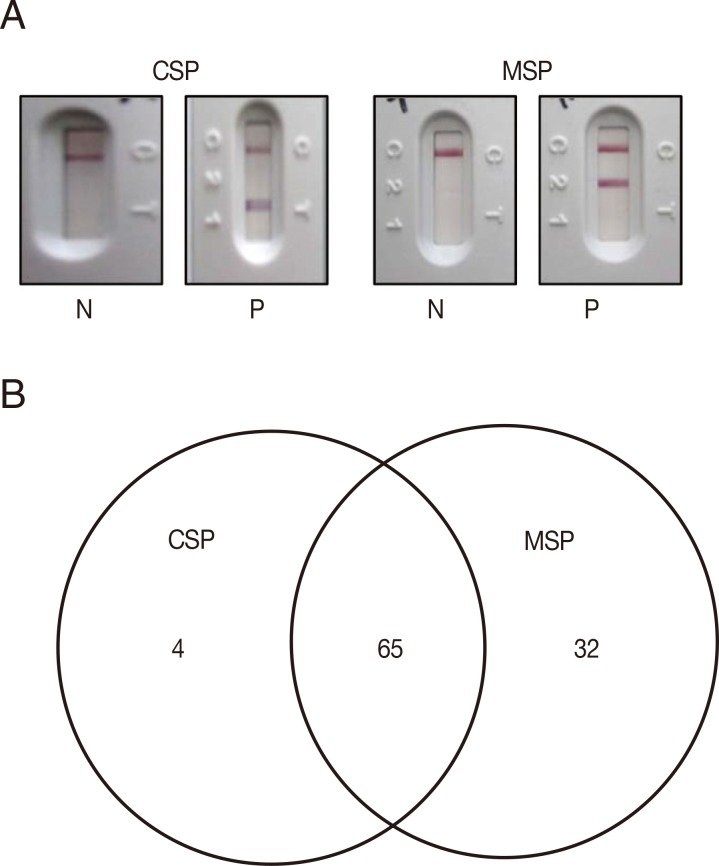
To test for usage as a malaria diagnostic antigen, PvCSPA and PvCSPB recombinant proteins were mixed and loaded to RDT and compared with
P. vivax recombinant PvMSP loaded RDT (
Fig. 3A). The seroprevalence of
P. vivax CSP in malaria was 57.0% (69/121) compared to 80.2% (97/121) of PvMSP, of which 65 sera (53.7%) were reacted with both CSP and MSP antigens (
Fig. 3B).
To compare the genotype of CSP VK210 in a
P. vivax endemic area, genomic DNA sequencing of CSP was performed and compared with PvCSPA and PvCSPB. There was only 1 difference in amino acids, at the 105th amino acid, in Ugandan (G: glycine) and Indian (R: arginine) isolates. However, 7 sites (PvCSPA: G→Ugandan and Indian: R) and 5 sites (PvCSPA: A: alanine→Ugandan and Indian: D: aspartic acid) were shown to have mutations (
Fig. 4).
DISCUSSION
For diagnosis of malaria, detection of P. vivax antigen is important but it is uneasy because of a low parasite load. Therefore, detection of antibodies against P. vivax is the method of choice in the diagnosis of symptomatic as well as asymptomatic patients or latent infections in temperate zones especially Korea. It is also necessary for radical cure in tropical and sub tropical areas. Meanwhile, antigen detection is more important comparing with antibody detection because of rapid depravation of symptoms after infection with P. falciparum. For these reasons, antibody detection against sporozoite antigens is a pivotal factor, and CSP is a well-known representative of the sporozoite stage antigen. In the present study, we attempted to evaluate the probability of exposure to CSP and antigen-antibody reactions during the relatively short period of translocation from the peripheral blood of biting site to liver parenchymal cells among Korean patients.
When CRD gene of CSP was cloned and sequenced, 2 types of genes were identified as PvCSPA and PvCSPB which belong to the VK210 genotype, which is the most popular genotype of malaria in Korea [
13]. In PvCSPA, the amino acid sequence of tandem repeats consisted of basic amino acid sequences of GDRADGQP/AA which were conserved well among Korean isolates [
9]. The amino acid sequences of PvCSPA were similar to those of SOL-101 with 17 repeats with GDRADGQPA, and PvCSPB had 19 tandem repeats with a mixed form of CSP, in which only 1 repeat was deleted at the 13th position comparing with KPVCSP 96-11, -21, and -33 [
9].
The antibody detection rate was 47.9% by using the recombinant PvCSPA or PvCSPB protein in western blot analysis of sera of vivax malaria patients; however, no reaction was shown with sera of
P. falciparum patients (data not shown). These recombinant PvCSPA or PvCSPB showed higher detection rates compared with the whole gene coding his-tagged CSP-1 antigen of 39.5% (60/152) by using ELISA [
14]. More increased CSP antibody detection rates (57.0%) were obtained in the RDT loaded PvCSPA and PvCSPB recombinant antigens. With these results, it is suggested that almost half of vivax malaria patients may complete the antigen-antibody reaction when exposed to CSP of sporozoites during the very short time of sporozoites before reaching to the liver tissue or through the repetitive exposure to CSP after consecutive biting of mosquitoes.
The CRD of CSP from Korean (PvCSPA), Ugandan, and Indian patients maintained homology in high degree only with a few minor mutations. During the preliminary search, GenBank provided us with about 260 sequences of CSPs all over the world. They were classified as VK210, VK247, and
P. vivax-like types according to the basic repeating unit in CRD. Generally, most endemic regions of
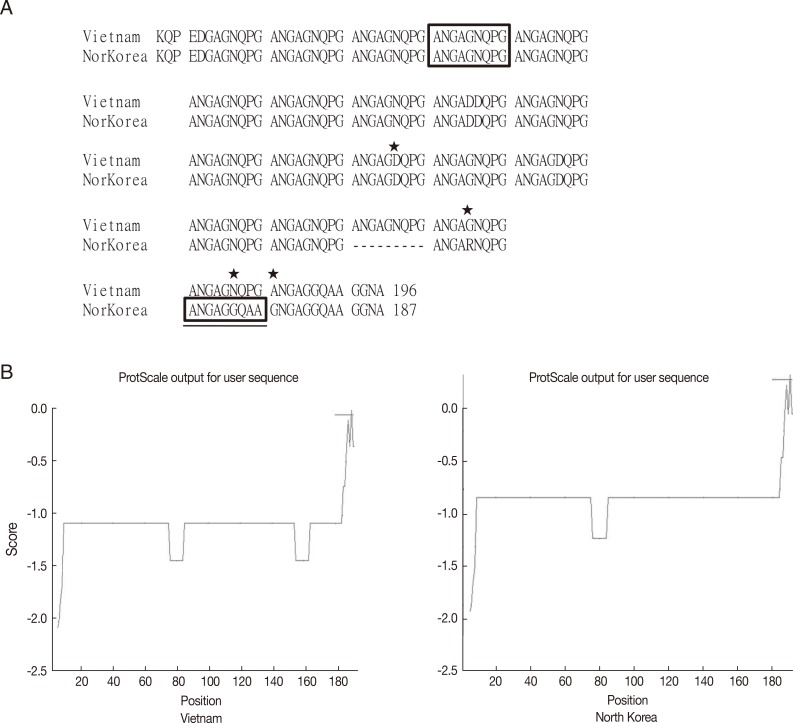
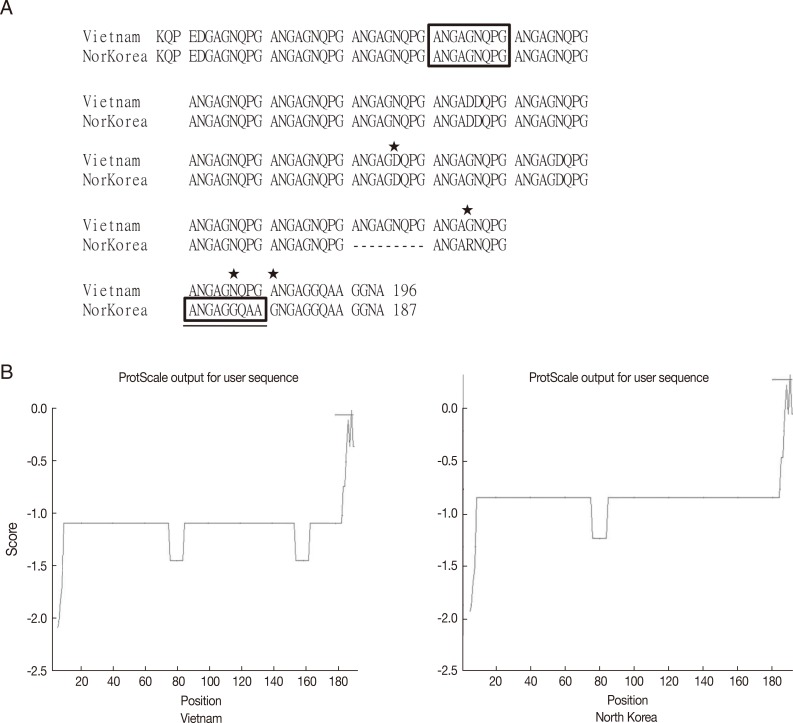
P. vivax infection showed approximately 1 genotype of VK210 with minor mutations, while the endemic regions in Indochina peninsula showed the VK247 genotype. Peculiarly, 2 sequences of CSPs are reported for North Korea, of which 1 belongs to VK210 (GenBank no. M20670.1) [
15] and the other to VK247 (GenBank no. EU401928) [
16]. Considering the current infection status in South Korea and general preferential distribution of the genotype, the former is regarded as the naïve strain and the latter as an imported case from Indochina when compared in
Fig. 5.
It is suggested that large regions may be occupied by the VK210 genotype which showed the same antigenic epitope of CRD; therefore, PvCSPA and PvCSPB loaded RDT kit may be useful for the diagnosis of P. vivax infection in these endemic areas. It is also suggested that detection of the incubation period is available by using the diagnosis of sera collected in winter which is inactive periods of mosquitoes and that the RDT diagnosis against CSP antibody is very helpful for radical cure of P. vivax when infected only and with P. falciparum in the area of tropical or subtropical zones.
National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MEST)2011-0028135
Inha University Research Grant
Notes
-
We declare that we have no conflict of interest related to this study.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean Government (MEST) (2011-0028135) and by an Inha University Research Grant.
References
- 1. Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol 1999;37:129-143.
- 2. Lee JS, Lee WJ, Cho SH, Ree HI. Outbreak of vivax malaria in area adjacent to the demilitarized zone, South Korea. Am J Trop Med Hyg 2002;66:13-17.
- 3. Lee JS, Kho WG, Lee HW, Seo M, Lee WJ. Current status of vivax malaria among civilians in Korea. Korean J Parasitol 1998;36:241-248.
- 4. Park JW, Klein TA, Lee HC, Pacha LA, Ryu SH, Yeom JS, Moon SH, Kim TS, Chai JY, Oh MD, Choe KW. Vivax malaria: a continuing health threat to the Republic of Korea. Am J Trop Med Hyg 2003;69:159-167.
- 5. Yeom JS, Ryu SH, Oh SJ, Lee WJ, Kim TS, Kim KH, Kim YA, Ahn SY, Cha JE, Park JW. Status of Plasmodium vivax malaria in the Republic of Korea during 2001-2003. Am J Trop Med Hyg 2005;73:604-608.
- 6. Yeom JS, Kim TS, Oh SJ, Sim JB, Barn JS, Kim HJ, Kim YA, Ahn SY, Shin MY, Yoo JA, Park JW. Plasmodium vivax malaria in the Republic of Korea during 2004-2005: changing patterns of infection. Am J Trop Med Hyg 2007;76:865-868.
- 7. Han ET, Lee DH, Park KD, Seok WS, Kim YS, Tsuboi T, Shin EH, Chai JY. Reemerging vivax malaria: changing patterns of annual incidence and control programs in the Republic of Korea. Korean J Parasitol 2006;44:285-294.
- 8. Escalante AA, Barrio E, Ayala FJ. Evolutionary origin of human and primate malarias: evidence from the circumsporozoite protein gene. Mol Biol Evol 1995;12:616-626.
- 9. Arnot DE, Barnwell JW, Tam JP, Nussenzweig V, Nussenzweig RS, Enea V. Circumsporozoite protein of Plasmodium vivax: gene cloning and characterization of the immunodominant epitope. Science 1985;230:815-818.
- 10. Lim CS, Kim YK, Lee KN, Kim SH, Hoffman KJ, Song KJ, Song JW. The analysis of circumsporozoites protein gene sequences from Plasmodium vivax isolates in the Republic of Korea. Ann Trop Med parasitol 2001;95:229-235.
- 11. Kim T, Kim YJ, Song KJ, Song JW, Cha SH, Kim YK, Shin YK, Suh IB, Lim CS. The molecular characteristics of circumsporozoite protein gene subtype from Plasmodium vivax isolates in Republic of Korea. Parasitol Res 2002;88:1051-1054.
- 12. Song KJ, Yang Z, Chong CK, Kim JS, Lee KC, Kim TS, Nam HW. A rapid diagnostic test for toxoplasmosis using recombinant antigen N-terminal half of SAG1 linked with intrinsically unstructured domain of GRA2 protein. Korean J Parasitol 2013;51:503-510.
- 13. Lee HW, Lee WJ, Lee JS, Lee HS. DNA sequencing and expression of the circumsporozoite protein of Plasmodium vivax Korean isolate in Escherichia coli. Korean J Microbiol 1999;37:234-242.
- 14. Kim S, Ahn HJ, Kim TS, Nam HW. ELISA detection of vivax malaria with recombinant multiple stage-specific antigens and its application to survey of residents in endemic areas. Korean J Parasitol 2003;41:203-207.
- 15. Arnot DE, Barnwell JW, Stewart MJ. Does biased gene conversion influence polymorphism in the circumsporozoite protein-encoding gene of Plasmodium vivax? Proc Natl Acad Sci USA 1988;85:8102-8106.
- 16. Ntumngia FB, McHenry AM, Barnwell JW, Cole-Tobian J, King CL, Adams JH. Genetic variation among Plasmodium vivax isolates adapted to non-human primates and the implication for vaccine development. Am J Trop Med Hyg 2009;80:218-227.
Fig. 1The deduced amino acid sequences and comparison of hydrophobicity of the circumsporozoite protein genes (PvCSPA and PvCSPB) of P. vivax. (A) Sequence alignment by using CLUSTAL 2.0.12 program. Underlined box indicates the basic repeating unit of VK210 and the other boxes indicate that proline substitutes to alanine in repeat sequences between PvCSPA and PvCSPB. (B) ProtScale analysis of the PvCSPA and PvCSPB protein, which showed more peaks above the hydrophobic scale of -0 in PvCSPB than in PvCSPA.

Fig. 2Western blot analysis of the recombinant PvCSPA and PvCSPB proteins. (A) Western blot of PvCSPA and PvCSPB against anti-His antibody (left) and with serum of malaria patient (right). (B) Western blot pattern against all the sera of malaria patients (121 cases). (C) Diagram of positives in western blot of PvCSPA and PvCSPB.
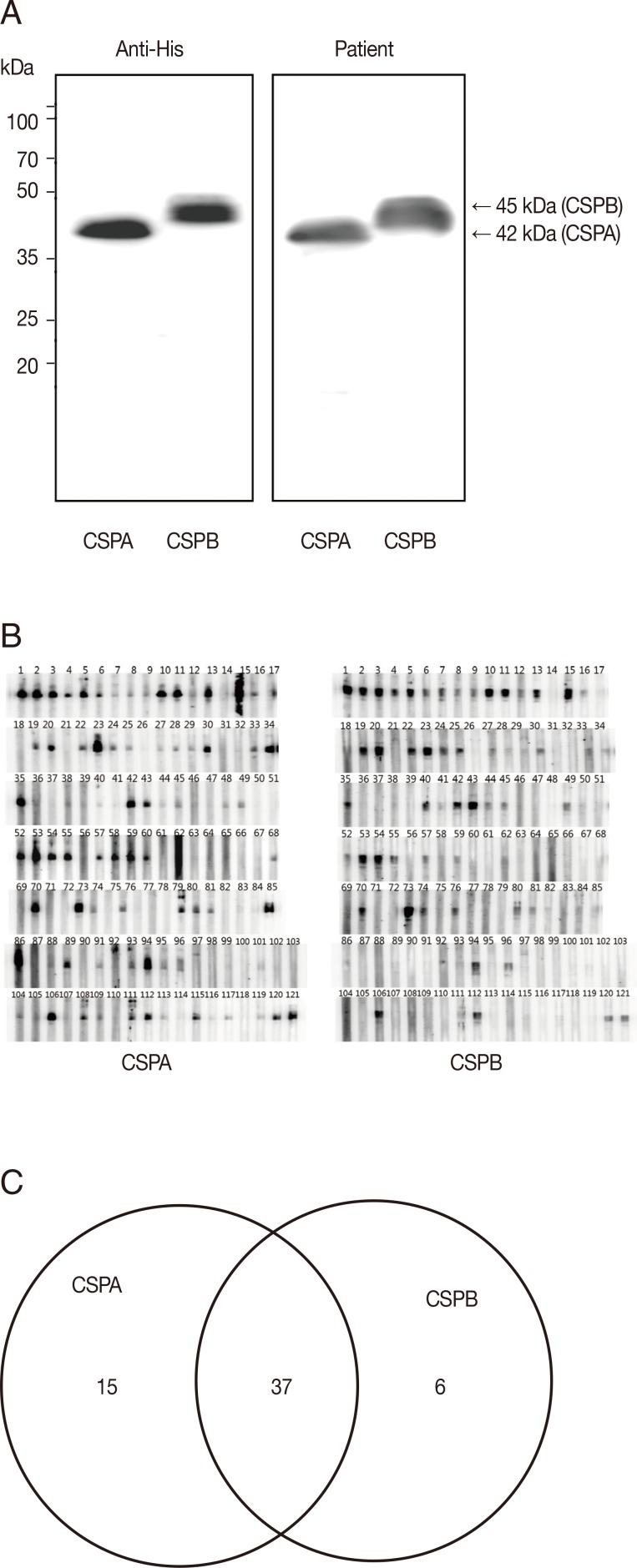
Fig. 3Interpretation and analysis of RDT results in malaria patients. (A) CSP or MSP loaded RDT windows of positive (P) and negative (N), of which the 2 bands appeared in the positives. (B) Diagram of the positive sera in RDT test among 121 malaria patients.
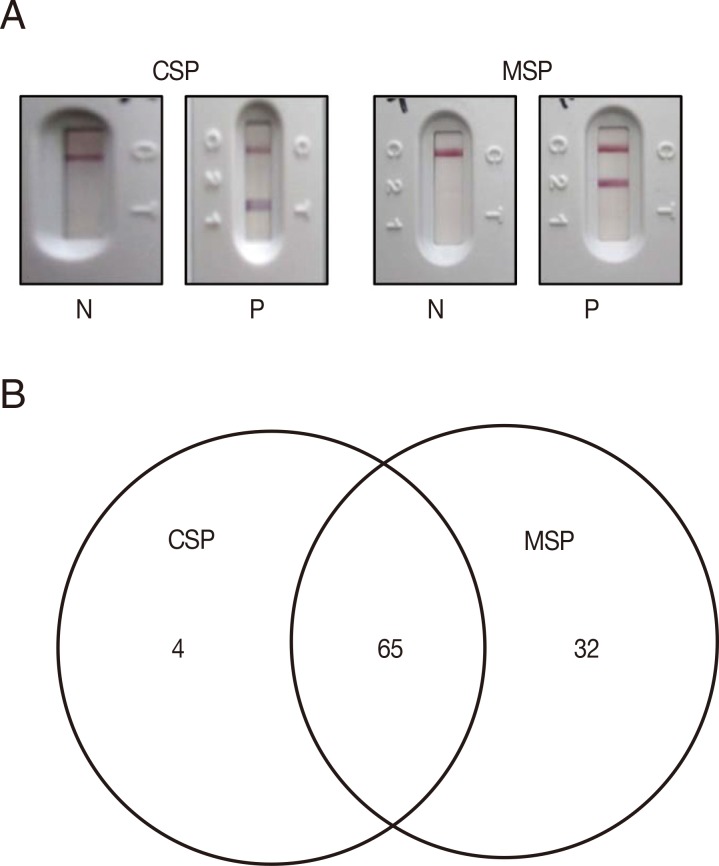
Fig. 4Comparison of the deduced amino acid sequences of CRD of CSP from Korean, Ugandan, and Indian patients. All belong to VK210 variant. Bold characters indicate different amino acids between Ugandan and Indian. Asterisks indicate genetic variations among the 3 samples.

Fig. 5Sequence alignment and hydrophobicity of the CSPs from Vietnam and North Korean strain. (A) Sequence alignment by using CLUSTAL 2.0.12 program. Box indicates the basic repeating unit specific for VK247 type. Asterisks indicate mutation sites. Underlined box is a peculiar mutation in the North Korean strain. (B) ProtScale analysis of CSPs showed similar pattern of hydrophobicity.