Abstract
The present study was designed to investigate polymorphism in Duffy binding protein (DBP) gene of Plasmodium vivax isolates of Korea. Thirty samples were obtained from P. vivax patients in Yonchon-gun, Kyonggi-do in 1998. The PCR products of the samples were subjected to sequencing and hybridization analyses of the regions II and IV of P. vivax DBP gene. Two genotypes, SK-1 and SK-2, were identified on the basis of amino acid substitution and deletion. The genotype of 10 isolates was SK-1 and that of 20 isolates was SK-2. Most of the predicted amino acids in the region II of DBP gene were conserved between the Korean isolates and Belem strain except for 4-5 amino acid substitutions. In the region IV of DBP, a 6-bp insert that was shown in the Sal-1 allele type was found in SK-1, and a 27-bp insert that was shown in the Papua New Guinea allele type was found in SK-2. In conclusion, the present findings suggest that two genotypes of P. vivax coexist in the endemic area of Korea.
-
Key words: Plasmodium vivax, Duffy binding protein, genotype, polymorphism, Korean isolates
INTRODUCTION
Malaria is one of the most serious diseases to affect peoples in developing countries with tropical and subtropical climates. Although large amounts of investment have been made in attempts to develop a vaccine against malaria, none have been developed so far. The major problem in vaccine development is the antigenic diversity of the vaccine candidates among parasites; thus, the variation of the immunodominant region is a serious obstacle (
Berzins and Anders, 1999;
Bold and Berzins, 2000). Therefore, the genetic variation study for the antigens of potential vaccine candidate, e.g. CSP (circumsporozoite protein),
MSP (merozoite surface protein), and DBP (Duffy binding protein), is very important and continues to grow steadily in both
Plasmodium faliciparum and
P. vivax (
Rich et al., 2000).
Erythrocytes of Duffy-negative individuals are resistant to invasion by
P. vivax (
Miller et al., 1976). The
P. vivax DBP (PvDBP) has been identified as the parasite ligand for the Duffy-glycoprotein receptor in human erythrocytes (
Barnwell et al., 1989). This fact could certainly compromise the potential of PvDBP as an asexual blood-stage malaria vaccine candidate (
Adams et al., 1990). The PvDBP is divided into seven regions: a leader peptide sequence (region I), cysteine rich regions (region II and VI), hydrophobic regions (region III to V), and a transmembrane domain (region VII). Among them the region II, amino terminal cysteine-rich region, has been proven that it is implicated in the binding process to human erythrocytes (
Barnwell et al., 1989;
Ranjan and Chitnis, 1999). Therefore the genetic polymorphism in the region should be considerable factor in the rational design of vaccines against asexual blood stages of
P. vivax. However, the only available information has been made from the Papua New Guinean (PNG) and Colombian isolates (
Tsuboi et al., 1994;
Ampudia et al., 1996). To add useful information for rational design of vaccine the polymorphism of PvDBP region II in the Korean isolates was investigated in this study. The polymorphism of region IV where the length polymorphism has been reported in the PNG isolates (
Tsuboi et al., 1994) also observed in the Korean isolates.
In addition, we have attempted oligonucleotide hybridization, which is economical and rapid compared to DNA sequencing, to identify the genotype for P. vivax of Korean isolates.
MATERIALS AND METHODS
Isolation of parasite genomic DNA
Thirty blood samples were collected from P. vivax patients who were detected in Yonchon-gun, Kyonggi-do in 1998. All patients were diagnosed by microscopic examination at the Institute of Malariology, Inje University.
As described previously, parasite DNA was extracted from 0.1 ml of EDTA treated blood by proteinase K digestion and then followed by two rounds of phenol/chloroform extraction (
Kho et al., 1999). The supernatant was mixed with 0.1 volume of 3 M sodium acetate (pH 5.2) and 3 volumes of ethanol, and incubated at -70℃ for 1 hour. The parasite DNA recovered were dissolved in 50 µl of sterilized water and used as template for PCR.
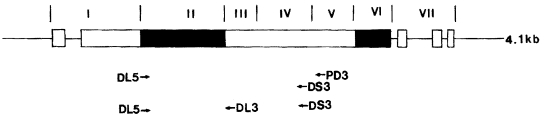
Three different primers were synthesized for amplification of DBP gene (
Fig. 1). The first set of primers was DL5 (5'-GGGAAAGAGATTGGGACTGT-3') and PD3 (5'-GCCCCGTTCTTTTCCGTGTC-3'). The second set of primers was DL5 and DS3 (5-AGTATCACCCGTAGCAGAGC-3). Amplification by the PCR of the fragment encoding regions II, III, and VI of the gene was carried out in 20 µl reaction mixture containing extracted
P. vivax DNA, 200 µM dNTP each, 0.5 pM primer each, 10 mM Tris-HCl, pH 8.0, 50 mM KCl, 1.0 mM MgCl
2, and 2.5 units of
Taq polymerase (Perkin Elymer, Norwalk, CT, USA). The reaction was subjected to 35 cycles of denaturation at 94℃ for 1 min, annealing at 58℃ for 1 min, and extension at 72℃ for 1 min 45 sec, and one at 72℃ for 5 min in a thermal cycler (GeneAmp PCR system 9700, Perkin-Elmer). A sample of 1/10
4 µl of the first PCR product was transferred to a new tube, followed by the addition of the second set of primers, fresh
Taq polymerase and fresh dNTPs. The semi-nested PCR reaction was performed as described above.
The 1.5 kb DNA fragments obtained by amplification of thirty blood samples were separated on a 0.8% agarose gel. The amplified DNA was purified from agarose gel using a QIAEX II gel extraction kit (Qiagen, Valencia, CA, USA) following the manufacturer's instruction. The nucleotide sequence was determined by dideoxynucleotide chain termination method using a sequenase kit (ABI PRISM Dye Terminator Cycle Sequencing Core Kit, Perkin Elmer) and an automated DNA sequencer (Applied Biosystems model 377A, Perkin Elmer). The DL5, DS3, and DL3 (5'-TCATTCTCAAAAGCCACCTC-3') primers were used for direct sequencing. In order to detect any possible nucleotide mis- incorporation due to false Taq polymerase activity, two of each amplified products were sequenced.
The DNA sequencing data were analyzed using DNASIS (Hitachi, Ver. 2.5, Japan) and the BLAST program of the NCBI databases (NIH, Bethesda, MD, USA). Our sequence data were also compared with published sequences of
P. vivax isolates (
Tsuboi et al., 1994;
Ampudia et al., 1996).
To distinguish the difference between SK-1 and SK-2 genotypes, two different oligonucleotide probes were used in this study. Two oligonucleotides, D4-AF1 (5'-TGATAGCG ATGGACC-3') and D4-BF3 (5'-GAATTTGC AGAATCTACGAAAT-3'), were designed and synthesized to detect 6-bp insert of the Sal-1 and 27-bp insert of the PNG isolate, respectively. The oligonucleotides were radiolabeled with 32P-dCTP by 5'-end labeling kit (Amersham-Pharmacia Biotech, Uppsala, Sweden) following the manufacturer's instruction. Unincorporated nucleotides were removed from the labeling mixture by a spin column (Sephadex G-25) chromatography.
One µg of PCR product for PvDBP gene was separated by 0.8% agarose gel electrophoresis and transferred to Hybond N+ membrane (Amersham-Pharmacia Biotech) by capillary action. These southern blots were hybridized with the radio-labelled oligonucleotides. The prehybridization and hybridization were carried out using Hybrid sol I (Oncer, Gaitherberg, MD, USA) in a hybridization incubator (Robbinson Scientific, Sunnyvale, CA, USA). After hybridization overnight at 42℃, the membrane was washed with 1 × SSC (10 × SSC; 1.5 M NaCl and 0.15 M sodium citrate) containing 0.2% SDS for 10 min at 50℃, then with 0.5 × SSC/0.2% SDS for 10 min at 55℃. The membrane was exposed to Kodak XAR-5 film overnight at -70℃ with intensifying screens.
RESULTS
PCR products of PvDBP gene
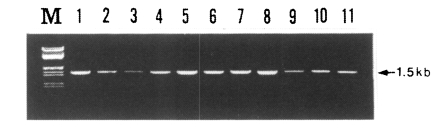
PCR amplification of the gene of PvDBP gene was attempted for 30 blood samples obtained from patients in the Republic of Korea. The primary PCR products showed a specific band in about 83% of samples (25/30 isolates), whereas the second round PCR showed a specific band pattern in all of the samples (30/30 isolates). The size of PCR product was 1.5 kb which includes the fragment encoding regions II (partial), III, and IV (partial) of the PvDBP gene (
Fig. 1). There was no difference in size, but a slight difference in band intensity was observed (
Fig. 2). There was not any specific product from un-infected donor (data not shown).
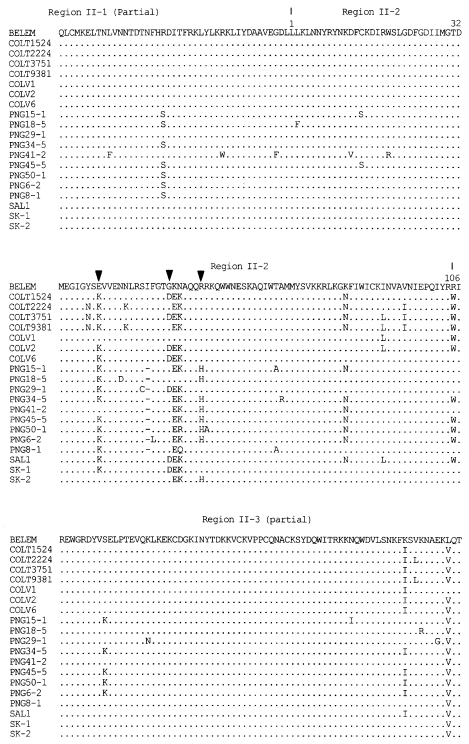
The DNA sequences of the 30 isolates were determined and deduced by amino acid sequences (
Fig. 3). Two genotypes, SK-1 (Genbank accession number AF215738) and SK-2 (Genbank accession number AF215738), were identified on the basis of grouping mutations in the nucleotides and their corresponding amino acids. Of 30 isolates, 10 isolates showed SK-1 genotype and 20 isolates SK-2 genotype. Most of the predicted amino acid sequence in the region II of DBP were conserved between the Korean isolates and the Belem strain. The amino acid substitutions occurred in the middle of the amino cysteinerich region (region II-2). Compared with the region II-2 of the Belem strain, 4 variations in SK-1 and 3 variations in SK-2 among 107 amino acids were found. Two of these variations in positions 54 (Lys/Glu) and 55 (Asn/Lys) were shared in SK-1 and SK-2. Variations at 40 (Glu/Lys) and 53 (Gly/Asp) were unique in SK-1 and variation at 59 (Gln/His) was found only in SK-2.
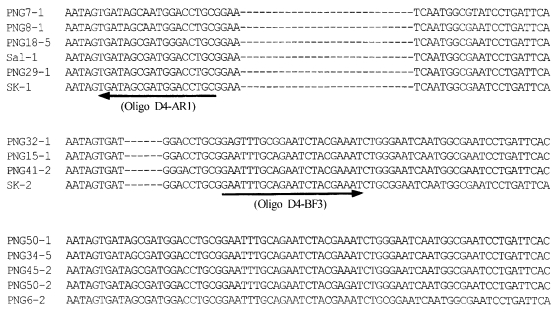
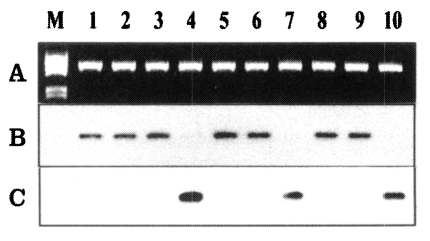
In the region IV of DBP, a 6-bp insert that shows the PNG allele type was found in SK-1, and a 27-bp insert that shows the Sal-1 allele type was found in SK-2 (
Fig. 4). The oligonucleotide probes (D4-AF1 and D4-BF3) were designed to identify the sequences of the two different types, SK-1 and SK-2. The D4-AF1 oligonucleotide probe was specific to SK-1 type, which characterized the 6-bp insert of the Sal-1 type, and hybridized to 10 of the 30 isolates (
Fig. 5B). The D4-BF3 oligonucleotide probe specific to SK-2 type hybridized to 20 of the 30 isolates (
Fig. 5C).
DISCUSSION
The central binding residues of PvDBP lay in 170 amino acids stretch between cysteines 4 and 7 of region II, between region II-1 and II-3, and participate in the binding process to human erythrocyte (
Ranjan and Chitnis, 1999). A previous study showed that the amino acid changes mainly observed in the middle of the cysteine-rich region (region II-2
Tsuboi et al, 1994). However, it is also demonstrated that three variants of the Duffy binding domain bind to RBCs in an identical fashion (
Chitnis et al., 1994). It seems more likely that the changes are a result of immune pressure or selection. Therefore, the conserved residues of this region are less likely to be subjected to immune pressure and possibly involved as a core part in erythrocyte binding.
In this study, part of region II including the central binding domain was studied in the Korean isolates. Most of the predicted amino acids in the region II of PvDBP were conserved between the Korean isolates and the Belem strain. Of the total 225 amino acids (part of region II), variations at 5 positions were found, corresponding to a polymorphism of 2.2% compared with the Belem strain (
Fig. 3). These 5 variations of the Korean isolates were also found in a previous study in PNG or Colombia (
Tsuboi et al., 1994;
Ampudia et al., 1996). It is proved that the variations in these positions do not affect erythrocyte specificity (
Barnwell et al., 1989;
Chitnis et al., 1994). Therefore, the variations of the PvDBP region II found in SK-1 and SK-2 genotypes are considered not to affect the binding affinity of PvDBP to RBCs.
Tsuboi et al. (
1994) classified the DNA sequence of region IV into three groups based on the inserted nucleotides: the first group has a 6-bp insert and no 27-bp insert; the second group has a 27-bp insert and no 6-bp insert; the third group has both 6-bp and 27-bp inserts. According to the Tsubois' criteria, SK-1 and SK-2 genotypes in region IV were identical to the first and second group, respectively. Results from this study support the postulation that the variations of
PvDBP region IV are formed by the tandem repeats (
Tsuboi et al 1994). The tandem-repeat polymorphism was identified in the merozoite microneme protein-1 (MP-1) genes of
P. knowlesi (
Prickett et al., 1994;
Chitnis et al., 1996) and
P. falciparum (
Ware et al., 1993). This result suggests that the
Plasmodium MP-1 family has a common mechanism for genetic exchange among different genotypes in a given species. To distinguish SK-1 and SK-2 genotypes, two different oligonucleotide probes were designed on the basis of PvDBP region IV nucleotide sequence. As shown in
Fig. 5, the two genotypes of SK-1 and SK-2 can be easily distinguished by the hybridigatin using these probes. The DNA sequencing data of the 30 specimens coincided very well with the hybridization results (data not shown). Therefore, the hybridization with these probes can be useful in distinguishing the two genotypes of
P. vivax found in Korea instead of using the gene sequencing technique.
In addition to vaccine development, the information of the genetic variation of an antigen can be utilized in various fields including genetics, and epidemiology. The genetic variation of some genes for
Plasmodium sp. is geographically related. Therefore, the information of genetic variation may be used in geophylogenetic studies and tracking the origin of
Plasmodium. For this purpose, we have reported the genotypes (SK-A and SK-B) of
P. vivax circumsporozoite (PvCSP) of the Korean isolates (
Kho et al., 1999). The analysis of
P. vivax genotype may demonstrate geographical linkage, which will help to find the origin of parasites. However, the geographical characteristic linkage of the PvDBP gene is unknown because it is purely understood at the molecular level. To date, the genetic study of PvDBP has only been done in PNG, Columbia, and Korea (
Tsuboi et al., 1994;
Ampudia et al., 1996). The PvDBP regions II and IV DNA sequences of the Korean isolates were found to have no identical sequence when compared with known data. To understand the PvDBP correlation according to the geographical distribution, further investigations of the isolates of Southeast Asia, China, and neighboring countries are required.
Concerning the extent of genetic polymorphism of PvDBP according to the geographical region, two genotypes were found from 30 patients in Korea while 12 genotypes from 50 patients in PNG and 17 genotypes from 20 patients in Colombia were found (
Tsuboi et al., 1994;
Ampudia et al., 1996). In this study, the number of positions where the change occurred was five from 255 amino acids while genetic variations were found at 37 positions from a total of 300 amino acids, corresponding to a polymorphism of 12% in PNG, and 19 positions from a total of 383 amino acids showed polymorphism (5%) in Colombia, respectively (
Tsuboi et al., 1994;
Ampudia et al., 1996). The difference both in the number of genotypes and positions changed in amino acid sequences between Korea and two other countries may be to the difference in the intensity of malaria transmission.
Recently, attempts are being made to evaluate the transmission intensity using genetic information; genetic variation in hyperendemic areas have been found to be larger than in hypo-endemic areas (
Babiker and Walliker, 1997;
Paul et al., 1998). Previous studies of the genetic variation within the amino terminal of the cysteine rich region in PvDBP show several patterns among isolates, also observed is a large genetic variation in the high transmission intensity areas (
Tsuboi et al., 1994). Because the variations may occur mainly due to a continuous immune pressure or selection. The both of two countries, PNG and Columbia, are malaria endemic areas and have shown high transmission intensity for a long time.
Although the recent intensity of malaria transmission in the Republic of Korea has not been scientifically measured, it is believed that the opportunity of malaria being transmitted by mosquitoes might be strictly limited since the transmission is possible during the limited season from May to October and the transmission ability of vector mosquito may be extremely low (
Sim et al., 1997;
Ree, 2000). When considering the epidemic characteristics of malaria, the genetic change may occur more slowly in Korea than in the high-endemic areas. In the Republic of Korea, one case of indigenous vivax malaria, which had disappeared since 1984, had been reported in 1993 and the number of cases has increased exponentially year after year. Currently about four thousand cases are reported annually (
Chai et al., 1994;
NIH, 2000). The result of this study may also be utilized as baseline data for monitoring the genetic change in an endemic area, because new malaria endemic recently begins in Korea. Therefore, it is thought that the results of this study in Korea may assist in elucidating the mechanism of polymorphisms due to immune pressure or selection and the mechanism of its spread in the gene.
In this study, we show that the PvDBP of Korean isolates has little polymorphism and that two genotypes of P. vivax coexist in the endemic areas of the Republic of Korea. The result shown in this study is significant in that it is the first report for PvDBP in the Far East Asia.
Notes
-
The authors wish to acknowledge the financial support of the Korea Research Foundation made in the program year of 1998.
References
- 1. Adams JH, Hudson DE, Torii M, et al. The Duffy receptor family of Plasmodium knowlesi is located within the micronemes of invasive malaria merozoites. Cell 1990;63:141-153.
- 2. Adams JH, Sim BK, Dolan SA, Fang X, Kaslow DC, Miller LH. A family of erythrocyte binding proteins of malaria parasites. Proc Natl Acad Sci 1992;89:7085-7089.
- 3. Ampudia E, Patarroyo MA, Patarroyo ME, Murillo LA. Genetic polymorphism of the Duffy receptor binding domain of Plasmodium vivax in Colombian wild isolates. Mol Biochem Parasitol 1996;78:269-272.
- 4. Babiker HA, Walliker D. Current views on the population structure of Plasmodium falciparum: Implication for control. Parasitol Today 1997;13:262-267.
- 5. Barnwell JW, Nichols ME, Rubinstein P. In vitro evaluation of the role of the Duffy blood group in erythrocyte invasion by Plasmodium vivax. J Exp Med 1989;169:1795-1802.
- 6. Berzins K, Anders RF. In Wahlgren M, Perlmann P eds, The malaria antigens. Malaria Molecular and Clinical Aspects. 1999, Switzerland. Harwood Academic Publishers; pp 181-216.
- 7. Bolad A, Berzins K. Antigenic diversity of Plasmodium falciparum and antibodymediated parasite neutralization. Scand J Immunol 2000;52:233-239.
- 8. Chitnis CE, Miller LH. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J Exp Med 1994;180:497-506.
- 9. Chitnis CE, Chaudhuri A, Horuk R, Pogo AO, Miller LH. The domain on the Duffy blood group antigen for binding Plasmodium vivax and P. knowlesi malarial parasites to erythrocytes. J Exp Med 1996;184:1531-1536.
- 10. Chai IH, Lim GI, Yoon SN, Oh WI, Kim SJ, Chai JY. Occurrence of tertian malaria in a male patient who has never been abroad. Korean J Parasitol 1994;32:195-200.
- 11. Kho WG, Park YH, Chung JY, et al. Two new genotypes of Plasmodium vivax circumsporozoite protein found in the Republic of Korea. Korean J Parasitol 1999;37:265-270.
- 12. Miller LH, Mason SJ, Clyde DF, McGinniss MH. The resistance factor to Plasmodium vivax in blacks: The Duffy-blood-group genotype, FyFy. N Engl J Med 1976;295:302-304.
- 13. National Institute of Health. Communicable Diseases monthly report. CDMR 2000;11:24. (in Korean).
- 14. Paul REL, Hackford I, Brockman A, et al. Transmission intensity and Plasmodium falciparum diversity on the northwestern border of Thailand. Am J Trop Med Hyg 1998;58:195-203.
- 15. Prickett MD, Smarz TR, Adams JH. Dimorphism and intergenic recombination within the microneme protein (MP-1) gene family of Plasmodium knowlesi. Mol Biochem Parasitol 1994;63:37-48.
- 16. Ranjan A, Chitnis CE. Mapping regions containing binding residues within functional domains of Plasmodium vivax and Plasmodium knowlesi erythrocyte-binding proteins. Proc Natl Acad Sci 1999;96:14067-14072.
- 17. Ree HI. Unstable vivax malaria in Korea. Korean J Parasitol 2000;38:119-138.
- 18. Rich SM, Ferreira MU, Ayala FJ. The origin of antigenic diversity in Plasmodium falciparum. Parasitol Today 2000;16:390-396.
- 19. Sim JC, Shin EH, Yang DS, Lee WK. Seasonal prevalence and feeding time of mosquitoes (Diptera: Culicidae) at outbreak regions of domestic malaria (P. vivax) in Korea. Korean J Entomol 1997;27:265-270. (in Korean).
- 20. Tsuboi T, Kappe SH, al-Yaman F, Prickett MD, Alpers M, Adams JH. Natural variation within the principal adhesion domain of the Plasmodium vivax Duffy binding protein. Infect Immun 1994;62:5581-5586.
- 21. Ware LA, Kain KC, Lee Sim BK, Haynes JD, BairdJK , Lanar DE. Two alleles of the 175-kilodalton Plasmodium falciparum erythrocyte binding antigen. Mol Biochem Parasitol 1993;60:105-109.
Fig. 1Schematic representation of the PvDBP gene and its region. The relative position of primers for PCR (DL5, DS3, and PD3) and sequencing (DL5, PD3, and DL3) are indicated beneath the map. The structurally distinct regions of the PvDBP gene are shown above the diagram in Roman numbers (
Adams et al., 1992): I, N terminus before amino cysteine-rich region; II, amino cysteine-rich region; III to V, hydrophilic regions; VI, carboxyl cysteine-rich region; VII, transmembrane domain region.
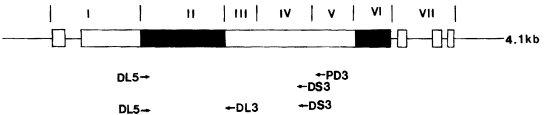
Fig. 2The PCR products from the PvDBP gene of Korean isolates. The semi-nested PCR products were separated on a 0.8% agarose gel and stained with ethidium bromide. M, λDNA/EcoR I + Hind III marker (MBI fermantas); lanes 1-11, SK isolates 1-10.
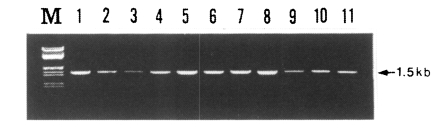
Fig. 3Alignment of deduced amino acid sequences of SK isolates comparing Sal-1, Belem, PNG (Papua New Guinea), COLV and COLT (Colombia) isolates in amino cysteine-rich region (region II, partial). The change between SK-1 and SK-2 genotype is showed by an arrow head (▼). The distinction of region II is showed above the amino acid sequence (
Tsuboi et al., 1994).
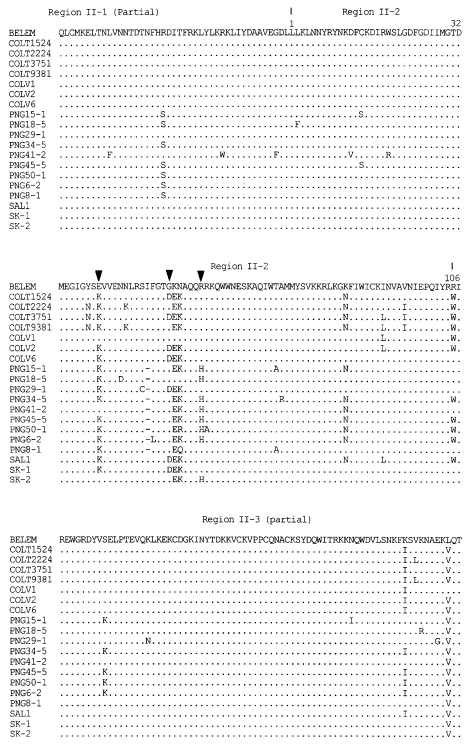
Fig. 4The nucleotide sequence comparisons for the region IV in PvDBP from South Korean isolates (SK-1 and SK-2), Papua New Guinea (PNG) isolates and Sal-1 allele (
Tsuboi et al., 1994). Gaps indicated by dashes (---) are introduced into the sequence to optimize the alignment. Nucleotide sequence corresponding to oligonucleotides D4-AF1 and D4-BF3 were marked by underlined and arrowed, respectively.
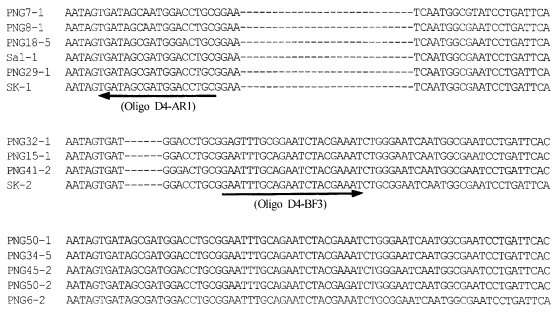
Fig. 5Analysis of PvDBP gene at the region IV by oligonucleotide hybridization. The PCR product (1 µg) transferred to nylon membrane and hybridized using the oligonucleotide primers. A. The PCR product separated on a 0.8% agarose gel. B. The transferred membrane was hybridized with the D4-BF3 oligonucleotide probe. C. The membrane was stripped and re-hybridized with the D4-AF1 probe.