Abstract
The diagnosis of cryptosporidiosis has been carried out using coprologic techniques in the Republic of Korea. However, antibody responses to Cryptosporidium have rarely been studied. Serum antibodies from HIV-positive/oocyst-positive Korean patients recognized significantly 31 and 27 kDa antigens, and HIV-negative/oocyst-positive individuals clearly reacted to 15/17 kDa antigens. Compared with oocyst-positive cases, 18.7% and 75.8% of sera from HIV-positive patients reacted to 31 and 27 kDa antigens. Only 11.1% of HIV-negative individuals reacted to 15/17 kDa. Based on these findings, serum antibody responses were different between HIV-positive and HIV-negative individuals infected with Cryptosporidium, and it is suggested that HIV-positive patients are more frequently exposed to C. parvum compared to HIV-negative individuals.
-
Key words: Cryptosporidium parvum, serologic response, antigenic protein, HIV
INTRODUCTION
Cryptosporidium is an intestinal protozoan causing self-limiting diarrhea in immunocompetent individuals, but can be life-threatening in immunocompromised patients [
1]. Hence, there is a need to evaluate serologic responses of immunocompromised patients against this pathogen, particularly in HIV-infected individuals. Sorvillo et al. [
2] reported that 120 (2.8%) HIV-positive patients were diagnosed as cryptosporidiosis among 4,247 examined, but the overall positive rate for
Cryptosporidium was 5.3% after follow-ups until death [
2]. In India, the positive rate was 9.2% among HIV-positive individuals with diarrhea [
3], and oocysts were detected up to 4% of AIDS patients in USA [
4]. According to the report of National Institute of Health, a total of 5,155 Koreans were infected with HIV as of January 2008, and the number has been rapidly increasing [
5]. There were few reports on opportunistic parasitic infections in the Republic of Korea; oocysts of
Cryptosporidium parvum or
Isospora belli were 3.1% of HIV-infected individuals [
6], and Guk et al. [
7] reported that 10.5% of AIDS patients suffered from
C. parvum infection. However, the reports on
C. parvum were insufficient considering its epidemiologic and clinical significances.
The diagnosis of cryptosporidiosis is usually based on examination of fecal smears with acid-fast stains [
8]. However, fecal examinations may underestimate the prevalence of
Cryptosporidium infection, not only because this infection is not a part of standard parasitological examinations, but because the duration of oocyst shedding can be short and intermittent [
9]. Thus, serologic assays may be an alternative way for investigating the epidemiology of
Cryptosporidium infection. Although detection of specific antibodies should not be necessarily regarded as an active infection, some antigens identified by immunoblot analysis are considered as excellent markers of infection, especially for 15/17 kDa and 27 kDa proteins [
10]. The sensitivity and specificity of ELISA using 27 kDa antigen (CP23) were 0.86 and 0.86, respectively, for predicting its infection, and a serological assay using 27 kDa antigen was proved to be more accurate regarding the community levels of
Cryptosporidium infection [
11,
12]. By assaying the antibody responses against 27 kDa and 17 kDa antigens, Sandhu et al. [
13] emphasized a high risk of HIV-infected population to
Cryptosporidium infection [
13].
In the Republic of Korea, however, epidemiological surveys for Cryptosporidium infection using serological assays have never been undertaken either on healthy population or on HIV-infected patients. The present study was performed to investigate serum antibody responses in HIV-positive and HIV-negative individuals compared with the results of oocyst examinations. This report may provide basic data to evaluate the risk of exposure to Cryptosporidium infection in each group, and to study the pathogenic proteins of Cryptosporidium.
MATERIALS AND METHODS
Collection of sera
Serum specimens were obtained from the following groups;
1) Eight oocyst-confirmed cryptosporidiosis patients living in Ssangbong-ri, Iyang-myon, Jeollanam-do, an endemic area of cryptosporidiosis. Their ages were distributed from 50 to 79 yr, and they discharged C. parvum oocysts at routine examinations over 3 times (positive control 1). The samples were collected during 1996-1997.
2) Four HIV-positive patients visiting Seoul National University Hospital during 1995-1998, who were confirmed to be heavily infected with C. parvum by microscopic examinations (positive control 2); all of them were males, and 29-34 yr in age range.
3) Ninety-one HIV-positive patients, who were not examined for C. parvum infection and whose HIV infections were confirmed by western blotting in Department of AIDS, National Institute of Health, Republic of Korea, from October 1993 to April 1995. The sex ratio and age could not be investigated (control group 1).
4) Twenty-seven healthy individuals not examined for C. parvum infection, all of whom were students in their 20s, attending Seoul National University College of Medicine. The sex ratio was not investigated, and the samples were collected in 1997 (control group 2).
5) In each test, human sera uninfected with any parasites were used as a negative control.
Antigen preparation
The oocysts were purified by ether extraction and discontinuous sucrose gradients from the feces of calves, experimentally infected with the Korean isolate of
C. parvum. The feces were collected daily, mixed with equal volumes of 2.5% potassium dichromate (K
2Cr
2O
7), and stored at 4℃. Oocysts were isolated by means of discontinuous sucrose gradients [
14]. Briefly, Sheather's solution containing sucrose was diluted with 0.025 M phosphate-buffered saline (PBS) supplemented with 1% Tween 80 to make 1 : 2 and 1 : 4 gradient solutions. After centrifugation, the oocysts were collected from the interface of the 2 gradients. Purified oocysts were washed with PBS and then used for the antigen preparation. Antigens of
C. parvum were prepared as previously described [
15]. In brief, oocysts were disrupted by sonication followed by 5 freeze-thaw cycles. The lysate was clarified by centrifugation, and the supernatant was used as the crude antigen.
The soluble fraction was separated by SDS-PAGE, and electrophoretically transferred onto a polyvinylidene fluoride (PVDF) membrane (Immobilon, Millipore Corp., Bedford, Massachusetts, USA) at 0.5 mA for 1-2 hr. The membranes were blocked in 0.2% skim milk/PBS (pH 7.4) for 2 hr, and incubated with serum samples for 12 hr. The secondary antibody was peroxidase-conjugated goat anti-human IgG (Cappel, Cochraville, Pennsylvania, USA), color developed using a substrate solution (0.6% chloronaphtol, 0.2% diaminobenzidine, 0.02% H2O2/PBS). All examinations were repeated 2-3 times.
RESULTS
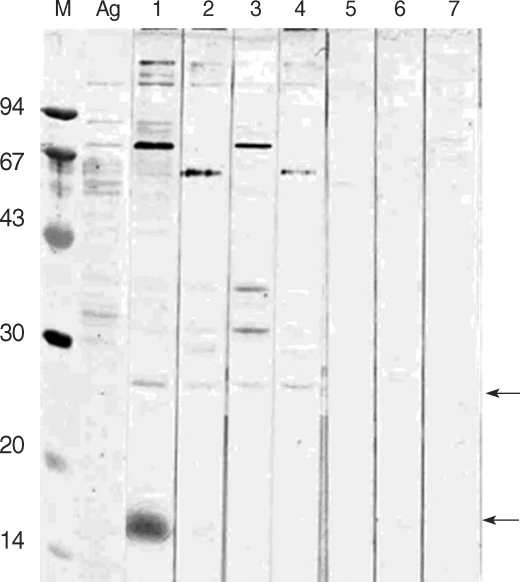
The soluble extract of
C. parvum showed multiple bands ranging 14-148 kDa in 12.5% SDS-PAGE gel. The sera from HIV-positive/oocyst-positive patients reacted with several protein bands, ranging 15/17-128 kDa. The positive rate to either of 62, 31 or 27 kDa band was 100%. The
C. parvum-negative sera cross-reacted with 128, 124, 114, and 62 kDa bands (
Fig. 1).
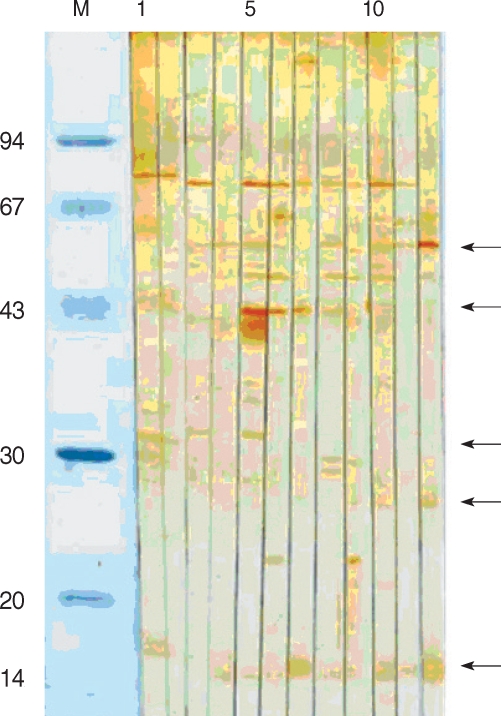
The 15/17 kDa antigen was recognized by 2 sera (50%). All sera (100%) from HIV-negative/oocyst-positive patients reacted against 15/17 kDa, and the reaction rates were higher than 60% against 55, 48, and 44 kDa antigens (
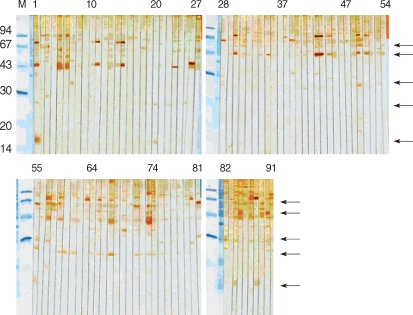
Fig. 2). The 31 kDa and 27 kDa antigens were recognized only by 3 sera (37.5%), the rate of which was significantly lower compared to the sera of HIV-positive/oocyst-positive patients. Overall, reaction to 15/17 kDa antigen was the highest (10/12), followed by 44 kDa (8/12), 55, 31, and 27 kDa (all were 7/12) antigens in the positive control groups. In particular, in control group 1, the sera of the patient numbers 1, 84, and 88 strongly reacted with 15/17 kDa antigen (
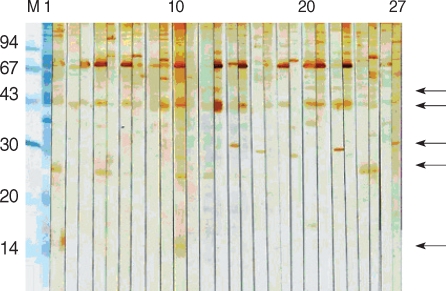
Fig. 3). Compared with the results of positive groups, reactions against 44 kDa (84/118), 27 kDa (73/118), 55 kDa (34/118), and 15/17 kDa (14/118) were observed in control 1 and 2 groups (
Fig. 3,
4). Collectively, the sera from HIV-positive/oocyst-positive patients significantly reacted to 27 and 31 kDa, whereas the sera of HIV-negative/oocyst-positive individuals reacted mostly to 15/17 kDa antigens.
The sera of HIV-positive individuals in control group 1 reacted against more proteins than the sera of HIV-negative individuals in control group 2. The 27 kDa antigen was the most frequently recognized by the sera of control group 1 (75.8%), whereas the 31 kDa antigen was recognized by 18.7% of the sera. In control group 2, the 31 kDa antigen was not reactive, whereas the 27 kDa was recognized by 14.8% of sera. The sera of 11.1% reacted weakly to the 15/17 kDa antigen.
The 27 and 15/17 kDa antigens were recognized by sera 100 % and 50%, respectively, in HIV-positive/oocyst-positive individuals, and 37.5% and 100%, respectively, in HIV-negative/oocyst-positive individuals. Furthermore, the combination of 31 and 27 kDa antigens were recognized 100% by HIV-positive/oocyst-positive sera, and 18.7% in control group 1.
DISCUSSION
The present study is the first to study the serologic responses of humans to
Cryptosporidium antigens in the Republic of Korea. Although many proteins were identified, a particular emphasis must be focused on 27 and 15/17 kDa antigens, which reacted significantly in oocyst-positive groups and are also known as serologic markers [
10-
13]. According to Sandhu et al. [
13],
Cryptosporidium infections are supposed to be common among HIV-positive individuals, whereas the absence of overt clinical cryptosporidiosis is due to the presence of intact immune responses among HIV-positive individuals [
13]. In the present study, immunoblot results demonstrated that sera from HIV-positive individuals recognized more protein fractions of
C. parvum than those from HIV-negative individuals. It has been reported that strong serological responses to 27 kDa antigen in immunosuppressed individuals were associated with a reduced risk of developing diarrhea [
16]. In the present study, it has been shown that 31 and 27 kDa antigens were significantly reacted in HIV-positive/oocyst-positive group. These results indicate that we could estimate
C. parvum-infection in HIV-positive patients through the reaction of 27 kDa antigen and the combination of 27 and 31 kDa antigens. In particular, 61.9% of control groups 1 and 2 (
C. parvum-infection was not examined) reacted with 27 kDa antigen. Only with these results, the real infection rate of
C. parvum infection cannot be explained. However, in control group 1, the combination of 27 and 31 kDa antigens were recognized by 18.7% and it is suggested that they have a possibility of
C. parvum infection.
However, serologic assays cannot solely determine
C. parvum infection. Given there was little agreement between serum ELISA and fecal tests on samples collected from adult cows [
17], serological assays should be used to support fecal examinations. The reported infection rate of randomly selected inhabitants in Seoul was only 0.5% [
18], whereas another report identified that the majority (94.6%) of oocyst-positive cases were people over 50 yr of age [
19]. Furthermore, Seo et al. [
20] reported that
C. parvum infections were confined to the over 30-yr age group [
20]. In the present study, it is considered that HIV-negative/oocyst-positive individuals were repeatedly infected with
C. parvum in the endemic area. Their sera reacted with mainly 55, 44, and 15/17 kDa antigens, and we focused on the reaction with 15/17 kDa antigen. Since members of control group 2 were young (in their 20s) medical college students, living in Seoul, their
C. parvum infection rate should have been low. Their sera showed 11.1% reactivity against 15/17 kDa antigens.
In humans infected with
Cryptosporidium, most oocyst-derived glycoproteins and sporozoite surface proteins, including 31, 27, 15/17 kDa, were recognized by serum antibodies [
10]. A study of laboratory-confirmed cryptosporidiosis patients showed that IgG levels against 27 kDa antigen tended to remain above the detection threshold for at least 2 yr after infection, whereas the levels of antibody against 17 kDa antigen decreased rapidly [
11]. The higher reactivity against 27 kDa of HIV-positive sera supports this phenomenon. It has been reported that HIV-positive individuals were more likely to have at least one infection during an 8.5-yr follow-up period compared to HIV-negative individuals (59% vs 30%) [
16]. Since most infections are acquired from water or food contaminated with infectious oocysts [
21], these results may reflect continuous exposure of people to contaminated environments. Although the results of the present study are important, they must be considered in light of methodological limitations, such as limited microscopically confirmed cases of cryptosporidiosis, and no information on their clinical status. Based on the findings of the present study, it is suggested that serum antibody responses were different between HIV-positive and HIV-negative individuals infected with
Cryptosporidium. In addition, it is possible that HIV-positive individuals may be more frequently exposed to
Cryptosporidium infection than immunocompetent people in the Republic of Korea.
ACKNOWLEDGEMENTS
This work was funded by Medical Science Research Center of Dankook University Medical Center in 2007. We are also thankful to Dr. M.H. Choi (Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, Seoul, Korea) for collection of blood samples from HIV+ patients.
References
Fig. 1
Cryptosporidium parvum antigenic proteins recognized by sera from HIV-positive/C. parvum oocyst-positive patients. M, molecular weight (kDa); Ag, oocyst antigen; lanes 1-4, HIV-positive/oocyst-positive patients; lanes 5-7, HIV-positive/oocyst-negative control patients. Arrows point to 27 and 15/17 kDa bands.

Fig. 2
C. parvum proteins recognized by sera from HIV-negative/oocyst-positive individuals. M, molecular weight (kDa); lanes 1-4, negative control; lanes 5-12, HIV-negative/oocyst-positive individuals. Arrows point to 55, 44, 31, 27, and 15/17 kDa bands.

Fig. 3Immunoblot with sera of HIV-positive patients. Arrows point to 55, 44, 31, 27, and 15/17 kDa bands.

Fig. 4Immunoblot with sera of HIV-negative individuals. Arrows point to 55, 44, 31, 27, and 15/17 kDa bands.