Abstract
A novel recombinant Bacille Calmette-Guerin (rBCG) vaccine co-expressed Eimeria tenella rhomboid and cytokine chicken IL-2 (chIL-2) was constructed, and its efficacy against E. tenella challenge was observed. The rhomboid gene of E. tenella and chIL-2 gene were subcloned into integrative expression vector pMV361, producing vaccines rBCG pMV361-rho and pMV361-rho-IL2. Animal experiment via intranasal and subcutaneous route in chickens was carried out to evaluate the immune efficacy of the vaccines. The results indicated that these rBCG vaccines could obviously alleviate cacal lesions and oocyst output. Intranasal immunization with pMV361-rho and pMV361-rho-IL2 elicited better protective immunity against E. tenella than subcutaneous immunization. Splenocytes from chickens immunized with either rBCG pMV361-rho and pMV361-rho-IL2 had increased CD4+ and CD8+ cell production. Our data indicate recombinant BCG is able to impart partial protection against E. tenella challenge and co-expression of cytokine with antigen was an effective strategy to improve vaccine immunity.
-
Key words: Eimeria tenella, recombinant BCG, rhomboid, chIL-2, immunity
INTRODUCTION
Coccidiosis is an economically important disease in poultry industry by intracellular infection of protozoan parasites of the genus
Eimeria, which infect the epithelial cells of intestinal lining [
1]. Economic losses due to coccidiosis include mortality, malabsorption, inefficient feed utilization, impaired growth rate in broilers, and reduction of egg production in layers [
2]. Anticoccidial drugs, as well as live and attenuated parasite vaccines, are available to control the disease [
3], but have been limited by the emergence of drug resistance, the increasing regulatory restrictions on antibiotic use in poultry production, and the risks of reversion to highly virulent strains in the host [
4,
5]. Therefore, with the progress of molecular biology, recombinant vaccines that elicit specific immunity are eminently preferable as alternatives [
6].
Cellular immune responses are required for protective immunity against
Eimeria tenella. Immunization strategies using live intracellular bacteria (such as Bacille-Calmette Guerin strain of
Mycobacterium bovis) expressing heterologous antigens can induce cellular immune responses [
7]. IL-2 plays an important role in function of the immune system. Chicken IL-2 (chIL-2) gene has been cloned [
8], and its biological function as a potent growth factor for a variety of cell types including T cell differentiation, B cell development, and NK cell activation has been characterized [
9,
10,
11]. ChIL-2 could enhance protective immunity against avian pathogens, which could introduce a strategy on the control of infectious diseases of poultry.
In previous studies, we demonstrated the construction of the integrative vector pMV361-rho, and the result indicated that
M. bovis BCG was a novel vaccine vector to express and present foreign antigens, and rBCG had a potential as a vaccine in chickens [
12,
13]. Yang et al. [
13] reported the
E. tenella rhomboid protein could stimulate lymphocyte production of infected chickens and immunization with recombinant rhomboid could produce protection against
E. tenella infection. Xu et al. [
14] reported vaccination of chickens with a chimeric DNA vaccine encoiding
E. tenella TA4 and chIL-2 induced protective immunity against coccidiosis, and the ACI was much higher in the group co-expressed TA4 and chIL-2. Lillehoj et al. [
15] documented co-injection of the IL-2 gene with the 3-1E coccidia gene enhanced the host response to the recombinant vaccination. Pasquini et al. [
16] reported that IL-2, IL-4, IL-12, and IFN-γ amplified immune responses to genetic vaccines.
Here we combined the advantages and report the construction of a recombinant BCG (rBCG) co-expressing rhomboid of E. tenella and chIL-2 gene and its efficacy against coccidiosis in chickens. Both intranasal and subcutaneous routes of rBCG pMV361-rho and pMV361-rho-IL2 vaccination had been investigated. The protective effects of immunization were evaluated by mortality, cecal lesion scores, oocysts output, and growth performance after a challenge with E. tenella oocysts. To our knowledge, little report has been published on rBCG used in avian species against coccidiosis.
MATERIALS AND METHODS
Experimental chickens
One-day-old specific-pathogen-free (SPF) chickens, purchased from Center of Laboratory Animal in Jilin Province (Jinlin, China), were reared in a coccidia-free environment in wire cages. Feed and water were supplied ad libitum.
Parasite and plasmids
A highly virulent
E. tenella F2 hybird strain isolated in China (Guangzhou, Baoding, and Changchun) was used in the present study [
13,
17], and sporulated oocysts were stored in 2% potassium dichromate solution at 4℃. Plasmids pMD-rhomboid containing the full length of rhomboid gene [
17] and plasmid pMD18-T-IL2 with the complete chIL-2 gene was provided by Dr. Li XR, College of Veterinary Medicine, Nanjing Agricultural University, China. BCG integrative expression vector pMV361 [
18] was kindly provided by Dr. Xu Heng, Institute of Biological Sciences, Sichuan University, China.
PvuII and XbaI sites were respectively added at the 5' and 3' ends of rhomboid gene primers. The sequences of the primers were: forward 5'-CTGA
CAGCTGATGTCGGACATCGAATCCCAGAG-3'; reverse: 5'-GCT
TCTAGATGCGCATCCCATGGGCAAAGGA-3'.
XbaI and
ClaI sites were added at the 5' and 3' ends of chIL-2 gene primers. The sequences of the primers were: forward 5'-CCG
TCTAGAATGTCTCTATCATCAG-3'; reverse: 5'-GCC
ATCGATTTATTTTTGCAG-3'. The above 2 DNA fragments were ligated together using a DNA Ligation Kit (Company Name, City, State, Country) and subcloned into the pMV361 expression vector, digested with
PvuII and
ClaI restriction enzymes previously. PCR amplification of rhomboid was performed using primers adding
PvuIIand
ClaI sites and subcloned into pMV361 expression vector as the same method to produce pMV361-rho. The recombinant plasmids pMV361-rho and pMV361-rho-IL2, with correct structures verified by restriction analysis and PCR amplification, were electrotransfected into BCG and selected by kanamycin as described previously [
12,
19]. The rBCG pMV361-rho and rBCG pMV361-rho-IL2 strains were routinely grown in Middlebrook 7H9 medium (Company Name, City, State, Country) supplemented with an albumin-dextrose complex enrichment. The rBCG vaccines were prepared from mid-log-phase liquid cultures of selected clones.
The cultivation and inducing expression of rBCG pMV361-rho and pMV361-rho-IL2 were carried out as described previously [
12,
19]. After a 2-hr induction period at 45℃, the rBCG strains were harvested by centrifugation and treated with Tris-EDTA containing 25% saccharose and lysozyme (100 µg/ml), and distrupted on ice with ultrasound sonication. For immunoblot analysis, bacterial pellets were directly resuspended in sodium dodecyl sulfate (SDS) sample buffer and proteins were separated by SDS-PAGE using 5-12% gradient gels. The Western bolt was done to detect the aim proteins with the 1:1,000 dilution of mouse antiserum against sporozoites of
E. tenella as the first antibody.
For challenge assays, chickens were immunized with 107 colony forming units (CFU) fresh cultures of exponentially growing rBCG. A total of 180 one-day-old SPF chickens were divided into 6 groups and immunized at day 7 and 14 of age as follows: chickens in group I was intranasal immunization with rBCG pMV361-rho-IL2, and group II was vaccinated subcutaneously with rBCG pMV361-rho-IL2. Chickens in group III were vaccinated intranasally with rBCG pMV361-rho, and group IV were vaccinated subcutaneously with rBCG pMV361-rho. Chicken in group V were immunized intranasally with PBS as control, and group VI were immunized BCG. Infection was administered 14 days after the second immunization. Chickens were carefully reared to prevent coccidian contamination during immunization period, and feces from each group were periodically examined for the oocysts.
Proportions of splenocyte subsets by flow cytometry
Spleens were collected randomly from 5 chickens of each group 2 weeks after the final immunization for the splenocytes subsets assay. Briefly, splenocytes were separated and washed twice with PBS by centrifugation, and 106 cells in 100 µl PBS were incubated with fluorescein-conjugated mouse anti-chicken CD4 antibody (0.5 µg/µl) and R-phycoerythrin-conjugated mouse anti-chicken CD8α antibody (0.1 µg/µl) (Southern Biotech Associates, Inc., City, State, Country) for 40 min at room temperature in the dark. The proportion of CD4+ and CD8+ cells were measured by flow cytometry.
Evaluation of immune protection
All chickens except the unchallenged control group VI were challenged with 3×10
4 sporulated oocysts of
E. tenella 1 week after the booster dose and were observed daily for clinical signs and mortality. Feces from each group were collected separately between the 5th and 8th days post-challenge. The oocyst shedding per gram of feces was determined using McMaster's egg counting technique. Each sample was counted 3 times. Five chickens from each group were sacrificed on day 6 postchallenge to evaluate the lesion score as described by Johnson and Reid [
20]. Body weight gain of the chickens in each group was determined before oocyst inoculation and 8 days post-inoculation during infection.
Data were statistically analyzed by variance (ANOVA) and Student's t-test using SPSS 14.0 software. Difference between groups was considered significant if the P-value was less than 0.05.
RESULTS
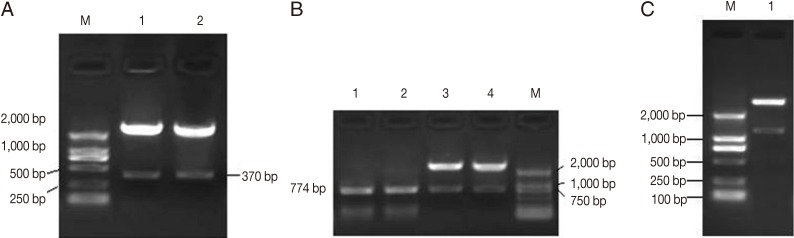
Identification of rBCG vaccines
A fragment of approximately 770 bp of rhomboid gene was resulted by the digested recombinant plasmid pMV361-rho with
PvuII and
ClaI. The digestion of recombinant plasmid pMV361-rho-IL2 with
PvuII and
ClaI produced a fragment of approximately 1,200 bp, which is equal to the molecular mass summation of rhomboid gene and chIL-2 gene. The rhomboid and chIL-2 gene were successfully amplified by PCR using rBCG pMV361-rho and pMV361-rho-IL2 as templates (
Fig. 1).
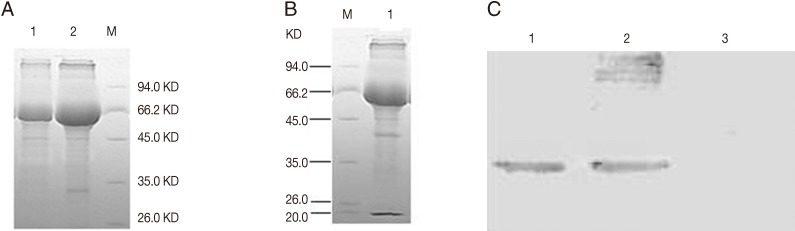
Expression of fusion proteins of rhomboid and chIL-2 were obtained after 2 hr of incubation in 45℃. SDS-PAGE of the whole cell preparation of the rBCG strains pMV361-rho and pMV361-rho-IL2 showed clear bands of -30 kDa for the rhomboid and -40kDa for the fusion protein of rhomboid-chIL-2, respectively. Western blotting showed that the rhomboid and rhomboid-chIL-2 were successfully expressed in rBCG pMV361-rho and pMV361-rho-IL2 (
Fig. 2).
As the effectors of primary immune responses following coccidian infection are primarily T-cells, the proportion of CD4
+ and CD8
+ cells were evaluated. As shown in
Table 1, all rBCG vaccinated groups induced significantly greater percentages of CD4
+ and CD8
+ cells than that of PBS-immunized control group (
P<0.05), especially higher in the rBCG pMV361-rho-IL2 vaccinated groups. The ratio of CD4
+/CD8
+increased simultaneously.
The immunizing efficacy of the rBCG vaccines, evaluated on the basis of cecal lesion scores, oocyst output, and body weight gains are described in
Table 2.
All chickens immunized with rBCG survived from the infection with E. tenella. Five chickens in each group were sacrificed on day 6 after challenge to assess the lesion score. A significant alleviation in cecal lesions was observed in the rBCG immunized chickens compared with that of challenge group (P<0.05). Cecal lesion scores were noted to be significantly lower in rBCG pMV361-rho-IL2 intranasal vaccination group as compared with others (P<0.05). Chickens immunized with rBCG pMV361-rho or pMV361-rho-IL2 revealed a significant decrease in the oocyst output (P<0.05). The highest reduction was recorded in the pMV361-rho-IL2 intranasally immunized group (64.1% relative to the challenge control group). Body weight gain was assessed on days before oocyst inoculation and 8 days post-challenge. Before oocyst inoculation, there was no significant body weight gain difference between rBCG immunized groups and unchallenged control group, indicating that rBCG vaccines are safe for chickens (date not shown). Body weight gain in all rBCG immunized chickens were higher than that of the challenged control group (P<0.05), especially higher in the group vaccinated intranasally with pMV361-rho-IL2. These results show that immunization with the rBCG strains did protect chickens from the coccidical infection-induced reduction in body weight gain.
DISCUSSION
Mycobacterium bovis BCG, the most widely used live bacterial vaccine in human tuberculosis, is a particularly attractive vector for the presentation of heterologous antigens. Therefore, BCG was used as a vaccine vector to co-express protective antigen of
E. tenella rhomboid and cytokine chIL-2 in this study. The main reasons that we chose rhomboid and chIL-2 genes fused and expressed in BCG system were as followsw: (1) ChIL-2 is a lymphokine that is responsible for the proliferation and differentiation of native T cells as well as for the activation of cytolytic T cells and natural killer cells [
21,
22]. (2) Rhomboid proteins, belonging to a class of serine proteases which play an important role in parasite invasion [
23,
24] have a potential role in microneme protein cleavage during host cell invasion.
E. tenella rhomboid protein has been identified and evaluated as an excellent candidate antigen for development of a coccidiosis vaccine in our previous report [
12,
17]. (3) BCG can not only stimulate the cellular immunity but also induce the humoral immunity, significantly improving the immunoprotection of expressed protein [
25,
26].
As the test results showed, when the chickens were vaccinated by intranasal and subcutaneous administration with different recombinant BCG and challenged with sporulated oocysts of
E. tenella, the OPG values of all test groups were significantly lower than the negative control group, especially in the group pMV361-rho-IL2 vaccinated using intranasal route (OPG=1.01±0.03×10
6). The OPG value plays a positive role in production. The reduction of OPG values usually indicates a reduction in the number of coccidia that can complete the life cycle in chickens, and therefore caused a reduction in infection intensity and immune stress, which is highly beneficial to improve production performance in chickens. Compared with the control group (CD4
+ with 12.3±0.30% and CD8
+ with 28.5±0.30%), all vaccinated groups (rBCG pMV361-Rho and rBCG pMV361-rho-IL2) induced significantly greater percentages of CD4
+ and CD8
+ cells (
P<0.05), especially in the pMV361-rho-IL2 intranasal vaccination group. These may reveal the rBCG which expressed cytokine can develop dual effects of BCG and cytokine, continuing to stimulate the proliferation and differentiation of CD4
+ and CD8
+ lymphocytes, accordingly enhancing the ability of resistance against coccidian infection. Our data showed that intranasal immunization with pMV361-rho-IL2 could induce stronger humoral and cellular immunity, and displayed higher level protection against challenge with
E. tenella than the group vaccinated subcutaneously. The reason may be that
E. tenella is an intestinal parasite, the route of intranasal immunization can deliver the recombinant vaccines to establish effective stimulation of local immunity and then elicit protective immune responses against mucosal infectious diseases [
27]. Intranasal immunization was proved to be superior to a subcutaneously immunization.
This research proved that coccidian recombinant BCG vaccine constructed under the laboratory conditions could significantly intensify chicken's immunity against coccidiosis and show effective protection against E. tenella challenge. However, there is a certain difference between the practical chicken-raising conditions and those in the laboratory, such as raising environment, management methods, and use of many kinds of vaccines and chemical drugs during the whole chicken-raising term. Would all these factors have any influence on the performance of the function of recombinant BCG vaccine to protect immunity of coccidiosis in chickens? How would be the extent of influence? To solve such a series of problems including the optimal dosage and immune time which are consistent with the production practice, further study on its field test needs to be launched to pave the way for recombinant BCG vaccine's actual application in chicken production.
National Hi-Tech Research and Development Program of China2011AA10A215
Hebei Normal University2010YB005
Notes
-
We have no conflict of interest related to this work.
ACKNOWLEDGMENTS
This work was supported by the National Hi-Tech Research and Development Program of China (863 program) under the grant number 2011AA10A215 and the Doctor Foundation of Hebei Normal University of Science and Technology, Hebei, China (2010YB005).
References
- 1. Subramanian BM, Sriraman R, Rao NH, Raghul J, Thiagarajan D, Srinivasan VA. Cloning, expression and evaluation of the efficacy of a recombinant Eimeria tenella sporozoite antigen in birds. Vaccine 2008;26:3489-3496.
- 2. Chapman HD, Cherry TE, Danforth HD, Richards G, Shirley MW, Williams RB. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int J Parasitol 2002;32:617-629.
- 3. Lillehoj HS, Lillehoj EP. Avian coccidiosis. A review of acquired intestinal immunity and vaccination strategies. Avian Dis 2000;44:408-425.
- 4. Vermeulen AN, Schaap DC, Schetters TP. Control of coccidiosis in chickens by vaccination. Vet Parasitol 2001;100:13-20.
- 5. Ding X, Lillehoj HS, Quiroz MA, Bevensee E, Lillehoj EP. Protective immunity against Eimeria acervulina following in ovo immunization with a recombinant subunit vaccine and cytokine genes. Infect Immun 2004;72:6939-6944.
- 6. Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun 2001;69:2527-2534.
- 7. Shen H, Wang C, Yang E, Xu Y, Liu W, Yan J, Wang F, Wang H. Novel recombinant BCG coexpressing Ag85B, ESAT-6 and mouse TNF-α induces significantly enhanced cellular immune and antibody responses in C57BL/6 mice. Microbiol Immunol 2010;54:435-441.
- 8. Sundick RS, Gill-Dixon C. A cloned chicken lymphokine homologous to both mammalian IL-2 and IL-15. J Immunol 1997;159:720-725.
- 9. Lillehoj HS, Min W, Choi KD, Babu US, Burnside J, Miyamoto T, Rosenthal BM, Lillehoj EP. Molecular, cellular, and functional characterization of chicken cytokines homologous to mammalian IL-15 and IL-2. Vet Immunol Immunopathol 2001;82:229-244.
- 10. Min W, Lillehoj HS, Burnside J, Weining KC, Staeheli P, Zhu JJ. Adjuvant effects of IL-1β, IL-2, IL-8, IL-15, IFN-α, IFN-γ, TGF-β4 and lymphotactin on DNA vaccination against Eimeria acervulina. Vaccine 2001;20:267-274.
- 11. Stepaniak JA, Shuster JE, Hu W, Sundick RS. Production and in vitro characterization of recombinant chicken interleukin-2. J Interferon Cytokine Res 1999;19:515-526.
- 12. Wang Q, Li J, Zhang X, Liu Q, Liu C, Ma G, Cao L, Gong P, Cai Y, Zhang G. Protective immunity of recombinant Mycobacterium bovis BCG expressing rhomboid gene against Eimeria tenella challenge. Vet Parasitol 2009;160:198-203.
- 13. Yang G, Li J, Zhang X, Zhao Q, Liu Q, Gong P. Eimeria tenella: construction of a recombinant fowlpox virus expressing rhomboid gene and its protective efficacy against homologous infection. Exp Parasitol 2008;119:30-36.
- 14. Xu Q, Song X, Xu L, Yan R, Shah MA, Li X. Vaccination of chickens with a chimeric DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 induces protective immunity against coccidiosis. Vet Parasitol 2008;156:319-323.
- 15. Lillehoj HS, Ding X, Quiroz MA, Bevensee E, Lillehoj EP. Resistance to intestinal coccidiosis following DNA immunization with the cloned 3-1E Eimeria gene plus IL-2, IL-15, and IFN-γ. Avian Dis 2005;49:112-117.
- 16. Pasquini S, Xiang Z, Wang Y, He Z, Deng H, Blaszczyk-Thurin M, Ertl HC. Cytokines and costimulatory molecules as genetic adjuvants. Immunol Cell Biol 1997;75:397-401.
- 17. Li J, Zhang X, Liu Q, Yin J, Yang J. Eimeria tenella: cloning of a novel Eimeria tenella cDNA encoding a protein related to rhomboid family from F2 hybrid strain. Exp Parasitol 2006;113:215-220.
- 18. Kumar D, Srivastava BS, Srivastava R. Genetic rearrangements leading to disruption of heterologous gene expression in mycobacteria: an observation with Escherichia coli beta-galactosidase in Mycobacterium smegmatis and its implication in vaccine development. Vaccine 1998;16:1212-1215.
- 19. Wang Q, Li J, Zhang X, Liu C, Cao L, Ren K, Gong P, Cai Y. Construction of EGFP-tagged rBCG of E. tenella and distribution in chickens. Sci China C Life Sci 2009;52:278-283.
- 20. Johnson J, Reid WM. Anticoccidial drug: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 1970;28:30-36.
- 21. Kogut M, Rothwell L, Kaiser P. Differential effects of age on chicken heterophil functional activation by recombinant chicken interleukin-2. Dev Comp Immunol 2002;26:817-830.
- 22. Klasing KC. Avian leukocytic cytokines. Poult Sci 1994;73:1035-1043.
- 23. Brossier F, Jewett TJ, Sibley LD, Urban S. A spatially localized rhomboid protease cleaves cell surface adhesins essential for invasion by Toxoplasma. Proc Natl Acad Sci USA 2005;102:4146-4151.
- 24. Dowse TJ, Soldati D. Rhomboid-like proteins in Apicomplexa: phylogeny and nomenclature. Trends Parasitol 2005;21:254-258.
- 25. Bastos RG, Borsuk S, Seixas FK, Dellagostin OA. Recombinant Mycobacterium bovis BCG. Vaccine 2009;27:6495-6503.
- 26. Gicquel B. BCG as a vector for the construction of multivalent recombinant vaccines. Biologicals 1995;23:113-118.
- 27. Lillehoj HS, Trout JM. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin Microbiol Rev 1996;9:349-360.
Fig. 1Identification of chIL-2 (A), rhomboid (B), and rho-chIL-2 genes (C) in the plasmid pMV361-rho-chIL-2 by restriction digestion. (A) Lanes 1, 2, chIL-2 gene digested by XbaI/ClaI. (B) Lanes 1, 2, PCR product of rhomboid gene using pMV361-Rho-IL-2 as temple. Lanes 3, 4, plasmid pMV361-Rho-IL-2 digested by PvuII/XbaI product rhomboid gene. (C)Lane 1, pMV361-Rho-IL-2 digested by PvuII/ClaI product Rho/chIL-2 fusion gene.
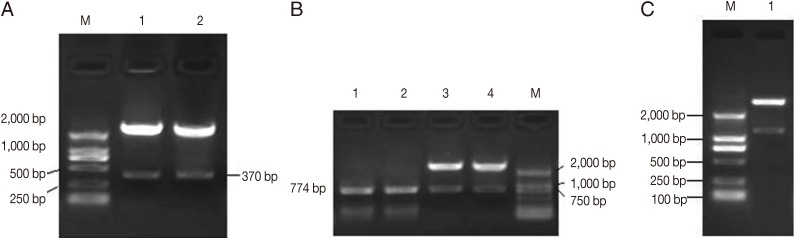
Fig. 2Expression of rBCG pMV361-Rho (A) and SDS-PAGE of rBCG pMV361-rho-IL2 (B) and Rho/chIL-2 fusion protein in rBCG pMV361-rho-IL2 analyzed by Western blotting (C). (A) Lane 1, negative control BCG; Lane 2, rBCG of pMV361-rho. (B) Lane 1, a band of -40 kDa was detected which corresponds to the expected size of the Rho/chIL-2 fusion protein in rBCG of pMV361-rho-IL2. (C)Western blotting of rBCG pMV361-Rho-IL2 (Lanes 1, 2), BCG as a control showed no immunoreactive band (Lane 3).
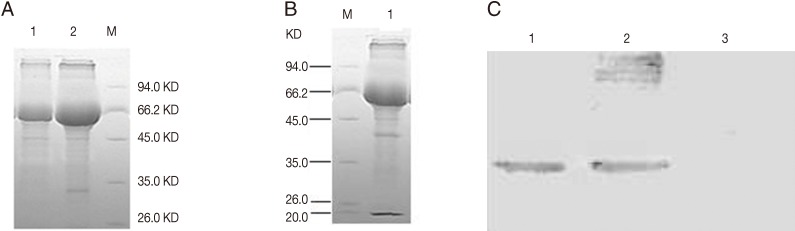
Table 1.The numbers of spleen CD4
+ and CD8
+ T lymphocyte in experimental and negative control groups
a
Table 1.
|
Group |
Vaccine |
CD4+ (%; mean±S.E.) |
CD8+ (%; mean±S.E.) |
CD4+/CD8+
|
|
I |
pMV361-rho-IL2 i.n. |
24.3±0.66A
|
36.5±0.65A
|
0.67 |
|
II |
pMV361-rho-IL2 s.c. |
21.9±0.20B
|
35.1 ±1.15A
|
0.62 |
|
III |
pMV361-Rho i.n. |
20.5±0.43C
|
34.8±0.42B
|
0.59 |
|
IV |
pMV361-Rho s.c. |
19.6±0.32C
|
34.5±0.33B
|
0.57 |
|
V |
PBS i.n. |
12.3±0.30 |
28.5±0.30 |
0.43 |
|
VI |
BCG |
17.7±0.2 |
32.8±0.42 |
0.54 |
Table 2.Comparison of protective effectiveness in chickens receiving different vaccines against
E. tenella challenge
a
Table 2.
|
Group |
Vaccine |
Total oocyst output in feces (x106) |
Reduction in oocyst output (%) |
Lesion scores |
Body weight gain post challenge (g) |
|
I |
pMV361-rho-IL2 i.n. |
1.01±0.03A
|
64.1 |
1.45±0.05A
|
86.0±3.3 |
|
II |
pMV361-rho-IL2 s.c. |
1.23±0.03B
|
56.2 |
1.92±0.02B
|
79.6±2.5 |
|
III |
pMV361-Rho i.n. |
1.36±0.02B
|
51.6 |
2.41±0.03C
|
73.5±3.7 |
|
IV |
pMV361-Rho s.c. |
1.54±0.03C
|
45.2 |
2.78±0.04D
|
75.7±2.6 |
|
V |
PBS i.n. |
2.81±0.08 |
- |
3.8±0.04 |
48.6±4.7 |
|
VI |
BCG |
1.62±0.04 |
42.3 |
2.32±0.03 |
64.2±3.3 |