Abstract
The mature domain of a cysteine protease of Spirometra erinacei plerocercoid larva (i.e., sparganum) was expressed in Escherichia coli, and its value as an antigen for the serodiagnosis of sparganosis was investigated. The recombinant protein (rSepCp-1) has the molecular weight of 23.4 kDa, and strongly reacted with the sparganum positive human or mice sera but not with negative sera by immunoblotting. ELISA with rSepCp-1 protein or sparganum crude antigen (SeC) was evaluated for the serodiagnosis of sparganosis using patient's sera. The sensitivity and specificity of ELISA using rSepCp-1 protein were 95.0% (19/20) and 99.1% (111/112), respectively. In contrast, the sensitivity and specificity of ELISA with SeC were 100% (20/20) and 96.4% (108/112), respectively. Moreover, in experimentally infected mice, the sensitivity and specificity of both ELISA assays were 100% for the detection of anti-sparganum IgG. It is suggested that the rSepCp-1 protein-based ELISA could provide a highly sensitive and specific assay for the diagnosis of sparganosis.
-
Key words: Spirometra erinacei, sparganum, cysteine protease, ELISA, serodiagnosis
INTRODUCTION
Sparganosis is a parasitic zoonosis caused by the plerocercoid larva of pseudophyllidean tapeworms,
Spirometra spp. This disease is prevalent in Asian countries, including China, Japan, Korea, and Thailand [
1], and Korea experiences more frequent occurrence of cases than other countries [
2]. Humans are a second intermediate host and become infected by drinking water containing infected
Cyclops, by ingesting raw or inadequately cooked flesh of second intermediate hosts such as frogs or snakes, and by applying poultices incorporating the raw flesh of frogs or snakes on open wounds [
3]. Most infections in humans usually present as subcutaneous nodules, but infections may also occur in any part of the body including the eye, spinal cord, and brain, resulting in blindness, seizures, and paralysis [
3].
In most cases, preoperative diagnosis is difficult because sparganosis does not present characteristic clinical features. Definitive diagnosis is usually made by surgical removal of parasites from infected tissues and examining the gross morphology. Imaging techniques are usually used for the preoperative diagnosis of sparganosis affecting the brain, spinal cord, abdomen, and other visceral organs [
4-
6]. However, using these techniques, misdiagnosis can be unavoidable as sparganosis is confused with soft tissue tumors, varicose vein, or conus medullaris [
4,
7,
8]. Therefore, development of a sensitive and specific immunological method would certainly accelerate the process of early detection and treatment of the disease and subsequently help prevent serious complications.
Among serological tests, ELISA has been useful for the diagnosis of sparganosis using the crude extract of sparganum as an antigen [
2,
6]. Although ELISAs using the whole extract or purified proteins have high sensitivities, they exhibit cross-reactions with the serum samples of individuals with other helminthiasis [
9]. Recently, an ELISA using the excretory-secretory antigen of sparganum has been shown to be highly sensitive and specific relative to the conventional ELISA using crude antigens [
10].
Cysteine proteases play major roles in the biology of parasites and have been exploited as serodiagnostic markers and vaccine targets because of their immunogenicity [
11]. Purified or recombinant cysteine proteases have been used for the diagnosis of human schistosomiasis [
12], fascioliasis [
13], clonorchiasis [
14], and paragonimiasis [
15]. Cysteine proteases of spargana have been identified, and their biological roles, biochemical properties, and structural nature have been characterized [
16,
17]. These cysteine proteases have been shown to react strongly with the sera of sparganum-infected patients.
In this study, the mature domain of a cysteine protease of the plerocercoid larva of Spirometra erinacei was expressed in Escherichia coli. The diagnostic potential of the recombinant cysteine protease was investigated by immunoblotting and ELISA and compared with that of ELISA using the crude antigen.
MATERIALS AND METHODS
Collection of spargana and preparation of the crude antigen
Plerocercoid larvae were collected from naturally infected snakes, Rhabdophis tigrinus tigrinus. The collected spargana were chopped up and homogenized in ice-cold PBS and centrifuged at 13,000 rpm for 30 min at 4℃. The supernatant was filtered using 0.20 µm Acrodisc® Syringe Filters (Pall Corporation, Ann Arbor, Missouri, USA). The concentration of crude antigen was measured using the BCA™ Protein Assay Kit (Pierce, Rockford, Illinois, USA), and the crude antigen was stored at -70℃ until use.
Serum samples
A total of 132 human serum samples of parasitologically and clinically proven cases were used for the evaluation of ELISA. Positive control serum samples (n=20) were obtained from patients with sparganosis that had been confirmed by either clinically or surgically. Negative control sera (n=65) were collected from healthy Korean subjects without any parasitological infections. For evaluation of cross-reactions, 47 serum specimens from patients with other parasitic infections were also used: these infections included toxocariasis (n=10), clonorchiasis (n=19), cysticercosis (n=5), paragonimiasis (n=8), and anisakiasis (n=5).
Serum specimens from experimentally infected (n=10) and uninfected mice (n=10) were also used for evaluation of ELISA. Serum samples were collected from mice after 2 months of oral infection with sparganum scolices.
Cloning of a cysteine protease gene
The gene encoding the mature domain of a sparganum cysteine protease was amplified by PCR using the sparganum cDNA. The primers were designed based on previously reported full-length cDNA sequences of
Spirometra erinacei plerocercoid cysteine protease (GenBank accession no. D63670.1) [
17] as follows: forward primer, 5'-GAGAGGTACCCTTCCCGACAGCGTAAACTGGCGC-3' and reverse primer, 5'-GAGAAAGCTTCACGGTTGGATAGCTTGCCATGCT-3'. Restriction sites for
Kpn I and
Hind III were added at the 5' ends of the forward and reverse primers, respectively. The PCR reaction was performed in a 50 µl reaction volume that contained 5 µl of 10x PCR buffer (Takara Shuzo Col, Kyoto, Japan), 0.5 µM each primer, 200 µM dNTP, 1.5 U of Taq polymerase, and 2 µl of 1:5 diluted cDNA. The PCR amplification reaction consisted of a Taq DNA polymerase activation step at 95℃ for 5 min followed by 35 cycles of denaturation at 95℃ for 30 sec, annealing at 68℃ for 30 sec, and extension at 72℃ for 60 sec. The amplified PCR products were purified and digested with restriction enzymes. The resulting insert was ligated into the pQE30 expression vector, which was then transformed into
E. coli M15 (pREP4) competent cells (Qiagen, Hilden, Germany) by standard methods. Transformants containing the pQE30 plasmid with the insert were screened by restriction analysis and confirmed by sequencing.
Expression of the recombinant S. erinacei plerocercoid cysteine protease (rSepCp-1) was induced by isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 1 mM at 37℃ for 4 hr. Cells were harvested by centrifugation at 4,000 g for 20 min. The rSepCp-1 protein expressed as inclusion bodies was solubilized completely with 8 M urea in 10 mM Tris-Cl and 100 mM NaH2PO4 buffer (pH 8.0). Then, the histidine-tagged protein was purified using a nickel-nitrilotriacetic acid (Ni-NTA) agarose column according to the manufacturer's instructions (Qiagen).
SDS-PAGE and immunoblotting
The recombinant protein was electrophoresed on 12% acrylamide gels. After electrophoresis, proteins were stained with Coomassie blue or transferred to nitrocellulose membranes (Millipore, Bedford, Massachusetts, USA). For immunoblotting analysis, membranes were blocked with 0.05% Tween-20 in PBS (PBST) containing 5% skim milk and were reacted with a mouse anti-histidine monoclonal antibody (1:4,000) (R&D Systems Inc., Minneapolis, USA), sparganum-positive human or mouse serum samples or negative serum samples (1:4,000) for 2 hr at room temperature. After 3 washes with PBST, membranes were incubated with peroxidase-conjugated secondary antibody against mouse IgG (1:4,000) (DakoCytomation, Glostrup, Denmark) or human IgG (1:4,000) (DakoCytomation) for 1 hr at room temperature, followed by detection with an immunoblotting detection reagent (West-Zol® Plus, Intron Biotechnology, Sungnam, Korea).
ELISA
Polystyrene microtiter plates (Costar, Corning, New York, USA) were coated with 0.5 µg/well (100 µl/well) of rSepCp-1 protein or sparganum crude extract (SeC) diluted in 0.1 M carbonate/bicarbonate buffer, pH 9.6, and incubated at 4℃ overnight. After washing 3 times with PBST, plates were blocked with 1% bovine serum albumin for 1 hr at 37℃. After washing, 100 µl of human or mouse serum samples diluted 1:500 were loaded into each well of the plates, and the plates were incubated for 2 hr at 37℃. Then, 100 µl of horseradish peroxidase-conjugated goat anti-human IgG (1:10,000) (ICN Pharmaceuticals Inc., Aurora, Ohio, USA) or rabbit anti-mouse IgG (1:5,000) (DakoCytomation) was loaded into each well. After incubation for 1 hr at 37℃, the reaction was developed with 1-Step™ Ultra TMB-ELISA substrate (Pierce) at room temperature, and the absorbances were read at 450 nm. All samples were run in duplicate. The cut-off values were defined as the mean absorbance plus 3 SDs of the values obtained from the sera of healthy negative controls.
Statistical analysis
The statistical significance of ELISA results was evaluated by the Student's t-test, and differences were considered significant when the P-values were less than 0.05.
RESULTS
The gene encoding the mature domain of a sparganum cysteine protease was amplified by PCR from the full-length cDNA sequence of
S. erinacei plerocercoid larva as previously reported [
17]. The amplicon was 648 bp and encoded a protein of 216 amino acid residues, with a molecular weight of approximately 23.4 kDa. The predicted amino acid sequence of rSepCp-1 showed identities of 98.9%, 98.6%, and 99.1% with the mature domains of cysteine proteases from
S. erinacei plerocercoid corresponding to GenBank accession numbers BAB62816, BAA09820, and BAB62718, respectively. Moreover, the amino acid sequence of rSepCp-1 protein has 85.6% homology to the mature domain of a cysteine protease of
Spirometra mansonoides (GenBank accession no. AAB17051).
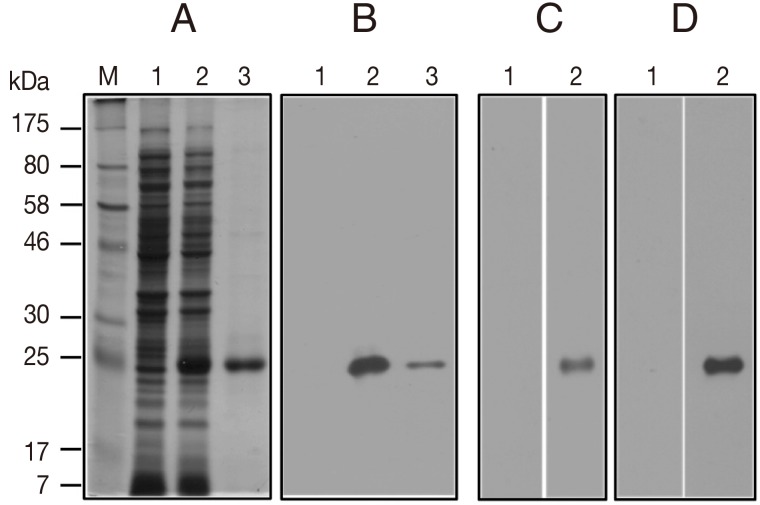
On the SDS-PAGE gel, the rSepCp-1 protein of approximately 23.4 kDa was observed in an IPTG-treated sample (
Fig. 1A, lane 2) and was purified to a single band by Ni-NTA affinity chromatography (
Fig. 1A, lane 3). Expression of histidine-tagged rSepCp-1 protein was also confirmed by immunoblotting with a mouse anti-histidine monoclonal antibody (
Fig. 1B). By immunoblotting assay, rSepCp-1 protein highly reacted with the sparganum-positive human or mouse sera but not with sparganum-negative sera (
Fig. 1C, D).
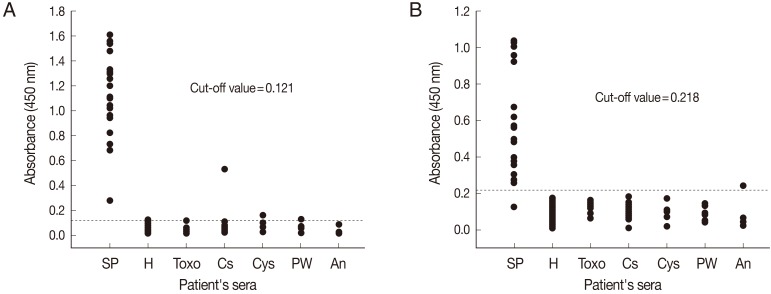
The diagnostic efficacy of ELISA for the detection of anti-sparganum IgG antibodies in human sera using crude antigens (SeC-ELISA) or rSepCp-1 protein (rSepCp-1-ELISA) was evaluated. The mean absorbances of SeC-ELISA for healthy negative control sera and for sparganosis patient's sera were 0.052±0.023 and 1.140±0.342, respectively, whereas those of rSepCp-1-ELISA were 0.095±0.041 and 0.571±0.284, respectively. The cutoff values for SeC-ELISA and rSepCp-1-ELISA were determined to be 0.121 and 0.218, respectively (
Fig. 2). The mean absorbances of the serum samples obtained from patients with other parasitic diseases were not significantly different from those obtained from healthy negative controls for both SeC-ELISA (
P=0.167) and rSepCp-1-ELISA (
P=0.531). Whereas, mean absorbances of the sera obtained from sparganosis patients were significantly higher than those obtained from individuals infected with other parasitic diseases (
P<0.05).
The sensitivities of SeC-ELISA and rSepCp-1-ELISA were 100% (20/20) and 95% (19/20), respectively. The specificity of ELISA was 96.4% (108/112) by crude antigen and 99.1% (111/112) by rSepCp-1 protein. SeC-ELISA cross-reacted with the sera of clonorchiasis, cysticercosis, paragonimiasis, and also with 1 serum sample of a healthy control (
Fig. 2A), while rSepCp-1-ELISA cross-reacted only with 1 serum sample of anisakiasis (
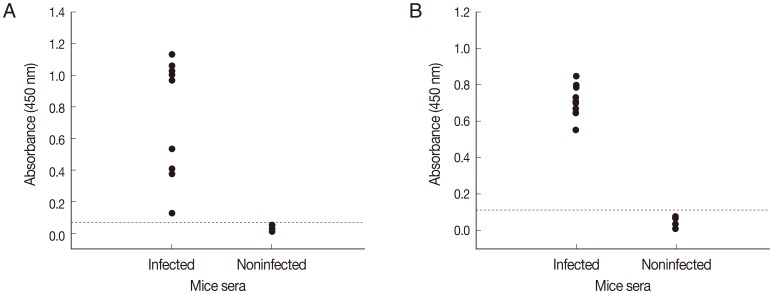
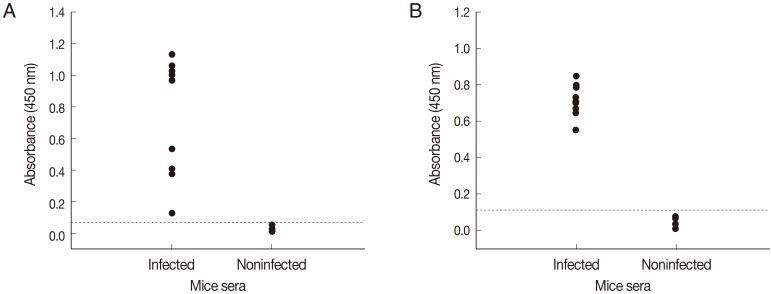
Fig. 2B). When ELISAs using crude antigen or rSepCp-1 protein were tested against serum samples from infected and uninfected mice, sensitivities of both ELISA assays for detecting anti-sparganum IgG in infected mice were 100% (10/10). Specificities of both ELISA assays were 100% (10/10) as it did not show any positive response with sera from non-infected mice (
Fig. 3).
DISCUSSION
In sparganum, 21, 27, and 53 kDa proteins had been reported to be cysteine proteases which showed strong immune reactions with sera of sparganum-infected patients [
16]. In the present study, the recombinant protein of the mature domain of a sparganum cysteine protease strongly reacted with the sparganum-positive human or mice sera by immunoblotting, which is in agreement with the previous report [
17]. Immunodiagnostic properties using the mature form of recombinant cysteine proteases have also been reported for the detection of paragonimiasis [
18] and leishmaniasis [
19].
When ELISA was performed using the crude antigen of sparganum in the present study, it demonstrated higher sensitivity than the rSepCp-1-ELISA for the detection of anti-spargaum IgG in human sera. However, the main disadvantage of SeC-ELISA is that it cross-reacted with the sera of other parasite infections and 1 serum of negative control. On the contrary, rSepCp-1 protein ELISA only cross-reacted with 1 serum from anisakiasis patient. Cross-reactions with the sera of other helminthiasis had also been reported in previous studies when the crude antigen of sparganum was used for ELISA [
9,
10]. To reduce the cross-reactivity, the crude antigen was purified by affinity chromatography; however, the process was complicated, and cross-reaction was still observed [
9]. Whereas, in the present study, we developed an ELISA using rSepCp-1 protein, and it demonstrated higher specificity than the ELISA using crude antigen of sparganum. However, a large scale validation of specificity of the test should be done with more sera from other helminthiasis patients including those cross-reacted in the present study. In addition, a large number of positive control sera should be analyzed to confirm the value of sensitivity.
Differential diagnosis between sparganosis and cysticercosis involving the central nervous system (CNS) is important because of different treatment strategies for the 2 diseases. Neurocysticercosis can be treated with praziquantel or albendazole [
20] or by surgical removal. In contrast, there are still no effective drugs for the treatment of neurosparganosis [
21], and therefore surgical removal is the only modality for treatment. As the rSepCp-1-ELISA was found to be highly specific for sparganosis and did not exhibit cross-reactivity with the sera of cysticercosis, this ELISA could be applied for the differential diagnosis of these 2 parasitic diseases when the CNS is involved. Moreover, the preoperative use of rSepCp-1-ELISA would eliminate the possibility of misdiagnosis, which is typically possible through the use of different imaging techniques [
4,
7,
8].
Preparation of the crude extract of sparganum for ELISA requires collection of spargana from naturally infected intermediate hosts or in vivo infection of laboratory animals for maintenance of spargana, which is practically inconvenient in terms of cost, labor, and time. Recombinant proteins are a good alternative to the crude extract as they can be produced easily in large amounts using a bacterial expression system and can be used as an antigen in a sensitive, specific, and standardized ELISA to diagnose sparganosis.
In conclusion, this study demonstrated that the recombinant cysteine protease of the plerocercoid larva of S. erinacei is useful for a sensitive and specific diagnosis of sparganosis by ELISA. Thus, the use of recombinant cysteine proteinase may provide a new source of diagnostic reagent and thereby enhancing the diagnosis and treatment of sparganosis.
The SNUH Research Fund21-2005-0310
Notes
-
We have no conflict of interest related with this work.
ACKNOWLEDGMENT
This study was supported by grant no. 21-2005-0310 from the SNUH Research Fund.
References
- 1. Li MW, Song HQ, Li C, Lin HY, Xie WT, Lin RQ, Zhu XQ. Sparganosis in mainland China. Int J Infect Dis 2011;15:e154-e156.
- 2. Park JH, Park YS, Kim JS, Roh SW. Sparganosis in the lumbar spine: report of two cases and review of the literature. J Korean Neurosurg Soc 2011;49:241-244.
- 3. Nithiuthai S, Anantaphruti MT, Waikagul J, Gajadhar A. Waterborne zoonotic helminthiases. Vet Parasitol 2004;126:167-193.
- 4. Kwon JH, Kim JS. Sparganosis presenting as a conus medullaris lesion: case report and literature review of the spinal sparganosis. Arch Neurol 2004;61:1126-1128.
- 5. Lee JH, Yu JS, Park MS, Lee SI, Yang SW. Abdominal sparganosis presenting as an abscess with fistulous communication to the bowel. AJR Am J Roentgenol 2005;185:1084-1085.
- 6. Song T, Wang WS, Zhou BR, Mai WW, Li ZZ, Guo HC, Zhou F. CT and MR characteristics of cerebral sparganosis. AJNR Am J Neuroradiol 2007;28:1700-1705.
- 7. Koo JH, Cho WH, Kim HT, Lee SM, Chung BS, Joo CY. A case of sparganosis mimicking a varicose vein. Korean J Parasitol 2006;44:91-94.
- 8. Chae SW, Choi JH, Lee DJ, Lee HM. Sparganosis presenting as a lateral neck mass. Head Neck 2003;25:74-76.
- 9. Kong Y, Kang SY, Cho SY. Single step purification of potent antigenic protein from sparganum by gelatin-affinity chromatography. Korean J Parasitol 1991;29:1-7.
- 10. Cui J, Li N, Wang ZQ, Jiang P, Lin XM. Serodiagnosis of experimental sparganum infections of mice and human sparganosis by ELISA using ES antigens of Spirometra mansoni spargana. Parasitol Res 2011;108:1551-1556.
- 11. McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol 2006;1:497-536.
- 12. El-Sayed LH, Ghoneim H, Demian SR, El-Sayed MH, Tawfik NM, Sakr I, Abou-Basha LM, Renganathan E, Klinkert MQ, Abou-Rawash N. Diagnostic significance of Schistosoma mansoni proteins Sm31 and Sm32 in human schistosomiasis in an endemic area in Egypt. Trop Med Int Health 1998;3:721-727.
- 13. Carnevale S, Rodriguez MI, Guarnera EA, Carmona C, Tanos T, Angel SO. Immunodiagnosis of fasciolosis using recombinant procathepsin L cystein proteinase. Diagn Microbiol Infect Dis 2001;41:43-49.
- 14. Nagano I, Pei F, Wu Z, Wu J, Cui H, Boonmars T, Takahashi Y. Molecular expression of a cysteine proteinase of Clonorchis sinensis and its application to an enzyme-linked immunosorbent assay for immunodiagnosis of clonorchiasis. Clin Diagn Lab Immunol 2004;11:411-416.
- 15. Ikeda T, Oikawa Y, Nishiyama T. Enzyme-linked immunosorbent assay using cysteine proteinase antigens for immunodiagnosis of human paragonimiasis. Am J Trop Med Hyg 1996;55:435-437.
- 16. Kong Y, Kang SY, Kim SH, Chung YB, Cho SY. A neutral cysteine protease of Spirometra mansoni plerocercoid invoking an IgE response. Parasitology 1997;114:263-271.
- 17. Liu DW, Kato H, Nakamura T, Sugane K. Molecular cloning and expression of the gene encoding a cysteine proteinase of Spirometra erinacei. Mol Biochem Parasitol 1996;76:11-21.
- 18. Yang SH, Park JO, Lee JH, Jeon BH, Kim WS, Kim SI, Yun KJ, Jeong ET, Lee KW, Kim YM, Lee MH, Park H. Cloning and characterization of a new cysteine proteinase secreted by Paragonimus westermani adult worms. Am J Trop Med Hyg 2004;71:87-92.
- 19. Rafati S, Nakhaee A, Taheri T, Ghashghaii A, Salmanian AH, Jimenez M, Mohebali M, Masina S, Fasel N. Expression of cysteine proteinase type I and II of Leishmania infantum and their recognition by sera during canine and human visceral leishmaniasis. Exp Parasitol 2003;103:143-151.
- 20. Matthaiou DK, Panos G, Adamidi ES, Falagas ME. Albendazole versus praziquantel in the treatment of neurocysticercosis: a meta-analysis of comparative trials. PLoS Negl Trop Dis 2008;2:e194.
- 21. Kim DG, Paek SH, Chang KH, Wang KC, Jung HW, Kim HJ, Chi JG, Choi KS, Han DH. Cerebral sparganosis: clinical manifestations, treatment, and outcome. J Neurosurg 1996;85:1066-1071.
Fig. 1SDS-PAGE and immunoblot analysis of recombinant Spirometra erinacei plerocercoid cysteine protease (rSepCp-1). (A) Histidine-tagged rSepCp-1 protein visualized by SDS-PAGE and Coomassie blue staining. Lane M, molecular weight marker; lane 1, E. coli lysate without isopropyl-β-D-thiogalactopyranoside (IPTG) induction; lane 2, E. coli lysate with IPTG induction; lane 3, rSepCp-1 protein purified by Ni-NTA affinity chromatography. (B) Immunoblot analysis of the same protein samples as in 'A' using a mouse anti-histidine monoclonal antibody. (C) Immunoblotting of the rSepCp-1 protein with sparganum-negative (lane 1) and sparganum-positive (lane 2) human serum samples. (D) Immunoblotting of the rSepCp-1 protein with sparganum-negative (lane 1) and sparganum-positive (lane 2) mouse serum samples.

Fig. 2Comparison of the absorbances of the ELISA using the crude antigen (A) and the ELISA using the recombinant Spirometra erinacei plerocercoid cysteine protease (rSepCp-1) (B) for the detection of anti-sparganum IgG in human serum samples. The cut-off values are indicated by the dashed horizontal lines. Sp, sparganosis; H, healthy control; Toxo, toxocariasis; Cs, clonorchiasis; Cys, cysticercosis; PW, paragonimiasis; An, anisakiasis.
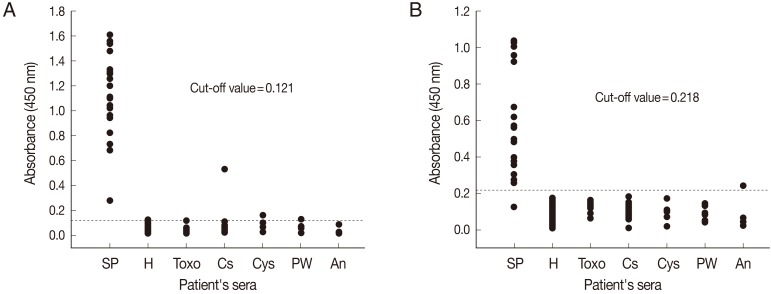
Fig. 3Comparison of absorbances by ELISA using the crude antigen (A) and ELISA using the recombinant Spirometra erinacei plerocercoid cysteine protease (rSepCp-1) (B) for the detection of anti-sparganum IgG in serum samples of infected and uninfected mice. The cut-off values are indicated by the dashed horizontal lines.