Abstract
Trichomonad species inhabit a variety of vertebrate hosts; however, their potential zoonotic transmission has not been clearly addressed, especially with regard to human infection. Twenty-one strains of trichomonads isolated from humans (5 isolates), pigs (6 isolates), rodents (6 isolates), a water buffalo (1 isolate), a cow (1 isolate), a goat (1 isolate), and a dog (1 isolate) were collected in Indonesia and molecularly characterized. The DNA sequences of the partial 18S small subunit ribosomal RNA (rRNA) gene or 5.8S rRNA gene locus with its flanking regions (internal transcribed spacer region, ITS1 and ITS2) were identified in various trichomonads; Simplicimonas sp., Hexamastix mitis, and Hypotrichomonas sp. from rodents, and Tetratrichomonas sp. and Trichomonas sp. from pigs. All of these species were not detected in humans, whereas Pentatrichomonas hominis was identified in humans, pigs, the dog, the water buffalo, the cow, and the goat. Even when using the high-resolution gene locus of the ITS regions, all P. hominis strains were genetically identical; thus zoonotic transmission between humans and these closely related mammals may be occurring in the area investigated. The detection of Simplicimonas sp. in rodents (Rattus exulans) and P. hominis in water buffalo in this study revealed newly recognized host adaptations and suggested the existence of remaining unrevealed ranges of hosts in the trichomonad species.
-
Key words: Pentatrichomonas hominis, trichomonad species, molecular taxonomy, internal transcribed spacer, zoonotic transmission, low vacuum SEM
INTRODUCTION
Trichomonad species are a flagellated type of protozoan parasites that inhabit a variety of vertebrate hosts, including humans. Although 2 species of those trichomonads,
Trichomonas vaginalis and
Dientamoeba fragilis, have been thought to be pathogens in humans as causative agents of vaginitis and diarrhea, respectively, recent molecular identification studies have raised novel notions of trichomonad infections in humans.
Pentatrichomonas hominis, which has been recognized as a harmless commensal gastrointestinal protozoan in humans [
1], was detected in 2 cases of diarrhea in children and suspected as the potential etiological agent of the gastrointestinal symptoms [
2]. Moreover, atypical types of infections with trichomonads in humans have been also reported.
Trichomonas vaginalis,
Trichomonas tenax, and
P. hominis, and also
Tetratrichomonas gallinarum (avian trichomonad) and
Tritrichomonas foetus (bovid trichomonad) have been isolated from the respiratory tract of patients with empyema and pulmonary diseases, such as acute respiratory distress syndrome [
3]. Considering these recent findings, trichomonads should be carefully monitored as a public health issue for humans and related domestic animals from the viewpoints of potential zoonotic transmission and unrecognized pathogenicity, in contrast to the previous commensal image of these organisms. Indeed, nearly all published studies have focused mainly on
T. vaginalis in Indonesia [
4] and molecular studies for other types of trichomonad species in human and animal hosts were not performed.
The present study thus targeted and identified gastrointestinal trichomonads using molecular taxonomy to evaluate the species distributions in humans and closely related mammalian hosts in a parasite endemic area.
MATERIALS AND METHODS
Sample collection
The fecal samples of humans and proximate mammals (pigs, rodents, cows, water buffaloes, goats, and dogs) were collected from 2010 to 2013 at Wainyapu area, East Nusa Tenggara Province, Indonesia. All fecal samples were directly collected just after excretion on the ground except for those of rodents, in which the cecum contents were directly collected from captured wild rodents.
Culture conditions and examination
Approximately 0.2 g of stool was inoculated into the culture examination medium; 1.5 ml of Ringer's solution containing 10% horse serum [
5] and 0.1% asparagine, i.e., the liquid part of Tanabe-Chiba method [
6] without rice starch, in a 1.5 ml screw-capped tube. After incubation of the culture at 35℃ for 3 to 4 days, the culture sediment was examined microscopically for moving trophozoites of trichomonads. Subsequently, all positive samples were sub-cultured into the fresh medium and also 200 µl of sediment was added to 800 µl of DNAzol® reagent (Thermo Fisher Scientific, Waltham, Massachusetts, USA) in a 1.5 ml tube and preserved at room temperature (in the field) and at -20℃ (in the laboratory) for further analyses. In addition to the culture examination, the above-mentioned medium supplemented with 10% Luria-Bertani (LB) broth (Sigma-Aldrich Japan, Tokyo, Japan) was used at 35℃ to maintain the isolates in a xenic culture with fecal bacterial flora for further analyses.
Genomic DNA was extracted from the DNAzol® mixture according to the manufacturer's instructions with some modifications. Briefly, the sample was treated by 2 freeze and thaw cycles and continual overnight proteinase K (final concentration 0.4 mg/ml) digestion at 55℃ before being subjected to the standard ethanol precipitation protocol for DNAzol®. The final DNA precipitate was resuspended in 80 µl of 10 mM Tris-HCl (pH 8.0) containing 1 mM EDTA and preserved at -20℃ until use.
PCR amplification
The internal transcribed spacer 1 (ITS1)/5.8S small subunit ribosomal RNA (rRNA) gene/ITS2 genomic region was amplified using trichomonad-specific primers [
7] with slight modifications. The first PCR primer set was TRICHO-F (5'-CGG TAG GTG AAC CTG CCG TT-3') and TRICHO-R (5'-TGC TTC AGT TCA GCG GGT CT-3'), and the second PCR primer set was TRICHO-FBIS (5'-GGT GAA CCT GCC GTT GGA TC-3'). The newly designed MK32 (5'-TTC AGT TCA GCG GGT CTT CC-3') was modified from the original TRICHO-RBIS primer by adding 'T' at the 5'-end and deleting 'T' at the 3'-end to improve the detection potential. These primers were used for the nested PCR. The PCRs were performed in a final volume of 10 or 20 µl (the first and second PCR, respectively) using 1X LA Taq® PCR buffer, including 0.5 or 1.0 U of LA Taq® DNA polymerase (Takara Bio Inc., Shiga, Japan), approximately 1 µg of genomic DNA or 0.5 µl of the first PCR solution as templates, each primer at 0.2 µM, each deoxynucleoside triphosphate (dNTP) at 2.5 mM, and 2.5 mM MgCl
2 under the following conditions: a denaturation step of 94℃ for 1 min followed by 35 cycles of 94℃ for 30 sec, annealing at 54-55℃ for 15 sec, and extension at 72℃ for 40 or 20 sec. The post-extension was completed at 72℃ for 5 min.
When ITS1/5.8S rRNA/ITS2 locus amplification was not successful, the partial 18S rRNA gene locus (approximately 1,350 bp) was analyzed as an alternative target. According to the alignment results using reference sequences of trichomonads, a novel 18S rRNA PCR method was designed. The first PCR using MK1 (5'-GTA GGC TAT CAC GGG TAA CG-3') and MK6 (5'-GTT GAC ACA CAT TTA CAA GGG ATT CC-3') and the second PCR using MK5 (5'-GCA GCA GGC GCG AAA CTT AC-3') and MK4 (5'-GGA CAT CAC GGA CCT GTT ATT GCT AC-3') were conducted as nested PCR. Both the first and second PCR reactions were performed in the same volume of a 10 µl reaction mixture using 1X PrimeSTAR® buffer containing 0.5 U of PrimeSTAR® HS DNA Polymerase (Takara), 1 µg of the extracted sample DNA or 0.5 µl of the first PCR solution as the template, each dNTP at 0.8 mM, and each primer at 0.3 µM under the following conditions: a denaturing step 98℃ for 30 sec followed by 30 cycles of 98℃ for 10 sec, annealing at 53℃ for 5 sec in both the first and the second PCR, and extension at 72℃ for 81 or 55 sec, with post-extension at 72℃ for 2 min. The PCR products were electrophoresed on 2% (ITS1/5.8S rRNA/ITS2) and 1.2% (18S rRNA) agarose gels with 0.2 µg/ml ethidium bromide and visualized on a Gel Doc™ EZ UV trans-illuminator (BioRad Laboratories, Tokyo, Japan).
DNA sequencing analysis
The target bands were then excised from the gel and purified using the FastGene® gel/PCR extraction kit (Nippon Genetics, Tokyo, Japan) according to the manufacturer's instructions. The direct sequencing of each purified PCR product was conducted with the ABI Prism BigDye® Terminator v3.1 Cycle Sequencing Kit (Life Technologies Japan, Tokyo, Japan) on an Applied Biosystems 3130 Genetic Analyzer (Life Technologies). All nucleotide sequences were confirmed using both the forward and reverse reading data, and ambiguous nucleotides were precisely confirmed by conducting the sequence procedures repeatedly.
Sequence identification and phylogenetic analysis
In total, 14 reference sequences of the ITS1/5.8S rRNA/ITS2 region of trichomonads were obtained from the DNA data bank of Japan (DDBJ) using a homology search with the sample sequence data in this study (
Table 1). The phylogenetic reconstruction was conducted using the 14 reference sequences and the novel 4 sequence variations targeting 264-349 bp of the ITS1/5.8S rRNA/ITS2 locus with MEGA5 [
8], and finally a total of 204 positions at the sequences were analyzed. For comparative analyses of phylogenetic reconstructions, the maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) methods were used, and each bootstrap statistical value for the ML, MP, and NJ tree was calculated with 1,000 replications. While, the 18S rRNA gene identification, a homology search, was conducted using the BLASTn algorithm on DDBJ.
The newly identified partial sequences of the ITS1/5.8S rRNA/ITS2 regions (4 sequences) and partial sequences of the 18S rRNA gene (2 sequences) were deposited in the DDBJ/European Molecular Biology Laboratory (EMBL)/GenBank nucleotide sequence databases under accession numbers of AB931157-AB931162.
Low vacuum scanning electron microscopy (LVSEM)
Trichomonads contained in the 150 µl maintenance culture solution were centrifuged at 100 g for 5 min, and the pellet fraction was washed with 300 µl of PBS (pH 7.4) warmed to 35℃. After spin-down of the trophozoites in the same conditions and removal of the supernatant, the pellet was resuspended with 100 µl of warmed PBS. The entire trophozoite solution was subsequently placed into 500 µl of ice-cold 4% paraformaldehyde in phosphate buffer (pH 7.0), drop by drop. The fixation was continued at 4℃ over night. The fixed trophozoites were washed twice by PBS and then stained with TI blue solution (Nisshin EM, Tokyo, Japan) for 2 min at room temperature according to the manufacturer's instructions on a nano-percolator filter (JEOL Ltd., Tokyo, Japan). The sample on the filter was mounted directly to the specimen holder and examined using a Miniscope® TM-3000 scanning electron microscope (Hitachi High-Technologies Corporation, Tokyo, Japan).
RESULTS
Trichomonads isolation
We isolated 21 strains of flagellate species (
Table 1) from humans (5 isolates, IdnH1-5), pigs (6 isolates, IdnP1-6), rodents (6 isolates, IdnR1-6; the species of all rodents, identified using cytochrome
c oxidase subunit 1 sequencing, was
Rattus exulans; data not shown), a water buffalo (1 isolate, IdnB1), a cow (1 isolate, IdnC1), a goat (1 isolate, IdnG1), and a dog (1 isolate, IdnD1). All fecal sample conditions were observed as formed stools, and no watery diarrheal case was found. Among the initially isolated strains, only limited strains, such as all human isolates (IdnH1-5), some of the rodent isolates (IdnR2, 3), and a water buffalo isolate (IdnB1), have been culture-maintained to date using the modified liquid layer of Tanabe-Chiba medium supplemented with 10% LB described in the Materials and Methods.
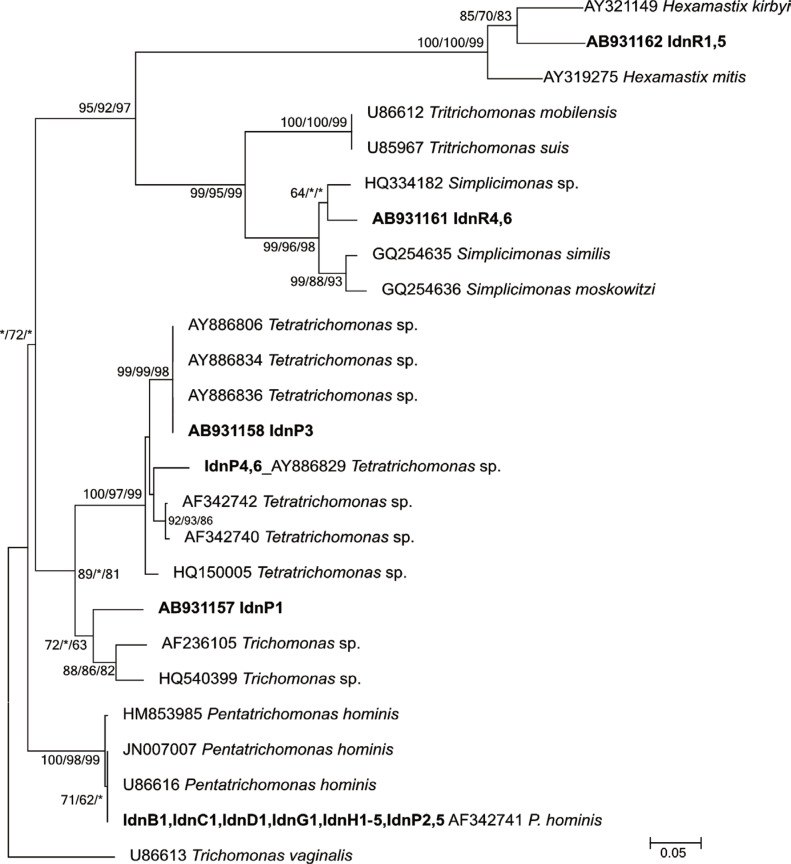
Among the 21 strains isolated in this study, the sequence results (297-349 bp) of the ITS1/5.8S rRNA/ITS2 locus identified 19 strains phylogenetically (
Fig. 1); the remaining 2 strains were from rodents (IdnR2, 3) and used the 18S rRNA gene locus (888 and 893 bp, respectively) for molecular identification (
Table 1).
The reconstructed phylogenetic tree of ITS1/5.8S rRNA/ITS2 consisted of 5 well-resolved clades supported by moderate to high bootstrap values. The first clade included a rodent isolated haplotype (IdnR1, 5) that clustered with Hexamastix mitis and Hexamastix kirbyi. The second clade consisted of the other rodent haplotype (IdnR4, 6) that grouped with Simplicimonas sp., which was positioned as a daughter clade of the Tritrichomonas species. The third clade consisted of comparatively diversified Tetratrichomonas species along with the 2 independent haplotypes (IdnP3, IdnP4, 6) from pigs. The fourth clade consisted of 1 pig isolate (IdnP1) with 2 references, which were registered as Trichomonas sp. and no specific name was recorded. The fifth and the last clade consisted of 3 references of P. hominis reported from humans and the bovine isolate, which was 100% identical to the confirmed sequence of isolates from humans (IdnH1-5), pigs (IdnP2, 5), the dog (IdnD1), the water buffalo (IdnB1), the cow (IdnC1), and the goat (IdnG1).
The remaining 2 rodent isolates (IdnR2 and IdnR3), whose 18S rRNA gene sequences differed from each other at only 1 substitution within the 888 bp overlap, showed the highest homology (94.0%) to
Hypotrichomonas sp. (HQ149966) isolated from
Otolemur garnettii (order Primates) [
9].
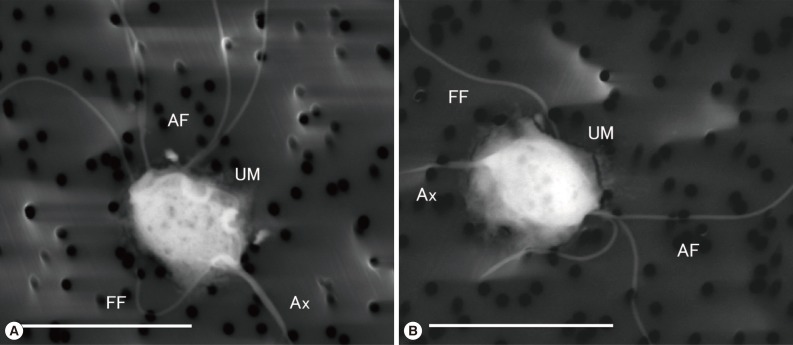
LVSEM clearly demonstrated the morphological features of
P. hominis (IdnH1) and
Hypotrichomonas sp. (IdnR2). In IdnH1 (
Fig. 2A), specific morphological features of
P. hominis [
1] were confirmed, such as the 5 anterior free flagella that apparently showed the typical 4+1 arrangement, which is specific to the genus
Pentatrichomonas. Moreover, a recurrent flagellum, which incorporated into the free margin of the undulating membrane along the whole oval shaped body and ended as a free posterior flagellum next to the axostyle, was observed. In IdnR2 (
Fig. 2B),
Hypotrichomonas sp. showed 3 anterior flagella and 1 recurrent flagellum along the undulating membrane, which was shorter than the body, and the free flagellum appeared to extend toward the lateral side from the end of the undulating membrane [
10].
DISCUSSION
The usability of the easily prepared modified Tanabe-Chiba medium supplemented with 10% LB was confirmed. Methodologically, trichomonad analyses depended on the culture examination, as microscopic detection using fixed staining samples, especially from stool samples, are quite difficult for this non-cyst-forming protozoan. Currently, various xenic culture methods, i.e., cultures with unknown microbiota, for microscopic examinations are available, such as the use of Rakoff's and Ringer's solution with blood serum and Les medium [
11,
12]. We employed a modified Tanabe-Chiba culture as the initial examination medium, which included the supplementation of 10% LB for the maintenance medium as described in the Materials and Methods. This simple protocol was effective for the initial culture examination and was partially successful for further maintenance cultivation of trichomonads. The culturable trichomonads in the maintenance medium were designated molecularly as
P. hominis and
Hypotrichomonas sp.; however, other species, such as
Tritrichomonas sp.,
Simplicimonas sp., and
Tetratrichomonas sp. were not maintained. Although the medium appeared to have a certain limitation regarding the culturable species variation,
P. hominis, which is only a trichomonad reported from human feces, could grow in the maintenance medium for long periods. Therefore, this method could be a convenient option for clinical diagnosis and further analyses of intestinal trichomoniasis for human isolates. It is noteworthy that the isolated strains of
P. hominis and
Hypotrichomonas sp. have been maintained under the condition described in the study for over 2 years till date.
Various host specificities and potential zoonotic transmissions of trichomonads were confirmed in the study (
Table 1;
Fig. 1). The only trichomonad species detected in non-human mammals were
Simplicimonas sp. (IdnR4, 6) and
Hexamastix sp. (IdnR1, 5) from rodents and
Tetratrichomonas sp. (IdnP3, 4, 6) and
Trichomonas sp. (IdnP1) from pigs.
Simplicimonas spp. has been reported from a gecko (
Uroplatus lineatus), a chameleon (
Chamaeleo cristatus) [
13], and a chicken [
14]. The isolates (IdnR4, 6) from rodents (
R. exulans) thus showed a novel host range for
Simplicimonas species. In the phylogeny analysis (
Fig. 1), the rodent haplotype (AB931161) formed a cluster with the poultry haplotype although at a comparatively low bootstrap value; nevertheless, the clustering of all Simplicimonas spp. was statistically supported.
Regarding the
Hexamastix species, 2 reptile isolates have been reported:
H. mitis from a Cuban rock iguana (
Cyclura nubile) and
H. kirbyi from an agamid lizard (
Uromastyx sp.). The phylogenic position of the ITS1/5.8S rRNA/ITS2 locus also has been precisely identified [
15,
16]. However, for
Hexamastix muris, which has been reported in rats and other rodents [
17,
18], and also
Hexamastix caviae and
Hexamastix robuslus, which were found morphologically in the cecum of the guinea pig [
19], genetic references remain unavailable. Because the strains (IdnR1, 5) did not grow in the maintenance medium, the morphological data could not be confirmed, we thus tentatively addressed the haplotype identification as
Hexamastix species based only on the molecular taxonomy.
The genus
Tetratrichomonas consists of approximately 10 valid species and has been detected in a broad spectrum of mammals, including ruminants, suiformes, rodents, and non-human primates [
20]. The
Tetratrichomonas isolates from pigs, IdnP3 and IdnP4, 6, were separately clustered with a chimpanzee haplotype [
9] and domestic-pig/peccary haplotypes [
20], respectively. Additionally, the bovine haplotypes [
21] were characterized as a daughter cluster that was clearly separated from the cluster of the presently collected pig isolates that clustered with the pig/peccary and chimpanzee types.
Trichomonas sp. (IdnP1) from pigs was clustered with a duck [
22] and pig-haplotypes [
14]. This study as well as previous studies could not identify the morphological characteristics of the isolates specific to this unique cluster. Further data regarding morphological characteristics are required to confirm if the cluster represents a novel sub-cluster of
Tetratrichomonas spp. or an unnamed new species. However, the sequence data of IdnP1 clearly addressed the phylogenetic position as a daughter cluster of
Tetratrichomonas species (
Fig. 1).
The reported host range of
P. hominis in the field is quite wide, including humans, dogs, cats, cattle, primates, rodents (guinea pig) [
23], boas, goats, and owls [
24], while, experimental infection trials in laboratory mammals have also supported a part of such wide range of host species [
25]. In this study,
P. hominis was detected also in a wide variety of host species, i.e., humans (IdnH1-5), pigs (IdnP2, 5), a dog (IdnD1), a water buffalo (IdnB1), a cow (IdnC1), and a goat (IdnG1). The potential zoonotic transmission of the species was suggested in the above-mentioned references morphologically, and also by molecular identifications in mammals, except humans [
24]. The zoonotic potential was confirmed using the high-resolution gene locus of the ITS regions molecularly (
Fig. 1), and also the species identification confirmed morphologically (
Fig. 2A). All
P. hominis strains isolated in this investigation were genetically identical; thus zoonotic transmission between humans and these closely related mammals may be occurring in the area.
In the analysis of rodent isolates (IdnR2 and IdnR3), we could not amplify the ITS1/5.8S rRNA/ITS2 region, and used the 18S rRNA gene locus alternatively to identify the species. Taken the results together with the fact that the reference data of ITS1/5.8S rRNA/ITS2 region has not been available for
Hypotrichomonas sp., the targeted primer regions may include some crucial substitutions for the ITS1/5.8S rRNA/ITS2 PCR. Although the confirmed 18S rRNA gene sequences showed only 94.0% homology to the reference sequence of
Hypotrichomonas sp. (
Table 1), the morphological analysis using LVSEM could confirm the identification (
Fig. 2B). Thus, the sequence data could be novel diversified references for the species.
To the best of our knowledge, the detection of
Simplicimonas sp. in
R. exulans and
P. hominis in the water buffalo revealed newly recognized host adaptations, and indicated a previously unknown potential host range of trichomonads. Considering the existence of such hidden host specificities and intra/inter-species genetic diversity of the trichomonad species, as seen in an unnamed unique daughter cluster of
Tetratrichomonas species and also comparatively diversified newly recognized
Hypotrichomonas variations, our current knowledge regarding the genetic and host diversity of the trichomonad species seems quite limited. In this study, the zoonotic transmissions of
P. hominis indicated that closely related animals are potential sources of human infection with
P. hominis. Therefore,
P. hominis should be monitored as a potential pathogen [
2] along with other trichomonads, such as
T. gallinarum (avian trichomonad) and
T. foetus (bovid trichomonad), which were reported as causal agents of atypical human infections [
3]. In contrast to the previous commensal image of intestinal trichomonads, the organisms could be harmful pathogens to humans and related animals. To address this novel issue, further data based on the robust and accurate taxonomy identification using molecular and morphological methodologies are required.
Notes
-
We have no conflict of interest related to this work.
ACKNOWLEDGMENTS
We would like to acknowledge the support of the Directorate General of Higher Education (DIKTI) Indonesia through the Joint Scholarship Program DIKTI Indonesia-Kanazawa University, Japan. A part of this work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research. We also appreciate Editage for providing editorial assistance.
References
- 1. Honigberg BM. Trichomonad found outside the urogenital tract of humans. In Honigberg BM ed, Trichomonads Parasitic in Humans. New York, USA. Springer-Verlag; 1990, pp 342-393.
- 2. Meloni D, Mantini C, Goustille J, Desoubeaux G, Maakaroun-Vermesse Z, Chandenier J, Gantois N, Duboucher C, Fiori PL, Dei-Cas E, Duong TH, Viscogliosi E. Molecular identification of Pentatrichomonas hominis in two patients with gastrointestinal symptoms. J Clin Pathol 2011;64:933-935.
- 3. Duboucher C, Pierce RJ, Capron M, Dei-Cas E, Viscogliosi E. Recent advances in pulmonary trichomonosis. Trends Parasitol 2008;24:201-202.
- 4. Adriyani Y. Trichomonas vaginalis, protozoa pathogen saluran urogenital. Sumatra. USU Institutional Repository, Library of North Sumatra University; 2006, Available from: http://repository.usu.ac.id/handle/123456789/3501
- 5. Rakoff AE. Results of intra-peritoneal injections of laboratory animals with various trichomonad flagellates (protozoa). Am J Epidemiol 1934;19:502-513.
- 6. Tanabe M, Chiba E. A new culture medium for Entamoeba histolytica. Acta Med Keijo 1928;11:1-4.
- 7. Duboucher C, Caby S, Dufernez F, Chabé M, Gantois N, Delgado-Viscogliosi P, Billy C, Barré E, Torabi E, Capron M, Pierce RJ, Dei-Cas E, Viscogliosi E. Molecular identification of Tritrichomonas foetus-like organisms as co-infecting agents of human Pneumocystis pneumonia. J Clin Microbiol 2006;44:1165-1168.
- 8. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731-2739.
- 9. Smejkalová P, Petrželková KJ, Pomajbíková K, Modrý D, Čepička I. Extensive diversity of intestinal trichomonads of non-human primates. Parasitology 2012;139:92-102.
- 10. Mattern CF, Daniel WA, Honigberg BM. Structure of Hypotrichomonas acosta (Moskowitz) (Monocercomonadidae, Trichomonadida) as revealed by electron microscopy. J Protozool 1969;16:668-685.
- 11. Diamond LS. In vitro cultivation of the Trichomonadidae: a state of the art review. Acta Univ Carol Biol 1986;30:221-228.
- 12. Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 2002;15:329-341.
- 13. Cepicka I, Hampl V, Kulda J. Critical taxonomic revision of parabasalids with description of one new genus and three new species. Protist 2010;161:400-433.
- 14. Lollis L, Gerhold R, McDougald L, Beckstead R. Molecular characterization of Histomonas meleagridis and other parabasalids in the United States using the 5.8S, ITS1, and ITS2 rRNA regions. J Parasitol 2011;97:610-615.
- 15. Hampl V, Cepicka I, Flegr J, Tachezy J, Kulda J. Critical analysis of the topology and rooting of the parabasalian 16S rRNA tree. Mol Phylogenet Evol 2004;32:711-723.
- 16. Hampl V, Cepicka I, Flegr J, Tachezy J, Kulda J. Morphological and molecular diversity of the monocercomonadid genera Monocercomonas, Hexamastix, and Honigbergiella gen. nov. Protist 2007;158:365-383.
- 17. Kirby H, Honigberg B. Flagellates of the Caecum of Ground Squirrels. 1st ed. Berkeley, California, USA. University of California; 1949, pp 315-366.
- 18. Wenrich DH. Culture experiments on intestinal flagellates; trichomonad and other flagellates obtained from man and certain rodents. J Parasitol 1946;32:40-53.
- 19. Nie D. Morphology and taxonomy of the intestinal protozoa of the guinea-pig, Cavia porcella. J Morphol 1950;86:381-494.
- 20. Cepicka I, Hampl V, Kulda J, Flegr J. New evolutionary lineages, unexpected diversity, and host specificity in the parabasalid genus Tetratrichomonas. Mol Phylogenet Evol 2006;39:542-551.
- 21. Walker RL, Hayes DC, Sawyer SJ, Nordhausen RW, Van Hoosear KA, BonDurant RH. Comparison of the 5.8S rRNA gene and internal transcribed spacer regions of trichomonadid protozoa recovered from the bovine preputial cavity. J Vet Diagn Invest 2003;15:14-20.
- 22. Crespo R, Walker RL, Nordhausen R, Sawyer SJ, Manalac RB. Salpingitis in Pekin ducks associated with concurrent infection with Tetratrichomonas sp. and Escherichia coli. J Vet Diagn Invest 2001;13:240-245.
- 23. Gookin JL, Birkenheuer AJ, St John V, Spector M, Levy MG. Molecular characterization of trichomonads from feces of dogs with diarrhea. J Parasitol 2005;91:939-943.
- 24. Dimasuay KGB, Rivera WL. Molecular characterization of trichomonads isolated from animal hosts in the Philippines. Vet Parasitol 2013;196:289-295.
- 25. Wenrich DH. Morphology of the intestinal trichomonad flagellates in man and of similar forms in monkeys, cats, dogs, and rats. J Morphol 1947;74:189-211.
- 26. Reinmann K, Müller N, Kuhnert P, Campero CM, Leitsch D, Hess M, Henning K, Fort M, Müller J, Gottstein B, Frey CF. Tritrichomonas foetus isolates from cats and cattle show minor genetic differences in unrelated loci ITS-2 and EF-1α. Vet Parasitol 2012;185:138-144.
- 27. Felleisen RS. Comparative sequence analysis of 5.8S rRNA genes and internal transcribed spacer (ITS) regions of trichomonadid protozoa. Parasitology 1997;115:111-119.
Fig. 1Representative neighbor-joining tree reconstructed with the ITS1/5.8S rRNA/ITS2 sequences of trichomonad species. A total of 204 positions at the sequences of the isolated strains and references of trichomonads were analyzed as described in the Materials and Methods. The values on the nodes are bootstrap values (1,000 replicates) inferred from comparative analyses of the maximum likelihood (ML), maximum parsimony (MP), and neighbor-joining (NJ) methods. The evolutionary distances are shown in units of the number of base substitutions per site. Bootstrap support less than 60% is shown by the asterisk marks. The analysis used 25 nucleotide sequences, including Trichomonas vaginalis as an out-group.

Fig. 2Representative LVSEM images of culture-isolated Pentatrichomonas hominis and Hypotrichomonas species. (A) Pentatrichomonas hominis (IdnH-1), (B) Hypotrichomonas sp. (IdnR-2). Scale bar=10 µm. AF, anterior flagella; Ax, axostyle; FF, free flagellum; UM, undulating membrane.

Table 1.Gene locus of ITS1/5.8S rRNA/ITS2 and 18S rRNA of trichomonad
Table 1.
|
Species name |
Accession number |
Strain ID |
Host |
Reference |
|
ITS1/5.8S rRNA/ITS2 gene locus |
|
|
|
|
|
Hexamastix kirbyi
|
AY321149 |
T |
Uromastyx
|
[15] |
|
Hexamastix mitis
|
AY319275 |
CYCL |
Cyclura nubila
|
[15] |
|
Hexamastix sp. |
AB931162 |
IdnR1, 5 |
Rattus exulans
|
This study |
|
Pentatrichomonas hominis
|
JN007007 |
ATCC 30000 |
Homo sapiens
|
[23] |
|
Pentatrichomonas hominis
|
HM853985 |
PM |
Homo sapiens
|
[2] |
|
Pentatrichomonas hominis
|
U86616 |
R51 |
Homo sapiens
|
[25] |
|
Pentatrichomonas hominis
|
AF342741 |
2000-0006 (IdnB1,C1,D1,G1,H1-5,P2, 5)a
|
Bovine |
[24] |
|
Simplicimonas similis
|
GQ254635 |
ULI |
Uroplatus lineatus
|
[13] |
|
Simplicimonas moskowi
|
GQ254636 |
CRIST1 |
Chamaeleo cristatus
|
[13] |
|
Simplicimonas sp. |
HQ334182 |
GABC1 |
Backyard chicken |
[14] |
|
Simplicimonas sp. |
AB931161 |
IdnR4 |
Rattus exulans
|
This study |
|
Tetratrichomonas sp. |
AF342742 |
1999-5000 |
Bovine |
[24] |
|
Tetratrichomonas sp. |
AF342740 |
2000-0017 |
Bovine |
[24] |
|
Tetratrichomonas sp. |
AY886829 |
PEKB (IdnP4, 6)a
|
Tayassu pecari
|
[20] |
|
Tetratrichomonas sp. |
HQ150005 |
PAN11 |
Pan troglodytes
|
[9] |
|
Tetratrichomonas sp. |
AY886806 |
PDOU12 |
Sus scrofa f. domestica
|
[20] |
|
Tetratrichomonas sp. |
AY886834 |
PDOU7 |
Sus scrofa f. domestica
|
[20] |
|
Tetratrichomonas sp. |
AY886836 |
PEKPR |
Pecari tajacu
|
[20] |
|
Tetratrichomonas sp. |
AB931158 |
IdnP3 |
Sus scrofa f. domestica
|
This study |
|
Trichomonas sp. |
AF236105 |
DUCK |
Duck |
[26] |
|
Trichomonas sp. |
HQ540399 |
NCT5 |
Turkey |
[14] |
|
Trichomonas sp. |
AB931157 |
IdnP1 |
Sus scrofa f. domestica
|
This study |
|
Tritrichomonas suis
|
U85967 |
C19F |
Sus scrofa f. domestica
|
[25] |
|
Tritrichomonas mobilens
|
U86612 |
M776 |
Squirrel, Monkey |
[25] |
|
18S rRNA gene locus |
|
|
|
|
|
Hypotrichomonas sp. |
HQ149966 |
KOMBG1 |
Otolemur garnettii
|
[9] |
|
Hypotrichomonas sp. |
AB931159 |
IdnR2 |
Rattus exulans
|
This study |
|
Hypotrichomonas sp. |
AB931160 |
IdnR3 |
Rattus exulans
|
This study |