Abstract
In acute uncomplicated falciparum malaria, there is a continuum from mild to severe malaria. However, no mathematical system is available to predict uncomplicated falciparum malaria patients turning to severe malaria. This study aimed to devise a simple and reliable model of Malaria Severity Prognostic Score (MSPS). The study was performed in adult patients with acute uncomplicated falciparum malaria admitted to the Bangkok Hospital for Tropical Diseases between 2000 and 2005. Total 38 initial clinical parameters were identified to predict the usual recovery or deterioration to severe malaria. The stepwise multiple discriminant analysis was performed to get a linear discriminant equation. The results showed that 4.3% of study patients turned to severe malaria. The MSPS = 4.38 (schizontemia) + 1.62 (gametocytemia) + 1.17 (dehydration) + 0.14 (overweight by body mass index; BMI) + 0.05 (initial pulse rate) + 0.04 (duration of fever before admission) - 0.50 (past history of malaria in last 1 year) - 0.48 (initial serum albumin) - 5.66. Based on the validation study in other malaria patients, the sensitivity and specificity were 88.8% and 88.4%, respectively. We conclude that the MSPS is a simple screening tool for predicting uncomplicated falciparum malaria patients turning to severe malaria. However, the MSPS may need revalidation in different geographical areas before utilized at specific places.
-
Key words: Plasmodium falciparum, uncomplicated malaria, severe malaria, predictive factors, discriminant score
INTRODUCTION
Malaria is a major infectious disease in tropical and subtropical countries. Malaria continues to be a major global health problem, with over 40% of the world's population - more than 2,400 million people - exposed to varying degrees of malaria risk in some 100 countries. The number of malaria cases worldwide appears to be growing, because of the increasing risk of transmission in areas where malaria control has declined and the increasing prevalence of drug-resistant parasite strains (e.g. chloroquine resistance). In a relatively few cases, because of increasing international travels, with modern rapid means of travel, large numbers of people from non-malarious areas are being exposed to infection, which may seriously affect them only after they return home (
Pasvol, 2005).
In some areas, the malaria mortality rate remains high for a number of reasons including limited access to healthcare and/or increased drug resistance (
Dzeing-Ella et al., 2005). Better classification of severe malaria could aid clinicians caring for patients with severe malaria to avoid delayed diagnosis, to identify severe malaria patients most likely to die, and thus to improve management by targeting resources to the sickest patients. The objectives of this study were 2-fold: to investigate the clinical factors affecting the severity of disease in acute uncomplicated falciparum malaria, and to devise a simple and reliable model of the Malaria Severity Prognostic Score (MSPS) to predict adult patients with acute uncomplicated falciparum malaria, who subsequently became severe malaria.
MATERIALS AND METHODS
Study site and recruitment procedures
The study was performed at the Bangkok Hospital for Tropical Diseases (BHTD), Mahidol University, Thailand. A total of 946 acute uncomplicated malaria patients were recruited into this study. Of the total, 600 cases had been enrolled into the score developing study, and 346 cases had been enrolled into the validation study. Patients who were admitted between January 2000 and January 2005 with a laboratory diagnosis of falciparum malaria were screened and enrolled into the score developing study. All patients were included who fulfilled the inclusion criteria; (1) acute uncomplicated falciparum malaria with microscopically confirmed
P. falciparum mono-infection, (2) either male or female, (3) body weight ≥ 40 kg and age ≥ 15 years, (4) ability to take orally antimalarial medication as part of clinical treatment for acute uncomplicated falciparum malaria, and (5) ability to remain in hospital for 7 days following the initiation of antimalarial therapy in order to evaluate the safety and efficacy of treatment and ability to return to the hospital for the scheduled follow up visit weekly until day 28 post dosing. We excluded severe malaria according to WHO criteria (
WHO, 2000), pregnant or lactating female, patients who had been pre-treated with any antimalarial drugs before admission, patients with significantly concomitant systemic diseases or diseases requiring therapy other than malaria, patients unable to attend follow-up visit, and patients who had any contraindication of blood sampling. Informed consents were obtained from the patients or their guardians. This study was reviewed and approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Patients were treated with artemisinin combination therapies (ACTs) as part of clinical protocols using alternative regimens of antimalarial drugs for acute uncomplicated falciparum malaria, e.g., artesunate 4 mg/kg/day together with mefloquine 8 mg/kg/day oral route once a day for 3 days. For any treatment failures, other antimalarial drugs (e.g. quinine plus doxycycline for falciparum) were used as appropriate.
General management was applied to all patients including reducing fever by tepid sponging and fanning. Careful attention for complications was also performed. All patients were treated symptomatically as indicated (e.g., intravenous fluid and antipyretics) according to the standard practice in the hospital. Clinical evaluation and parasite counts were performed 12-hourly until negative, then daily and at every scheduled visit. Clinical laboratory was performed on admission (before treatment) and weekly until day 28 post dosing as appropriate.
Data collection
Clinical variables potentially useful for identifying the prognosis of usual recovery or deterioration to severe malaria were recorded on admission before treatment. These were age, gender, Body Mass Index (BMI: normal weight was 18.5-24.9 kg/M2), body temperature before treatment, duration of fever before admission, initial pulse rate, past history of malaria in last 1 year, gametocytemia, schizontemia, hepatomegaly, splenomegaly, anemia (hemoglobin < 14 g/dL in males or < 12 g/dL in females, but not less than 7 g/dL), dehydration, weakness (but able to eat, drink, or walk), chill, headache, dizziness, abdominal pain, diarrhea, nausea, and anorexia. The clinically qualitative variables were scored as follows: absent = 0 and present = 1. The initially clinical blood laboratory data were also recorded as following: initial parasite density (both asexual and sexual forms, as available), hemoglobin, pack cell volume, white blood cell count, platelet count, bilirubin, alkaline phosphatase, aminotransferases, protein, blood urea, creatinine, glucose, cholesterol, sodium and potassium.
Outcome of treatment
Radical cure was defined as the absence of asexual parasite during 28 days of follow-up. Any resistant responses (RI, RII, or RIII) were categorized according to the WHO criteria (
1973). Severe malaria was defined according to the WHO criteria (
2000).
The quantitative and the qualitative data were expressed as the mean with standard deviation (SD) and as the number of observation with percentage (%), respectively. All P-values reported were from 2- tailed testing, and the statistically significant level was set at a 0.05 probability. Descriptive statistics were used to summarize baseline values and demography data. Chi-square or Fisher's exact test was used to compare proportions as appropriate and t-test to analyze continuous data. Risk verification was expressed with odds ratio (OR) and 95% confidence interval (CI). The stepwise multiple discriminant analysis was performed to get a linear discriminant equation showing the relationship between the discriminant score and the independent variables, the linear discriminant equation could be written as:
D = b1x1 + b2x2 + b3x3 + ...... + bnxn + a
Where, D = discriminant score, bi= discriminant coefficients (i = 1, 2, ... , n), xi = independent variables, a = constant; and D was labeled as the Malaria Severity Prognostic Score (MSPS).
Validation study
The validation study of MSPS, the linear discriminant equation, was applied to another group of malaria patients (n = 346) who had been admitted to the BHTD between May 2005 and April 2007, with the same conditions of inclusion and exclusion as the original study patients. The performance of the MSPS was determined with respect to the area under the Receiver Operating Characteristic (ROC) curve, sensitivity, specificity, and accuracy. The cut-off point of discriminant score was identified in the original multivariate study by using the best separation that was the highest sensitivity, together with the optimal specificity and accuracy, between the 2 study groups (uncomplicated malaria patients with becoming recovery and no severe malaria finding was found during treatment; UM group, and uncomplicated malaria patients turned to severe malaria during treatment; USM group).
RESULTS
Univariate and multivariate analyses and score developing study
Of the 946 acute uncomplicated malaria patients enrolled in this study, 600 cases were enrolled into this stage, 26 cases (4.3%) turned to severe malaria within the 4th day of hospitalization or with the median and range were 60 (18-78) hr after admission. The study patients, therefore, were divided into 2 groups; group 1: uncomplicated malaria patients with becoming recovery and no severe malaria finding was found during treatment (UM), and group 2: uncomplicated malaria patients turned to severe malaria during treatment (USM). After malaria treatment, we found 3 cases in group 1 having the reappearance of malaria during follow-up period, on day 17, 20 and 21. Since the patients in this study had returned to their home after treatment, re-infection could not be ruled out. However, no RII nor RIII resistance was observed. In cases of patients who turned to severe malaria or had reappearance of malaria, the standard treatment of hospital was given as appropriate. All patients had completed recovery by day 28 of treatment period. No patients received hemodialysis, mechanical ventilator support or any invasive intervention support. No fatal case was found.
An initial univariate analysis was made of the 38 clinical variables recorded on admission that potentially might affect to the severity of malaria disease. Only 15 out of 38 variables (39.5%) were shown the statistical significance between 2 study groups (
Tables 1 and
2). Risk analysis was verified, the results showed that schizontemia, dehydration, gametocytemia, overweight by BMI, and anorexia were identified as indicators of high risk with OR (95% CI) were 37.3 (5.9-234.2), 12.3 (3.6-41.5), 11.4 (3.2-39.9), 9.6 (2.3-39.8) and 4.5 (1.5-13.3), respectively.
The further analysis was performed in order to eliminate the confounding factors that might be affecting to the study results. To substantiate the initial results obtained, all 38 clinical variables were subjected to the stepwise multiple discriminant analysis. With this method, 8 variables were found to be significant for discrimination. The discriminant equation was showed as below formula:
Malaria Severity Prognostic Score (MSPS) = 4.38 (presented schizontemia) + 1.62 (presented gametocytemia) + 1.17 (presented dehydration) + 0.14 (overweight by BMI) + 0.05 (initial pulse rate) + 0.04 (duration of fever before admission) - 0.50 (past history of malaria in last 1 year) - 0.48 (initial serum albumin) - 5.66
Where, initial pulse rate was in beat per minute, duration of fever before admission was in day(s), initial serum albumin was in g/dL, presented schizontemia, gametocytemia, dehydration, overweight by BMI, and past history of malaria in last 1 year; when presented = 1, when absented = 0.
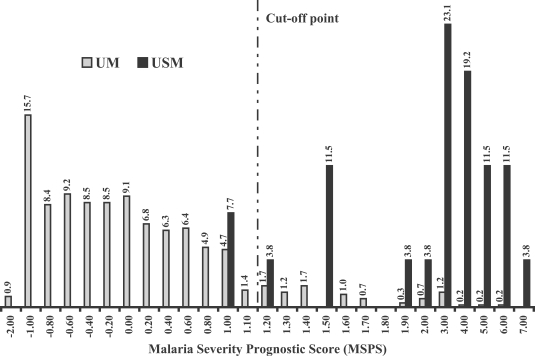
Fig. 1 showed the percentage frequency distribution of the discriminant score when the MSPS was calculated by using the clinical data from the original 600 malaria patients. From the discriminant scores' result, we found that median and 95% confidence interval of the scores in patients group 1 (UM) and 2 (USM) were -0.235 (-0.216 to -0.609) and 2.717 (2.346 to 3.786), respectively. With the cut-off point determination, we found that the score at 1.10 showed the best separation giving the highest sensitivity, together with the optimal specificity and accuracy between two study groups. Based on the score at 1.10, the sensitivity and specificity of the score tested in the original malaria patients were 92.3% and 90.7%, respectively, with an accuracy of 90.8% (
Table 3).
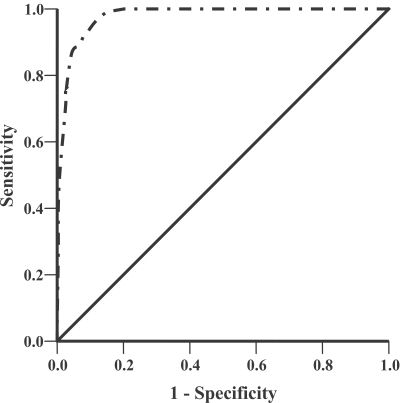
To evaluate the prognostic power of the discriminant score, we constructed the ROC curve for this MSPS based on the clinical data of the original 600 malaria patients. The results showed that prognostic power of our discriminatant score was very good, with area under the ROC curve (95% CI) was 0.97 (0.96-0.99) with
P-value < 0.001 (
Fig. 2).
The results of validation study in the other 346 acute uncomplicated falciparum malaria patients were shown in
Table 4. According to the same condition of original study, of 346 cases in validation study, 337 cases were complete recovery and no severe malaria finding was found during treatment and 9 cases turned to severe malaria during treatment. In the validation study's result, we found that the sensitivity and specificity of MSPS were 88.8% and 88.4%, respectively, with an accuracy of 88.4%.
DISCUSSION
Malaria is the major cause of mortality and morbidity in the tropical and subtropical regions in the world. However, deaths can be reduced by effective use of standard treatment procedures. Patients who require hospitalization and those who need intensive care can be identified promptly and treated before they develop complications. All cases of falciparum malaria, around 10% can be classified as severe malaria, among which the mortality is 10% but may rise to as high as 50% (
Pasvol, 2005). In the past recording data at BHTD, severe malaria after admission accounted for 3-5% of all cases of acute uncomplicated falciparum malaria (Looareesuwan, 2006: personal communication). It is well established that the prognosis of acute uncomplicated falciparum malaria patients who might be turned to severe malaria vary depending on the early diagnosis, prompt management and the presence of any complications of malaria disease (
Marsh et al., 1995;
White, 2003;
Mishra et al., 2006). Hence, it is crucial to prognosticate the cases accordingly. At present, there was no prognostic score to predict acute uncomplicated falciparum malaria patients who had not met WHO criteria for severe malaria but turned to severe malaria later. This study, to our knowledge, was the first predictive score for predicting acute uncomplicated falciparum malaria patients who turned to severe malaria later.
There was a study that investigated on the clinical and laboratory features in children with severe malaria (
Dzeing-Ella et al., 2005). The investigators reported that anemia was the most frequent feature of children patients with severe malaria, followed by respiratory distress, cerebral malaria, hyperlactataemia and hypoglycemia. There were some consistencies to another study showed low blood glucose level, hyperparasitemia and leukocytosis as the clinical features and prognostic indicators in pediatric cerebral malaria (
Molyneux et al., 1989). Moreover, Marsh and coworker emphasized the importance of respiratory complications as a simple bedside prognostic indicator in children with cerebral malaria; this could identify 84.4% fatal cases (
Marsh et al., 1995). Blood schizont and gametocyte associated with the severity of malaria disease (
Kwiatkowski, 1995;
Nacher et al., 2002;
Dondrop et al., 2004). The results of this study had agreed with those studies that presence of schizontemia and gametocytemia in malaria patients affecting to the severity of disease. Subcutaneous fatty tissue had been established as a factor indicated prognostic severity of malaria (
Wilairatana et al., 2000). When we compared with our results, this was a possible reason that overweight factor was included into the clinical factors affecting to the severity of malaria. It was not surprising that dehydration was one of the factors affecting to the severity of malaria as showed in our results. High fever and poor oral fluid intake on admission might contribute to dehydration as well described in the previous study (
Ibadin et al., 2000). In this study, we found that anorexia indicating the severity of disease. Anorexia might affect to the anitimalarial drugs absorption (
WHO, 2006). Our study result also showed that past history of malaria in last 1 year might be the protective factor to severe malaria. People who had past history of malaria in last 1 year might acquire immunity to malaria through natural exposure to malaria parasites (
WHO, 2006). Hematological changes during acute malaria were well described (
Wickramasinghe and Abdalla, 2000), our results showed that the number of white blood cell count had tendency to be lower in uncomplicated malaria patients who turned to severe malaria during treatment, with the statistically significant difference. This event was very well established in the previous study (
Ladhani et al., 2002). We also found that some serum biochemistries (e.g., albumin) were affecting to the severity of malaria disease as described in many studies (
Wilairatana et al., 1994;
Enwere et al., 1999;
Premaratna et al., 2001).
In an earlier study, the APACHE II scoring for predictive outcome in cerebral malaria had been conducted (
Wilairatana and Looareesuwan, 1995). There were many variables used in the score system e.g., vital signs, serum electrolytes, serum creatinine, hematocrit, etc. However, the results of the study suggested that the APACHE II system was useful for stratifying the prognosis of group outcome in cerebral malaria patients with the accuracy of 95.8%. Recently, there was a study focusing on the scoring for predictive outcome in adult patients with severe falciparum malaria (
Mishra et al., 2007). The study results had shown that severe anemia, acute renal failure, respiratory distress and cerebral malaria were the major factors influencing to the mortality rate of disease. The results of the study also showed a sensitivity of score was 89.9% and positive value was 94.1%. However, this index was applicable only for the adult patients with severe malaria.
Presenting of multiple complications in falciparum malaria patients after admission made the clinical management difficult. Even in the tertiary hospitals the clinical management difficulty becomes encounter as severe malaria deterioration could appear at anytime during the hospitalization. To predict the possibility of turning to severe malaria in acute uncomplicated falciparum malaria is therefore very difficult and demanding from the patient relatives, as predictive scoring system is not available yet. The previous literatures, the malaria patients' prognoses were investigated only on the outcome of death or survival. In this study, however, we focused on the difference outcome. We tried to establish the score for predicting the outcome of adult patients with acute uncomplicated falciparum malaria who might turn to severe malaria after treatment with ACTs treatment. If we can predict the turning to severe malaria in uncomplicated falciparum malaria, it may prevent the patients from any sequel complications of complicated malaria by e.g., frequent monitoring the patients or administering parenteral antimalarial drugs in high risk uncomplicated malaria patients. In our score, the laboratory tests and clinical evaluation were routine and readily available; both parameters were unambiguous without any subjective variations. Since the score would be as a screening tool, we needed the cut-off point giving the highest sensitivity. Therefore, 1.10 was the cut-off point showing the highest sensitivity, together with the optimal specificity and accuracy. According to the results of validation study in another malaria patient group, however, we found that all validity indices were slightly lower than the results which were showed by using the original malaria patients' data. This was possible cause from the effect of sample size in the validation study, as we had a limited time for patient recruitment.
Regarding to the reasonableness of the MSPS, we had looked for the possibility of the score using in the rural areas where the laboratory test was limited. According to our score, initial serum albumin was one factor included into the MSPS. We had tried to exclude the initial serum albumin out from the MSPS. Therefore, the score was accordingly modified to exclude only the initial serum albumin. The results from the modified score showed range from 0 to 9. The average accuracy was 85.3% for score 0-3.0, 93.5% for score 3.1-3.5, 95.6% for score 3.6-4.0, 96.8% for score 4.1 to 5.0 and 96.0% for score 5.1 or more. The score 3.5 was the best cut-off point, because the highest sensitivity together with the optimal specificity and accuracy (sensitivity, specificity and accuracy were 84.6, 95.8 and 95.3, respectively). Therefore, the modified score would be useful as a screening tool at bedside particularly in rural areas where some laboratory, e.g., serum albumin, was not available.
In conclusion, we have shown that the MSPS is appropriate as a simply screening tool for predicting the turning to severe malaria in adult patients with acute uncomplicated falciparum malaria at bedside in the general hospitals. It can help to assess the malaria patients as well as to communicate to the relatives of the patients about prognosis. The development of the MSPS was intended as an example for the future development of suitable model for prognostic purpose. The score also needs revalidation in different geographical areas and subsequently be utilized at the specific places.
Notes
-
This work received a financial support from the Faculty of Tropical Medicine Research Grant, Mahidol University, Bangkok, Thailand.
ACKNOWLEDGMENTS
We thank nurses and laboratory technicians of the Bangkok Hospital for Tropical Diseases for their excellent care of the patients, and their help in this study. We also thank Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand for a page charge support.
References
Fig. 1The percentage frequency distribution of Malaria Severity Prognostic Score (MSPS) in the original malaria patients. UM: acute uncomplicated falciparum malaria patients who became recovery and no severe malaria finding was found during treatment (n = 574). USM: uncomplicated malaria patients turned to severe malaria during treatment (n = 26).
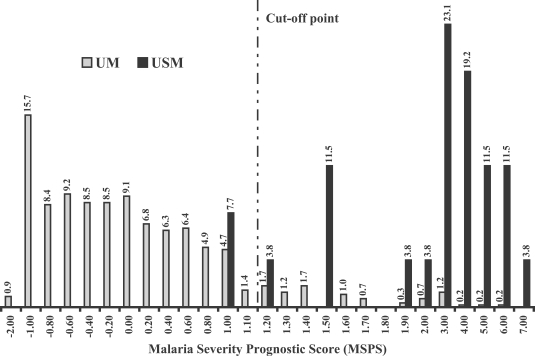
Fig. 2ROC curve of the MSPS based on the original malaria patients for discriminating acute uncomplicated falciparum malaria patients turned to severe malaria during treatment from those with becoming recovery and no any severe malaria finding was found during treatment. The area under the ROC curve (95% CI) was 0.97 (0.96-0.99), P < 0.001.
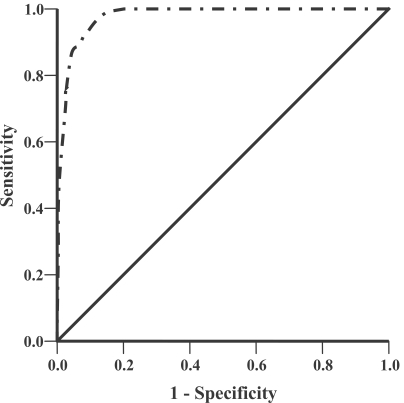
Table 1.Clinical characteristics of study patients on admission
Table 1.
|
UM (n = 574) |
USM (n = 26) |
OR (95% CI) |
|
Gender (male/female) |
449/125 |
20/6 |
1.1 (0.4-2.7) |
|
Age (years) |
|
|
|
|
Mean (SD) |
26.0 (9.0) |
25.0 (10.7) |
NA |
|
Body Mass Index [No. (%)] |
|
|
|
|
Underweight (< 18.5) |
153 (26.6) |
7 (26.9) |
1.1 (0.5-2.9) |
|
Overweight†† (> 24.9) |
8 (1.4) |
3 (11.5) |
9.6 (2.3-39.8) |
|
Geometric mean parasite count† (/µL) (Min-Max) |
4,861 (137-152,080) |
1,8062 (143-183,150) |
NA |
|
Fever [Mean(SD)] |
|
|
|
|
Before treatment†† (°C) |
37.9 (0.9) |
39.3 (0.7) |
NA |
|
Duration before admission (days) |
5.1 (4.0) |
6.6 (3.0) |
NA |
|
Pulse rate before treatment†† (bpm) |
|
|
|
|
Mean (SD) |
79.8 (11.2) |
101.6 (10.0) |
NA |
|
No.(%) of patients with: |
|
|
|
|
Past history of malaria in last 1 year†
|
283 (49.3) |
5 (19.2) |
0.2 (0.1-0.6) |
|
Presented gametocytemia†
|
9 (1.6) |
4 (15.4) |
11.4 (3.2-39.9) |
|
Presented schizontemia†
|
2 (0.3) |
3 (11.5) |
37.3 (5.9-234.2) |
|
Hepatomegaly |
116 (20.2) |
5 (19.2) |
0.9 (0.3-2.5) |
|
Splenomegaly |
43 (7.5) |
2 (7.7) |
1.0 (0.2-4.5) |
|
Anemia |
426 (74.2) |
20 (76.9) |
1.2 (0.4-2.9) |
|
Dehydration††
|
220 (38.3) |
23 (88.5) |
12.3 (3.6-41.5) |
|
Weakness |
512 (89.2) |
25 (96.2) |
3.0 (0.4-22.7) |
|
Chill |
295 (51.4) |
16 (61.5) |
1.5 (0.6-3.4) |
|
Headache |
521 (90.8) |
26 (100) |
1.0 (1.0-1.1) |
|
Dizziness |
292 (50.9) |
18 (69.2) |
2.1 (0.9-5.0) |
|
Abdominal pain |
137 (23.9) |
4 (15.4) |
0.5 (0.2-1.7) |
|
Diarrhea |
81 (14.1) |
4 (15.4) |
1.1 (0.4-3.2) |
|
Nausea |
281 (49.0) |
13 (50.0) |
1.0 (0.4-2.2) |
|
Anorexia†
|
314 (54.7) |
22 (84.6) |
4.5 (1.5-13.3) |
Table 2.Baseline laboratory data of study patients on admission
Table 2.
|
Hematology and biochemistry profiles; Mean (SD)
|
|
UM (n = 574) |
USM (n = 26) |
|
Hematology profiles |
|
|
|
Hemoglobin (g/dL) |
12.0 (2.3) |
11.9 (2.0) |
|
Pack cell volume (%) |
36.1 (6.4) |
35.5 (6.0) |
|
White blood cell count†(x103/µL) |
5.4 (1.7) |
4.9 (1.1) |
|
Platelet count (x103/µL) |
102.3 (48.0) |
97.4 (35.6) |
|
Biochemistry profiles |
|
|
|
Direct bilirubin (mg/dL) |
0.28 (0.09) |
0.31 (0.12) |
|
Total bilirubin (mg/dL) |
1.08 (0.34) |
1.17 (0.48) |
|
Alkaline phosphatase (U/L) |
102.7 (33.3) |
114.5 (37.8) |
|
Aspartate aminotransferase (U/L) |
36.0 (14.7) |
37.3 (15.5) |
|
Alanine aminotransferase (U/L) |
35.1 (11.4) |
38.6 (12.3) |
|
Albumin† (g/dL) |
3.6 (0.5) |
3.4 (0.4) |
|
Blood urea nitrogen (mg/dL) |
16.3 (5.9) |
18.4 (7.2) |
|
Creatinine (mg/dL) |
0.84 (0.12) |
0.86 (0.18) |
|
Glucose† (mg/dL) |
119.9 (15.3) |
103.2 (11.1) |
|
Cholesterol† (mg/dL) |
107.7 (18.9) |
99.8 (13.7) |
|
Sodium† (mmol/L) |
135.6 (4.2) |
133.4 (5.1) |
|
Potassium (mmol/L) |
3.7 (0.5) |
3.5 (0.3) |
Table 3.Validity results of different Malaria Severity Prognostic Scores (MSPSs) derived from the stepwise multiple discriminant analysis function based on the original malaria patients
Table 3.
|
Scores: Cut-off score |
Validity results
|
|
Sensitivity (%) |
Specificity (%) |
Accuracy (%) |
|
0.0 |
100 |
60.3 |
62.0 |
|
1.0 |
92.3 |
89.4 |
89.5 |
|
1.1*
|
92.3 |
90.7 |
90.8 |
|
1.2 |
88.5 |
92.5 |
92.3 |
|
1.3 |
88.5 |
93.7 |
93.5 |
|
1.4 |
88.5 |
95.5 |
95.1 |
|
1.5 |
76.9 |
95.5 |
94.6 |
|
1.6 |
76.9 |
96.5 |
95.6 |
|
1.7 |
76.9 |
97.2 |
96.3 |
Table 4.Validation study of MSPS in other acute uncomplicated falciparum malaria patients (n = 346) who became recovery and no severe malaria finding was found during treatment (UM) and uncomplicated malaria patients turned to severe malaria during treatment (USM)
Table 4.
|
Malaria Severity Prognostic Score (MSPS) |
Uncomplicated malaria patients becoming recovery and no any severe malaria finding was found during treatment (n = 337) |
Uncomplicated malaria patients turned to severe malaria during treatment (n = 9) |
|
Correctness of score for predicting UM |
298 |
1 |
|
Correctness of score for predicting USM |
39 |
8 |