Abstract
The aim of the present study is to determine the characteristics of genotype and phenotype of Echinococcus granulosus derived from wild sheep and to compare them with the strains of E. granulosus sensu stricto (sheep-dog) and E. granulosus camel strain (camel-dog) in Iran. In Khojir National Park, near Tehran, Iran, a fertile hydatid cyst was recently found in the liver of a dead wild sheep (Ovis orientalis). The number of protoscolices (n=6,000) proved enough for an experimental infection in a dog. The characteristics of large and small hooks of metacestode were statistically determined as the sensu stricto strain but not the camel strain (P=0.5). To determine E. granulosus genotype, 20 adult worms of this type were collected from the infected dog. The second internal transcribed spacer (ITS2) of the nuclear ribosomal DNA (rDNA) and cytochrome c oxidase 1 subunit (COX1) of the mitochondrial DNA were amplified from individual adult worm by PCR. Subsequently, the PCR product was sequenced by Sanger method. The lengths of ITS2 and COX1 sequences were 378 and 857 bp, respectively, for all the sequenced samples. The amplified DNA sequences from both ribosomal and mitochondrial genes were highly similar (99% and 98%, respectively) to that of the ovine strain in the GenBank database. The results of the present study indicate that the morpho-molecular features and characteristics of E. granulosus in the Iranian wild sheep are the same as those of the sheep-dog E. granulosus sensu stricto strain.
-
Key words: Echinococus granulosus, wild sheep, genotype, phenotype, Iran
INTRODUCTION
The pastoral cycle of echinococcosis/hydatidosis is endemic in most parts of the world, including Iran. Domestic dogs are known as the final host, while several hosts such as domestic ruminants, humans [
1], and donkey in Iran [
2] are identified as intermediate hosts. In the sylvatic cycle of
Echinococcus granulosus, the wild sheep and wild boar act as the intermediate host, whereas the golden jackal [
3,
4] acts as the final hosts. In Iran, based on morphological characteristics of
E. granulosus, there are 2 distinct strains, including the sheep-dog and the camel-dog strains [
5]. Ungulates like wild sheep, wild goats, and gazelle ingest the egg which hatches in the intestine. Consequently, oncospheres penetrate the wall of the intestine and travel through the blood stream to become lodged in the capillary beds, finally encapsulated in organs as hydatid cyst. Canids like wolves, jackals, and dogs eat hydatid cysts, and, therefore, scolices attach to their intestinal walls, grow into adult tapeworms, and produce eggs.
Based on intermediate and final hosts of
E. granulosus, 10 distinct genotypes of G1-G10 exist in the world [
6-
8]. For the first time in Iran, the genotype study of 16 isolates of
E. granulosus using mitochondrial DNA (mtDNA) markers from humans, sheep, goats, cattle, and camels revealed the existence of 2 distinct strains; the sensu stricto strain which infects sheep, goats, cattle, and humans, and the camel strain which infects camels [
9]. The sensu stricto strain (sheep-dog) was recently determined in buffalos throughout Iran, except in a very limited area in its west as the buffalo-dog or G3 strain [
10]. Further studies showed the possibility of interaction among the cycles of transmission.
Sheep is the most important host of
E. granulosus, and 5.1-74.4% prevalence has been reported [
11,
12]. The sheep strain was shown to be the most common genotype of
E. granulosus (sensu stricto). The majority of camels are occasionally infected by the camel strain [
1,
13]. Both morphological and molecular approaches could together provide more accurate and reliable information about the nature and extent of variants within
E. granulosus [
14-
16]. The objective of this study was to identify the morphological and molecular characteristics of
E. granulosus derived from the wild sheep (
O. orientalis) in Iran.
MATERIALS AND METHODS
Sample preparation
The hydatid cyst was collected from the liver of a dead wild sheep in Khojir National Park near Tehran (
Fig. 1). Protoscolices were collected in sterile saline containing antibiotics (0.4 µg/ml pencillin and 100 µg gentamycin). The vitality of protoscolices was determined by their overall aspects (motility, activity, and presence of calcareous corpuscles) and negative methylene blue staining. A 5-month-old puppy which had received the required vaccinations and anthelmintics was infected orally with 6,000 protosclices which were derived from the wild sheep. After 41 days, the dog was euthanized by an intravenous injection of an overdose of sodium pentobarbital. At the autopsy, adult
E. granulosus worms were immediately collected from the small intestine, washed extensively in PBS (pH 7.4), and fixed in 70% ethanol for molecular evaluation.
Protoscolices were mounted in polyvinyl lactophenol, and sufficient pressure was applied to the coverslip in order to flatten it without damaging the hooks. All measurements were taken on an Olympus microscope by the same individual using a calibrated ocular micrometer. The total length of large (LTL) and small (STL) hooks, blade length of large (LBL) and small (SBL) hooks, and the ratio of blade length to total length in large (LBL/LTL%) and small (SBL/STL%) hooks were measured. Three large and 3 small hooks were measured, and all hooks were counted from each of 6 protoscolices [
17]. The ratio of blade length was the percentage of blade length to total length of large (LBL/LTL%) and small (SBL/STL%) hooks. The shape and arrangement of hooks were noticed.
Genomic DNA was extracted from an adult individual E. granulosus which was derived from wild sheep (O. orientalis) using DNA kit (MBST, Tehran, Iran) and following the manufacturer’s recommendations. All the DNA samples were stored at -20˚C until they were used. DNA fragments of ribosomal ITS2 and mitochondrial COX1 were amplified using the primers, including EgI: (forward; 5´-GTCTGTCCGAGCGTCGGCTTGTAA-3´) and (reverse; 5´-CCCATCCACCACAGCATCCA-3´) for ITS2, and EgC: (forward; 5´-TAGGGTTTTTATGACCAAAG-3´) and (reverse; 5´-CACACCACATTTATCCAATT-3´) for COX1. The PCR mixture was prepared in a total volume of 50 µl containing 25 µl of Taq master mix (Amplicon, Pullman, Washington, USA), 2 µl of forward primer and 2 µl of reverse primer (final concentration of 0.4 µM), 10 µl of DNA, and 11 µl of deionized distilled water in an automated thermocycler (Biorad Italia, Milano, Italy). The PCR was performed using the following protocol: 5 min incubation at 94˚C to denature the double stranded DNA, 38 cycles for 45 sec at 94˚C (denaturing step), staying for 45 sec at 44˚C for COX1 primer or 45 sec at 56.6˚C for ITS2 specific primers (annealing step), and 45 sec at 72˚C (the extension step). Finally, the PCR was completed with an additional extension step for 10 min at 72˚C. The DNA size marker (Vivantis, Romania) was used to estimate the length of the ITS2 or the COX1 amplicons. The PCR products were analyzed on 1.2% agarose gels in 0.5×TBE buffer and visualized using Sybersafe staining (Cinaclone, Tehran, Iran) and a UV illuminator. Consequently, the PCR product was purified using a quick PCR product purification kit (MBST) according to the manufacturer’s recommendations.
Sequencing and phylogeny
Sequencing was performed in both directions for each of the PCR products based on Sanger’s method by the Kowsar Biotech Co. (Tehran, Iran), and was subjected to BLAST analysis through applying programs in the National Center for Biotechnology Information. The sequences were aligned and compared with each other and those of E. granulosus strains. The phylogenetic relationships of E. granulosus derived from wild sheep isolates and based on ITS2 and COX1 were studied employing Mega 5.
Pairwise comparisons of the level of sequence differences (D) were conducted by the formula D=1-(M/L), where M is the number of alignment positions at which the 2 sequences have a common base, and L is the total number of alignment positions with which the 2 sequences are compared [
18].
The results were statistically analyzed using the Student’s t-test, and SPSS software (version 18) was utilized as well. Furthermore, the P-value of <0.05 was considered to be statistically significant.
RESULTS
Morphometric parameters
The hydatid cyst recovered from the liver of a wild sheep was fertile and had enough protoscolices for study on the morphology and genotype characters. The rostellar hooks of the protoscolices consisted of 2 rows with alternating regular arrangements of large and small hooks, and their outlines were rough. Comparison was carried out between the measurements of different variables from sheep-dog (sensu stricto), camel-dog (intermedius) from Iran [
5], and wild sheep isolate. The results are summarized in
Table 1.
The results showed that the total length and the blade length of large and small hooks are almost similar to those of sensu stricto strain, while they have statistically significant differences from those of the camel strain (P <0.05).
Molecular characteristics
DNA was extracted from adult
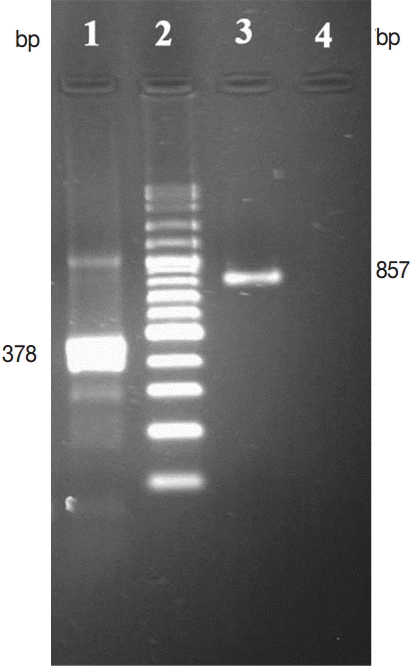
E. granulosus derived from the wild sheep. PCR was performed for 378 and 857 bp fragments of
E. granulosus ITS2 and COX1 genes, respectively, using specific primers. The results are shown in
Fig. 2. The distance based analysis, using neighbor-joining and parsimony analysis, was conducted for phylogenetic trees in order to evaluate the genetic relationships (
Figs. 3,
4). The sequences were submitted to GenBank (accession no. KR872308 for ITS2 and KR905709 for COX1).
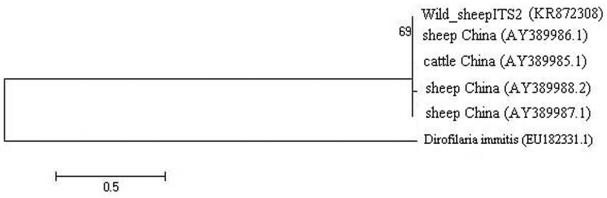
The similarity of these sequences with the recorded data in GenBank varied 99% for ITS2 and 98% for COX1 genes. The phylogenetic tree of
E. granulosus from wild sheep isolate based on ITS2 was first computed altogether and then with an outgroup of
Dirofilaria immitis to place the root (
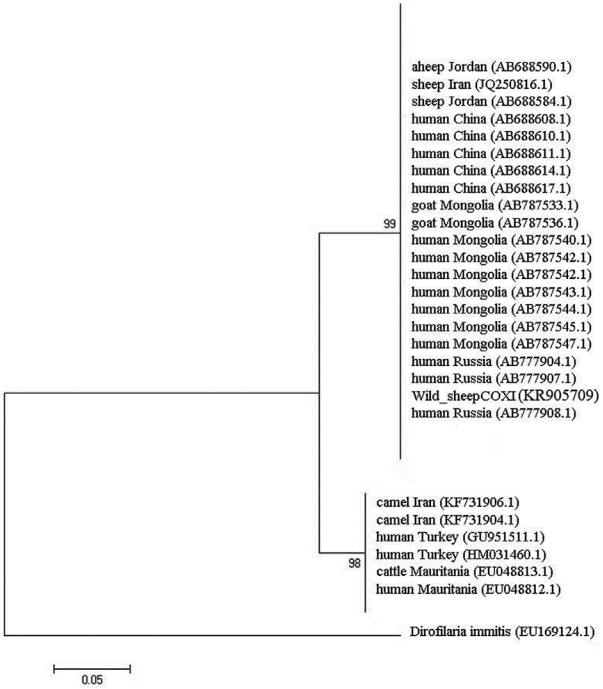
Fig. 3). The values on the nodes are bootstrap support from maximum likelihood and neighbor-joining analysis. The bootstrap supporting lower than 90% is not shown. COX1 nucleotide sequences were also used to construct a phylogenetic tree with an outgroup of
D. immitis (
Fig. 4). Two genotypes of
E. granulosus corresponding to the camel and sheep isolates showed 10% differences, and wild sheep isolate was grouped into a distinct cluster corresponding to the sensu stricto genotype.
DISCUSSION
Parasites play an important role for regulation of host population in a natural environment. Therefore, having enough knowledge about helminthic infections of wildlife is essential in every area. Echinococcosis is endemic in Iran and is maintained in 3 distinct cycles; a livestock dog domestic cycle, a desert cycle between dogs and camels, and a sylvatic cycle between wild carnivores and wild ruminants [
19]. The domestic cycle in Iranian sheep is the most important intermediate reservoir with the prevalence of 5.1-74.4% in sheep [
11,
12], 3.3-63.3% in farm dogs, and 5.0-50.5% in stray dogs [
11,
20]. In the sylvatic cycle, 5.0-8.7% of golden jackals [
3,
4] act as the final host. Intermediate hosts, including wild sheep and wild pigs [
2], were found to be infected with
E. granulosus in this cycle. The stray dogs, farm dogs, and wild carnivores are in close contact with farmers, their families, and their residential areas as well as the nomads and their black trends mainly during their wandering. Free-roaming dogs and other carnivores may be fed on or scavenge for discarded offal of domestic and wild ruminants like camels.
Drawing the hooks of protoscolices for measurements has several advantages. The total and handle lengths of both large and small hooks are considered as the most variable characteristics that could be used not only for differentiating parasite isolates from different host species but also for the original infection in the definitive host. Due to intraspecific variation of sheep and camel strains, using both morphologic and molecular tools concomitantly provides more accurate and reliable information about the phenotype and genotype characteristics of E. granulosus isolates and emphasizes the valuable tools in transmission and epidemiological studies as well.
In this study, the liver of a wild sheep infected with hydatid cysts from the Khojir National Park near Tehran was used in determining the
E. granulosus phenotype. It should be noted that this wild sheep isolate was highly similar to the sensu stricto strain (
P <0.05), which is highly important from the zoonotic point of view. The possibility of interaction between the pastoral and sylvatic cycles of
E. granulosus [
14-
16] which were found in Khojir National Park, Tehran, Iran in an examined wild sheep suggested the possibility of interaction of 3 different cycles existing worldwide.
The genotyping of 16 isolates of
E. granulosus from humans, sheep, goats, cattle, and camels revealed the existence of 2 distinct strains (sheep-dog and camel-dog strains) by mtDNA markers [
9]. As molecular analyses of the sylvatic cycle of echinococcosis, NADH dehydrogenase subunit 1 (ND1) and COX1 sequences were used to characterize deer isolates of
E. granulosus from Canada and Finland, respectively [
21,
22]. In the second part of the present study, ITS2 and COX1 genes of
E. granulosus derived from an Iranian wild sheep were amplified and sequenced. Our findings showed the sequence of fragments of 378 and 857 bp for ITS2 and COX1 genes, respectively. Two nucleotide differences of 5.1% (16/314) and 5.4% (16/295) were obtained for ITS2 and COX1 which were at 9 and 8 variable sequence positions, respectively. The COX1 nucleotide sequence obtained from deer isolate [
22] was different from what our findings (92% similarity) demonstrated. ITS2 and COX1 sequences indicated that
E. granulosus derived from the present wild sheep could be categorized as the sheep-dog genotype. Moreover, no variation was observed in the composition of the 2 obtained gene sequences (KR872308 and KR905709) deposited in GenBank.
In conclusion, our results showed that both morphological characters and molecular analyses were confirmed to be complementary for determination of
E. granulosus which was derived from an Iranian wild sheep (
O. orientalis) as a sensu stricto strain in Iran. To sum up,treatment of non-human intermediate hosts is not practical, although the frequented baiting in Australian wild definitive hosts containing praziquantel is likely to be practical [
23]. Human beings can ingest eggs if they fail to follow sanitary and hygienic practices, including wearing protective barriers like gloves and washing their hands well with soap and water after possible exposure to canid feces. In humans, this exposure could be most effectively curtailed by preventing the consumption of cervid viscera by free-roaming dogs. The parasites such as
E. granulosus strains should be identified and monitored. The results of the present study showed that the sensu stricto strain is also an infective strain in the wildlife, and the wildlife infection reflects the infections of domestic animals.
Notes
-
We have no conflict of interest related to this work.
Project supports were provided by the University of Tehran, Iran (grant no. 3/1/7502004). The authors are very grateful to Environmental Organization for help.
Fig. 1.Khojir National Park located in the northeast of Tehran, Iran.

Fig. 2.Agarose gel (1.2%), electrophoresis COX1, and ITS2 PCR products of E. granulosus derived from wild sheep isolates. Lane 1, ITS2 gene; lane 2, DNA size marker; lane 3, COX1 gene; lane 4, negative control.

Fig. 3.Phylogenetic relationships of E. granulosus which was derived from wild sheep isolate in Iran based on ITS2 sequences in comparison with different isolates.

Fig. 4.Phylogenetic relationships of E. granulosus which was derived from wild sheep isolates in Iran based on COX1 sequences in comparison with different isolates.

Table 1.Comparison between morphological characteristics of rostellar hooks of protoscolices of wild sheep, sheep, and camel isolates in Iran
Table 1.
|
Characteristics of hooks |
Wild sheep |
Sheep-dog straina
|
Camel-dog straina
|
|
Large hook |
|
|
|
|
Total length (LTL) (μm) |
23.6 ± 1.4 (22.4-25.2)b
|
23.3 ± 2.9 (21.4-24.8) |
28.9 ± 1.5 (24.8-32.5)b
|
|
Blade length (LBL) (μm) |
10.7 ± 1.1 (8.4-11.2)b
|
11.7 ± 0.9 (10.1-13.5) |
14.2 ± 2.9 (12.4-31.5)b
|
|
LBL/LTL (%) |
45.3 ± 5.2 (33.0-50.0) |
50.5 ± 2.3 (42.1-54.5) |
49.3 ± 5.5 (42.3-58.3) |
|
Small hook |
|
|
|
|
Total length (STL) (μm) |
19.6 ± 1.7 (16.7-22.4)b
|
18.1 ± 1.0 (16.9-20.3) |
21.6 ± 1.4 (18.0-23.0)b
|
|
Blade length (SBL) (μm) |
6.6 ± 1.3 (5.6-8.4)b
|
7.4 ± 0.8 (6.7-9.0) |
9.7 ± 1.1 (6.7-11.0)b
|
|
SBL/STL (%) |
33.4 ± 5.6 (28.5-43.0) |
40.6 ± 3.3 (33.3-45.2) |
45.2 ± 3.4 (30.0-48.0) |
|
Total number of hooks |
34.8 ± 1.9 (30.0-36.0) |
35.5 ± 4.2 (29.0-44.0) |
33.7 ± 2.6 (31.0-39.0) |
|
Outline |
Rough |
Rough |
Smooth |
|
Arrangement |
Alternate |
Alternate |
Alternate |
References
- 1. Fasihi Harandi M, Hobbs RP, Adams PJ, Mobedi I, Morgan-Ryan UM, Thompson RCA. Molecular and morphological characterization of Echinococcus granulosus of human and animal origin in Iran. Parasitology 2002;125:367-373.
- 2. Eslami A, Farsad-Hamdi S. Helminth parasites of wild boar, Sus scrofa, in Iran. J Wildl Dis 1992;28:316-318.
- 3. Meshgi B, Eslami A, Bahonar AR, Kharazian-Moghadam M, Gerami-Sadeghian A. Prevalence of parasitic infections in the red fox (Vulpes vulpes) and golden jackal (Canis aureus) in Iran. Iran J Vet Res Shiraz Univ 2002;10:387-391.
- 4. Sadighian A. Helminth parasites of stray dogs and jackals in Shahsavar area, Caspian Sea region. J Parasitol 1969;55:372-374.
- 5. Hosseini SH, Eslami A. Morphological and developmental characteristics of Echinococcus granulosus derived of sheep, cattle and camel in Iran. J Helminthol 1998;72:337-341.
- 6. Thompson RCA, McManus DP. Towards a taxonomic revision of the genus Echinococcus. Trend Parasitol 2002;18:452-457.
- 7. Zhang L, Eslami A, Hosseini SH, McManus DP. Indication of the presence of two distinct strains of Echinococcus granulosus in Iran by mitochondrial DNA markers. Am J Trop Med Hyg 1998;59:171-174.
- 8. Nakao M, McManus DP, Schantz PM, Craig PS, Ito A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology 2007;134:713-724.
- 9. Moks E, Jogisalu I, Valdmann H, Saarma U. First report of Echinococcus granulosus G8 in Eurasia and a reappraisal of the phylogenetic relationships of' ‘genotypes’ G5-G10. Parasitology 2008;135:647-654.
- 10. Aminpour A, Hosseini SH, Shayan P. Comparative genotyping of Echinococcus granulosus infecting buffalo in Iran using cox1 gene. Parasitol Res 2011;108:1229-1234.
- 11. Sharifi I. Seasonal prevalence of hydatid cyst in slaughterhouse of the city of Kerman. Iran J Public Health 1996;25:39-46.
- 12. Daryani A, Alaei R, Arab R, Sharif M, Dehghan MH, Ziaei H. The prevalence, intensity and viability of hydatid cyst in slaughtered animals in the Ardabil province ofIran. J Helminthol 2007;81:13-17.
- 13. Sharbatkhori M, Mirhendi H, Fasihi Harandi M, Rezaeian M, Mohebali M, Eshraghian M, Rahimi H, Bigomkia E. Echinococcus granulosus genotypes in livestock of Iran indicating high frequency of G1 genotype in camel. Experiment Parasitol 2010;124:373-379.
- 14. Eckert J. Intraspecific variation of Echinococcus granulosus and related species with emphasis on their infectivity to humans. Acta Trop 1997;64:19-34.
- 15. McManus DP, Bryant C. Biochemistry, physiology and molecular biology of Echinococcus. In Thompson RCA, Lymbery AJ eds, Echinococcus and Hydatid Disease. Walingford, Oxon, UK. CAB International; 1995, pp 135-181.
- 16. McManus DP, Bowles J. Molecular genetic approaches to parasite identification: their value in diagnostic parasitology and systematics. Int J Parasitol 1996;26:687-704.
- 17. Hobbs RP, Lymbery AJ, Thompson RCA. Rostellar hook morphology of Echinococcus granulosus (Batsch, 1786) from natural and experimental Australian hosts and its implications for strain recognition. Parasitology 1990;101:273-281.
- 18. Chilton NB, Gasser RB, Beveridge I. Differences in a ribosomal DNA sequence of morphologically indistinguishable species within the Hypodontus macropi complex (Nematoda: Strongyloidea). Int J Parasitol 1995;25:647-651.
- 19. Ahmadi N, Dalimi A. Characterization of Echinococcus granulosus isolates from human, sheep and camel in Iran. Infect Genet Evol 2006;6:85-90.
- 20. Eslami A, Hoseini SH. Echinococcus granulosus infection of farm dogs of Iran. Parasitol Res 1998;84:205-207.
- 21. Himsworth CG, Jenkins E, Hill JE, Nsungu M, Ndao M, Thompson RCA, Covacin C, Ash A, Wagner BA, Leighton FA, Skinner S. Emergence of sylvatic Echinococcus granulosus as a parasitic zoonosis of public health concern in an indigenous community in Canada. Am J Trop Med Hyg 2010;82:643-645.
- 22. Lavikainen A, Lehtinen MJ, Laaksonen S, Agren E, Oksanen A, Meri S. Molecular characterization of Echinococcus isolates of cervid origin from Finland and Sweden. Parasitology 2006;133:567-570.
- 23. Claridge AW, Mills DJ, Hunt R, Jenkins DJ, Bean J. Satellite tracking of wild dogs in south-eastern mainland Australian Forests: implications for management of a problematic top-order carnivore. Forset Ecol Manag 2009;256:814-822.