Abstract
Chloroquine remains the drug of choice for the treatment of vivax malaria in Thailand. Mixed infections of falciparum and vivax malaria are also common in South-East Asia. Laboratory confirmation of malaria species is not generally available. This study aimed to find alternative regimens for treating both malaria species by using falciparum antimalarial drugs. From June 2004 to May 2005, 98 patients with Plasmodium vivax were randomly treated with either artemether-lumefantrine (n = 47) or chloroquine (n = 51). Both treatments were followed by 15 mg of primaquine over 14 days. Adverse events and clinical and parasitological outcomes were recorded and revealed similar in both groups. The cure rate was 97.4% for the artemether-lumefantrine treated group and 100% for the chloroquine treated group. We concluded that the combination of artemether-lumefantrine and primaquine was well tolerated, as effective as chloroquine and primaquine, and can be an alternative regimen for treatment of vivax malaria especially in the event that a mixed infection of falciparum and vivax malaria could not be ruled out.
-
Key words: Plasmodium vivax, vivax malaria, chloroquine, artemether-lumefantrine, Thailand
INTRODUCTION
Malaria is still a major public health problem in Thailand. Apart from
Plasmodium falciparum parasite, which is resistant to nearly all antimalarial drugs,
P. vivax malaria is now receiving more attention. Rarely,
P. vivax can cause severe and even fatal complications, but it represents about 50% of all malaria infections, which is a heavy burden for Thailand. The prevalence of vivax malaria is increasing. Most vivax malaria is prevalent in areas of low endemicity with little immunity and affects all age groups. After treatment of falciparum malaria, 20-40% of cases will have detectable vivax parasitemia during 2-mo follow up. Vivax malaria in Thailand has a rate of relapse, estimated at between 20-40% in the 1-2 mo after an acute attack and eradication of the hypnozoite stage of the parasite requires sometimes high dose (30 mg/day) and long duration (14 days) of primaquine (
Looareesuwan et al., 1987;
Wilairatana et al., 1999).
In Thailand, chloroquine remains the drug of choice for the treatment of vivax malaria. Resistance to chloroquine has been reported in many part of the world, but so far no case has been confirmed in Thailand (
Luxemburger et al., 1999;
Looareesuwan et al., 1999;
Bair, 2004). Mixed infections of falciparum and vivax malaria are common and even increasing in South-East Asia. However, rapid diagnostic tests or microscopic confirmation of malaria species are not generally available. Therefore, clinical diagnosis of malaria is usually implemented and most of the cases will be treated using falciparum antimalarial drugs, despite availability of specific vivax malaria treatment. At present, many investigations are focused on trying to find alternative regimens for treating chloroquine-resistant vivax malaria. Artemisinin-based combination therapies (ACTs), which are currently recommended by the World Health Organization for the treatment of uncomplicated falciparum malaria, are also effective against vivax malaria. The present study aimed to find alternative regimens for treating the 2 malaria species using falciparum antimalarial drugs.
MATERIALS AND METHODS
Vivax malaria patients (n = 98) with the inclusion criteria (acute uncomplicated vivax malaria infections, either male or female; if female, pregnancy test negative before enrolment into the study, positive only asexual forms of P. vivax in blood smears, weight ≥ 40 kg and age ≥ 15 years) were enrolled in the study, and admitted to the Bangkok Hospital for Tropical Diseases for 28 days. Patients with severe malnutrition, pregnancy and lactation, concomitant febrile illness, which would interfere with follow-up, ingestion of antimalarial drugs in the past 14 days or known allergy and/or intolerance to drug(s) being tested were excluded from the study. Patients with G6PD deficiency were not given primaquine. All patients included in the study gave their informed consent. The study was approved by Ethics Committee of the Mahidol University. The patients were randomly assigned with one of the following treatments: group I artemether-lumefantrine (Coartem®, Novartis) 4 tablets, each tablet containing 20 mg artemether and 120 mg lumefantrine given orally at 0, 8, 24, 32, 48, and 60 hr (total 24 tablets) followed by 15 mg primaquine (manufactured by Government Pharmaceutical Organization) daily for 14 days in adults (children dose was adjusted according to the age); group II chloroquine (Government Pharmaceutical Organization) 25 mg base/kg given over 3 days followed by primaquine as group I. The baseline demographic data including complete blood count, G6PD status and biochemistry were obtained upon admission. Clinical assessment was performed every day, and the axillary temperature was recorded and blood smears were examined on admission and every 12 hr until parasite and fever clearance, and then at day 3, 7, 14, 21 and 28, respectively. Treatment failure was defined as i) clinical deterioration after treatment due to P. vivax illness requiring hospitalization in the presence of parasitemia, ii) presence of parasitemia and axillary temperature ≥ 37.5°C any time between day 3 and day 28, iii) presence of parasitemia on any day between day 7 and day 28, irrespective of clinical conditions.
RESULTS
The study was conducted from June 2004 to May 2005. Among the 98 patients, 47 were treated with artemether-lumefantrine and 51 with chloroquine. The baseline information included gender, age, height, weight, organomegaly, temperature, initial parasite count, and baseline laboratory results were shown in
Table 1. Eighteen patients were lost to follow-up at day 15, 16, 16, 21, 21, 23, 25, 26, and 27 for the artemether-lumefantrine treated group, and at day 13, 16, 16, 23, 23, 23, 26, 26, and 27 for the chloroquine treated group (
Table 2). Only one treatment failure was recorded in the artemether-lumefantrine treated group at day 26. Another patient in the artemetherlumefantrine treated group was diagnosed with
P. falciparum infection at day 1 and was successfully cured at day 28 for both species. The fever clearance time and parasite clearance time were slightly longer in chloroquine treated group (21.8 hr vs 25.3 hr,
P = 0.12; and 41.6 hr vs 55.8 hr,
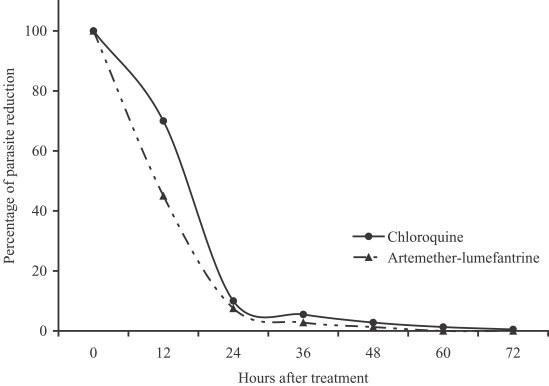
P < 0.01, respectively). The percentage of parasite reduction after treatment was shown in
Fig. 1. Both treatments were well tolerated. The main side effects in the artemether-lumefantrine group were headache (4.3%), dizziness (4.3%), and vomiting (2.1%), which could not be differentiated between the clinical signs and symptoms of malarial infection and drug-related. However, the severities of these side effects were only mild to moderate. No serious adverse event was found.
DISCUSSION
Annually, 1,500-2,200 malaria patients were admitted to the Bangkok Hospital for Tropical Diseases, and 51% of these patients had P. vivax infection and 2-4% had mixed infections with P. falciparum and P. vivax. In Thailand, chloroquine remains the drug of choice for the treatment of vivax malaria. In many parts of the world, several cases of so-called chloroquine-resistant P. vivax have been reported, but these cases were not systematically confirmed with the measurement of chloroquine and monodesethylchloroquine blood level at the day of failure, which can help to distinguish between chloroquine-sensitive and chloroquine-resistant isolates.
The artemisinin derivatives, such as artesunate, have been approved by the Thai FDA for the treatment of falciparum malaria in Thailand. These compounds are well tolerated; however, monotherapy for at least 5-7 days is required. Therefore, artemisinin derivatives should be used for the treatment of falciparum malaria in combination with long acting drugs. Artesunate has been used successfully for treatment of asexual forms of vivax malaria in combination with primaquine in Thailand (
Hamedi et al., 2004). Artemisinin was less effective in Vietnam (
Phan et al., 2002). Several ACTs are now available in co-formulated or in co-packaged presentations. Very few studies on the efficacy of ACTs for the treatment of vivax malaria are available so far. Efficacy of artesunate plus sulfadoxine-pyrimethamine in Indonesia was 89.5%, whereas dihydroartemisinin-piperaquine was 100% effective in Indonesia (
Tjitra et al., 2002). In the present study, artemether-lumefantrine was as effective as chloroquine. However, parasite clearance time was shorter in artemether-lumefantrine treated group. Only one case of failure was reported at day 26. In an earlier study in Indonesia, treatment failures after chloroquine therapy between day 17 and day 28 may be either recrudescence or relapse (
Baird et al., 1997). Unfortunately, no blood was sampled for lumefantrine dosage or molecular markers in the present study. Reinfection was excluded since patients were hospitalized during the follow-up.
Many countries have adopted ACTs as the first line drugs for the treatment of uncomplicated falciparum malaria. The present study confirms that artemether-lumefantrine is effective against vivax malaria and well tolerated in combination with primaquine. These results have implication for countries that have adopted artemether-lumefantrine as the first line drug, and where both malaria species are prevalent and parasitological differentiation is not carried out in routine practices.
Notes
-
This study was partially funded by the World Health Organization M50/370/30, Ministry of Health, Labor, and Welfare of Japan, and Mahidol University Research Grants.
ACKNOWLEDGMENTS
We are grateful to the staff of the Hospital for Tropical Diseases for their help.
References
Fig. 1Percentage of parasite reduction after treatment.
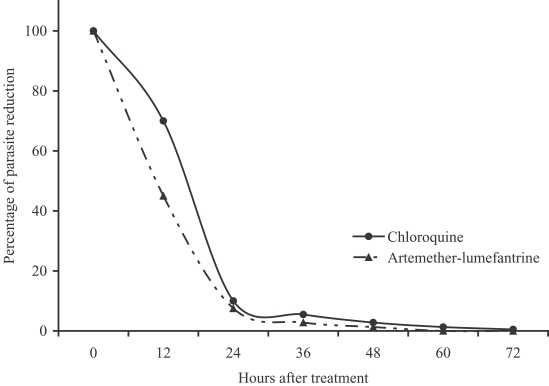
Table 1.Clinical and laboratory characteristics of study groups before treatments
Table 1.
|
Group I Artemether-lumefantrine (n = 47) |
Group II Chloroquine (n = 51) |
|
Male/Female |
35/12 |
35/16 |
|
Age (yr) |
|
|
|
Mean (SD) |
25.1 (6.9) |
24.3 (6.3) |
|
Range |
15-65 |
15-65 |
|
Mean (SD) height in cm |
162.3 (6.4) |
161.4 (7.0) |
|
Mean (SD) weight in kg |
52.9 (5.1) |
53.8 (6.3) |
|
Highest fever before treatment (°C) |
37.5 (0.8) |
37.8 (0.9) |
|
No. of patients with splenomegaly |
1 |
2 |
|
No. of patients with hepatomegaly |
12 |
11 |
|
Geometric mean parasite count per μl |
4,347.9 |
6,125.5 |
|
Range |
40-95,800 |
111-47,400 |
|
Laboratory data; mean (SD) |
|
|
|
Packed cell volume (%) |
36.2 (5.9) |
37.4 (6.1) |
|
Platelet count (/μl) |
91.3 (62.5) |
98.8 (62.0) |
|
Blood urea (mg/dL) |
16.0 (20.1) |
17.1 (18.9) |
|
Serum creatinine (mg/dL) |
0.8 (0.2) |
0.9 (0.1) |
|
Total bilirubin (mg/dL) |
1.1 (0.5) |
1.4 (0.8) |
|
Aspartate aminotrans-ferases (U/L) |
26.6 (14.5) |
29.8 (19.6) |
|
Alamine aminotrans-ferases (U/L) |
27.5 (16.7) |
32.7 (29.5) |
|
Albumin (g/L) |
3.7 (0.4) |
3.9 (0.5) |
|
Alkaline phosphatase (U/L) |
88.3 (49.0) |
90.6 (29.0) |
Table 2.
Table 2.
|
Group I Artemether-lumefantrine (n = 47) |
Group II Chloroquine (n = 51) |
|
No. of patients lost to follow-up |
9 (19.1%) |
9 (18%) |
|
No. of patients with 28 days follow-up |
38 (80.9%) |
42 (82.0%) |
|
No. (%) cured at day 28 |
37 (97.4%) |
42 (100%) |
|
Fever clearance time (hr) |
|
|
|
Mean (SD) |
21.8 (11.2) |
25.3 (10.8) |
|
Range |
4-70 |
4-90 |
|
Parasite clearance time (hr) |
|
|
|
Mean (SD)*
|
41.6 (7.2) |
55.8 (7.8) |
|
Range |
14-71 |
23-106 |
Citations
Citations to this article as recorded by

- Drug resistance markers in Plasmodium vivax isolates from a Kanchanaburi province, Thailand between January to May 2023
Thanawat Sridapan, Paweesuda Rattanakoch, Kaewkanha Kijprasong, Suttipat Srisutham, Kristan Alexander Schneider
PLOS ONE.2024; 19(7): e0304337. CrossRef - Adapted Guidelines for Malaria Case Management in Sudan
Samah Elhassan, Sahar Khalid Mohamed, Khlood Fathi Hassan Alnaeem, Ahmed Abdulgadir Noureddin, Samah Kamaleldeen Bakrri Abass, Fadwa Mohamed Saad, Technical Advisory Committee
Sudan Journal of Medical Sciences.2024; 19(4): 531. CrossRef - Plasmodium knowlesi as a model system for characterising Plasmodium vivax drug resistance candidate genes
Lisa H. Verzier, Rachael Coyle, Shivani Singh, Theo Sanderson, Julian C. Rayner, Paulo Pimenta
PLOS Neglected Tropical Diseases.2019; 13(6): e0007470. CrossRef - Management of relapsingPlasmodium vivaxmalaria
Cindy S Chu, Nicholas J White
Expert Review of Anti-infective Therapy.2016; 14(10): 885. CrossRef - Evaluation of Efficacy of Chloroquine for Plasmodium Vivax Infection Using Parasite Clearance Times: A 10-Year Study and Systematic Review
Hariharan Subramony, Noppadon Tangpukdee, Srivicha Krudsood, Kittiyod Poovorawan, Sant Muangnoicharoen, Polrat Wilairatana
Annals of the Academy of Medicine, Singapore.2016; 45(7): 303. CrossRef - Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review
Benjamin J Visser, Rosanne W Wieten, Daniëlle Kroon, Ingeborg M Nagel, Sabine Bélard, Michèle van Vugt, Martin P Grobusch
Malaria Journal.2014;[Epub] CrossRef - Malaria: an update on current chemotherapy
Benjamin J Visser, Michèle van Vugt, Martin P Grobusch
Expert Opinion on Pharmacotherapy.2014; 15(15): 2219. CrossRef - Effectiveness of Artemether/Lumefantrine for the Treatment of Uncomplicated Plasmodium vivax and P. falciparum Malaria in Young Children in Papua New Guinea
Nicolas Senn, Patricia Rarau, Doris Manong, Mary Salib, Peter Siba, John C. Reeder, Stephen J. Rogerson, Blaise Genton, Ivo Mueller
Clinical Infectious Diseases.2013; 56(10): 1413. CrossRef - Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria
Nithya Gogtay, Sridharan Kannan, Urmila M Thatte, Piero L Olliaro, David Sinclair
Cochrane Database of Systematic Reviews.2013;[Epub] CrossRef - Efficacy of artemether-lumefantrine as a treatment for uncomplicated Plasmodium vivax malaria in eastern Sudan
Tajeldin M Abdallah, Abdel Aziem A Ali, Mohammed Bakri, Gasim I Gasim, Imad R Musa, Ishag Adam
Malaria Journal.2012;[Epub] CrossRef - Therapeutic efficacy of artemether-lumefantrine for Plasmodium vivax infections in a prospective study in Guyana
Daniel Eibach, Nicolas Ceron, Karanchand Krishnalall, Keith Carter, Guillaume Bonnot, Anne-Lise Bienvenu, Stéphane Picot
Malaria Journal.2012;[Epub] CrossRef - Coartem®: a decade of patient-centric malaria management
Kamal Hamed, Heiner Grueninger
Expert Review of Anti-infective Therapy.2012; 10(6): 645. CrossRef - Open-label trial with artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria three years after its broad introduction in Jimma Zone, Ethiopia
Teferi Eshetu, Nasir Abdo, Kunuz H Bedru, Sintayehu Fekadu, Andreas Wieser, Michael Pritsch, Thomas Löscher, Nicole Berens-Riha
Malaria Journal.2012;[Epub] CrossRef - Primaquine in vivax malaria: an update and review on management issues
Deepika Fernando, Chaturaka Rodrigo, Senaka Rajapakse
Malaria Journal.2011;[Epub] CrossRef - Plasmodium vivax treatments
Ric N. Price, Nicholas M. Douglas, Nicholas M. Anstey, Lorenz von Seidlein
Current Opinion in Infectious Diseases.2011; 24(6): 578. CrossRef - Pyronaridine-Artesunate versus Chloroquine in Patients with Acute Plasmodium vivax Malaria: A Randomized, Double-Blind, Non-Inferiority Trial
Yi Poravuth, Duong Socheat, Ronnatrai Rueangweerayut, Chirapong Uthaisin, Aung Pyae Phyo, Neena Valecha, B. H. Krishnamoorthy Rao, Emiliana Tjitra, Asep Purnama, Isabelle Borghini-Fuhrer, Stephan Duparc, Chang-Sik Shin, Lawrence Fleckenstein, Lorenz von S
PLoS ONE.2011; 6(1): e14501. CrossRef - The Use of Artemether-Lumefantrine for the Treatment of Uncomplicated Plasmodium vivax Malaria
Quique Bassat, David Joseph Diemert
PLoS Neglected Tropical Diseases.2011; 5(12): e1325. CrossRef - Confirmed Vivax Resistance to Chloroquine and Effectiveness of Artemether-Lumefantrine for the Treatment of Vivax Malaria in Ethiopia
Ambachew M. Yohannes, Pascal Ringwald, Awash Teklehaimanot, Yngve Bergqvist
The American Journal of Tropical Medicine and Hygiene.2011; 84(1): 137. CrossRef - Efficacy and safety of chloroquine for treatment in patients with uncomplicated Plasmodium vivax infections in endemic countries
Cho Naing, Kyan Aung, Daw-Khin Win, Mak Joon Wah
Transactions of the Royal Society of Tropical Medicine and Hygiene.2010; 104(11): 695. CrossRef - Artemisinin combination therapy for vivax malaria
Nicholas M Douglas, Nicholas M Anstey, Brian J Angus, Francois Nosten, Ric N Price
The Lancet Infectious Diseases.2010; 10(6): 405. CrossRef - The 'non-falciparum' malarias: the roles of epidemiology, parasite biology, clinical syndromes, complications and diagnostic rigour in guiding therapeutic strategies
J. D. Maguire, J. K. Baird
Annals of Tropical Medicine & Parasitology.2010; 104(4): 283. CrossRef - Application of mobile-technology for disease and treatment monitoring of malaria in the "Better Border Healthcare Programme"
Pongthep Meankaew, Jaranit Kaewkungwal, Amnat Khamsiriwatchara, Podjadeach Khunthong, Pratap Singhasivanon, Wichai Satimai
Malaria Journal.2010;[Epub] CrossRef - Resistance to Therapies for Infection byPlasmodium vivax
J. Kevin Baird
Clinical Microbiology Reviews.2009; 22(3): 508. CrossRef