Abstract
Acanthoparyphium shinanense n. sp. (Digenea: Echinostomatidae) is described from chicks experimentally infected with the metacercariae encysted in 2 brackish water clam species, Ruditapes philippinarum and Coecella chinensis, in the Republic of Korea. The metacercariae were round to oval, armed with 23 collar spines, and 0.216 (0.203–0.226) mm in diameter. From 5 chicks experimentally infected each with 200 metacercariae, 34 juvenile (5-day-old worms) and 104 adult flukes (7-day-old worms) were harvested from their small intestines, with the average worm recovery rate of 13.8%. The adult flukes were 3.18 (2.89–3.55) mm long and 0.68 (0.61–0.85) mm wide, with an elongated, posteriorly tapering body, and a prominent head collar armed with 23 collar spines arranged in a single uninterrupted row. The posterior testis of A. shinanense was longitudinally elongated, which is similar to Acanthoparyphium spinulosum Johnston, 1917 but unique from the other closely related species, including Acanthoparyphium tyosenense Yamaguti, 1939, Acanthoparyphium kurogamo Yamaguti, 1939, and Acanthoparyphium marilae Yamaguti, 1934. The eggs of A. shinanense were larger than those of A. spinulosum, and the anterior extent of 2 lateral groups of vitellaria was slightly more limited in A. shinanense than in A. spinulosum. Molecular analysis of nuclear and mitochondrial genes revealed low homology with A. spinulosum from USA (96.1% in 5.8S rRNA) and Ukraine (97.9% in 28S rRNA), Acanthoparyphium n. sp. from USA (98.0% in 28S rRNA), and Acanthoparyphium sp. from Australia, Kuwait, and New Zealand. Biological characteristics, including its first intermediate host and natural definitive hosts, as well as its zoonotic capability, should be elucidated.
-
Key words: Acanthoparyphium shinanense n. sp., chick, intestinal fluke, brackish water clam, 5.8S rRNA, 28S rRNA, cox1, Republic of Korea
INTRODUCTION
The family Echinostomatidae Looss, 1899 (Digenea) is a group of intestinal parasites of predominantly birds, but also of mammals, including humans [
1–
3]. They are morphologically characterized by the presence of a head collar equipped with variable numbers and arrangements of collar spines [
1–
3]. At least 23 species have been reported to be able to infect humans; they include 8 species of
Echinostoma, 2 species of
Isthmiophora, 6 species of
Echinochasmus, 3 species of
Artyfechinostomum, and 1 species each of
Acanthoparyphium,
Echinoparyphium,
Himasthla, and
Hypoderaeum [
1,
3].
Flukes of the genus
Acanthoparyphium Dietz, 1909 are parasitic in aquatic birds of marine or brackish water in Tunisia, the Republic of Korea (=Korea), Japan, Australia, New Zealand, USA, Taiwan, the Philippines, Kuwait, and Ukraine [
4–
12]. At least 19 species have been described in this genus (see Discussion), although 4 of them were suggested to be synonyms of
Acanthoparyphium spinulosum Johnston, 1917, which was originally described from Australia [
13]. Later,
A. spinulosum was reported from Australia again [
6] and in Japan [
4], Taiwan [
14], USA [
7,
15–
17], Kuwait [
18], and Ukraine [
19]. The type and oldest species is
Acanthoparyphium phoenicopteri (Lühe, 1898) Dietz, 1909 described from the Tunisian flamingo
Phenicopterus roseus at Tunis [
20,
21]. In addition, it should be noted that based on molecular analyses several species-undetermined but genetically distinct
Acanthoparyphium flukes (
Acanthoparyphium sp.) have been reported from New Zealand [
12], USA [
19], Australia [
22], and Kuwait (GenBank, unpublished).
In Korea, 2 species,
Acanthoparyphium tyosenense Yamaguti, 1939 and
Acanthoparyphium marilae Yamaguti, 1934, have been found [
9,
11]. In Japan, 6 species, including
Acanthoparyphium charadrii Yamaguti, 1939 (synonymized with
A. spinulosum by Soota et al. [
23]),
Acanthoparyphium kurogamo Yamaguti, 1939,
A. marilae,
Acanthoparyphium melanittae Yamaguti, 1939,
Acanthoparyphium spinulosum suzugamo Yamaguti, 1939 (synonymized with
A. spinulosum by Skrjabin and Bashkirova [
24]), and
Acanthoparyphium squatarolae Yamaguti, 1934 have been described [
4,
5]. In China,
Acanthoparyphium loborchis Wang, 1977 and
Acanthoparyphium haematopi Ku and Chiu, 1979 were described [
25]. To date, only 1 species,
A. tyosenense was reported to infect humans, involving 10 cases from 2 coastal villages of Puan-gun in Korea [
9].
In the present study, we discovered a new group of Acanthoparyphium metacercariae having 23 collar spines from 2 species of brackish water clams, Ruditapes philippinarum and Coecella chinensis, on Aphae-do Island in Shinan-gun (County), Korea. The metacercariae were experimentally fed to chicks, and juveniles and adult flukes were recovered at days 5 and 7 post-infection, respectively. They were morphologically analyzed, and several points distinct from the pre-existing species, including A. spinulosum, A. marilae, and A. tyosenense, were recognized. We also performed molecular analysis, including sequencing of nuclear 5.8S and 28S rRNAs and mitochondrial cytochrome c oxidase 1 (cox1) genes. We concluded that our specimens are morphologically and molecularly unique from the pre-existing species, and here we propose our specimens as Acanthoparyphium shinanense n. sp.
MATERIALS AND METHODS
Surveyed area and clam collection
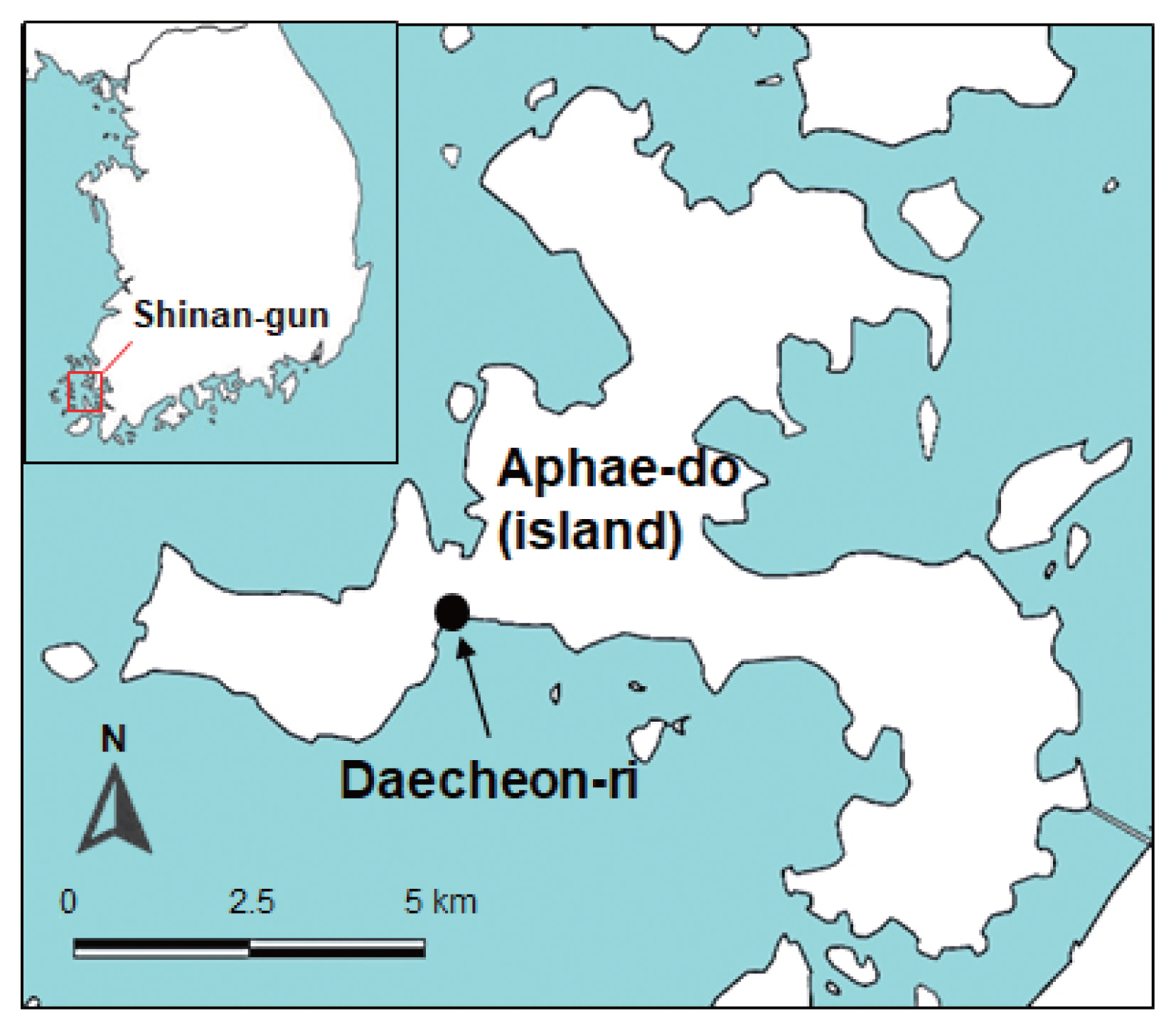
The surveyed area was a southwestern coastal region of Korea, i.e., Daecheon-ri (Village), Aphae-myon (Township), Shinan-gun, Jeollanam-do (Province) (latitude: 34.842; longitude: 126.274) (
Fig. 1). The Manila clam,
R. philippinarum (
Fig. 2A), about 1,000 in number, and the Chinese wedge clam
C. chinensis (
Fig. 2B), 987 in number, were collected from the estuary of the surveyed area in August 2020. They were quickly transported to the laboratory of Institute of Parasitic Diseases, Korea Association of Health Promotion, Seoul, Korea. After species identification, the clams were individually opened with a knife. Each of the animal part of the clam was chopped into around 10 pieces in a petri dish, and the chopped samples were pulverized for a short time (3 sec) using an electronic blender. The pulverized specimens were filtered through a thin layer of gauze, and 0.85% saline was added. Larval trematodes, in particular, metacercariae, were collected in a petri dish using a stereomicroscope.
The metacercariae in the petri dish were morphologically examined using a stereomicroscope and transferred onto a glass slide with a cover slip for light microscopic examinations. The metacercariae were tentatively identified according to their general morphological features, in particular, the morphology of the head collar and collar spines. Two hundred metacercariae were administered orally to each of 5 experimental chicks (Gallus domesticus), 3-day-old or 7-day-old, and the chicks were killed by cervical dislocation at day 5 or day 7 post-infection. Their small intestines were resected, opened longitudinally in a petri dish containing 0.85% saline, and searched for worms using a stereomicroscope. The animal experiment followed the Institutional Guidelines for Animal Care and User Committee, Institute of Parasitic Diseases, Korea Association of Health Promotion, Seoul, Korea.
Morphological observations of juvenile and adult flukes
The juvenile (5-day-old) and adult flukes (7-day-old) were fixed in 10% formalin under a coverslip pressure for morphological studies. Some specimens were preserved in 70–80% ethanol for molecular analyses. The formalin-fixed samples were washed in water, stained with Semichon’s acetocarmine, dehydrated in a graded series of ethanol, cleared in xylene, and mounted in Canada balsam. To keep the morphology of intrauterine eggs finely visible, some acetocarmine-stained samples in 70% ethanol were cleared in glycerin-alcohol and mounted in glycerin jelly. Twelve acetocarmine-stained and glycerin jelly-mounted specimens were used for morphological observations and measurements. They were compared with other
Acanthoparyphium spp. based on morphological characteristics suggested by previous authors (
Table 1) [
4,
5,
7,
9,
13]. The photos of adult flukes were taken with an Olympus DP72 digital camera (Tokyo, Japan) on an Olympus CKX41 microscope (Tokyo, Japan). The size of organs and structures were measured from digital images using ‘cellSens standard v1.5 image analysis software’ (Leica, Wetzlar, Germany).
The adult flukes preserved in 70–80% ethanol were further processed for molecular analysis. Genomic DNA was extracted from adult specimens according to the spin-column protocol described for DNeasy
® Blood & Tissue kit (QIAGEN, Hilden, Germany). Nuclear 5.8S rRNA and 28S rRNA genes and mitochondrial cytochrome
c oxidase 1 (
cox1) gene were amplified by PCR on a C1000 Touch Thermal Cycler according to the procedure reported previously [
17,
26,
27]. The forward and reverse primers for 5.8S rRNA were BDI-ITS_F (5′-GTC GTA ACA AGG TTC CGT A-3′) and 4S-ITS_R (5′-TCT AGA TGC GTT CGA AGT GTC GAT G-3′), respectively [
17], and the primers for 28S rRNA were dig12_F (5′-AAG CAT ATC ACT AAG CGG-3′) and Lo_R (5′-GCT ATC CTG AGR GAA ACT TCG-3′) [
26]. The primers for
cox1 were JB3_F (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and trem.
cox1.rrnl_R (5′-AAT CAT GAT GCA AAA GGT A-3′) [
27]. The basic local alignment search tool (BLAST;
http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to assess the genetic identity of the samples. The sequence obtained using Geneious
® version 11.1.5 (Biometers Ltd., Auckland, New Zealand) was compared with the GenBank reference sequences of the
Acanthoparyphium species or other Echinostomatidae family members. The phylogenetic trees were constructed using the maximum-likelihood method based on Tamura-Nei model of nucleotide substitution with 1,000 bootstrap replications with our samples and others deposited in GenBank and viewed by MEGA-X program.
RESULTS
Infection status of A. shinanense metacercariae in clams
About 2,000 A. shinanense metacercariae were harvested from a total of 1,000 R. philippinarum clams examined. Whereas, only 100 metacercariae of the same species were obtained from 987 C. chinensis clams examined. The clams were pooled in groups of 10 in number, and all (100%) of 100 groups of R. philippinarum were found infected, whereas only 10 (10.1%) of 99 groups of C. chinensis clams were infected with the metacercariae. The localities of the metacercariae in the clams were the foot muscle, gill, and mantle.
Recovery of A. shinanense worms from experimental chicks
From 2 chicks sacrificed at day 5 post-infection, total 34 juvenile flukes (13 and 21, respectively) were harvested from their small intestines, with the average recovery rate of 8.5%. Also, from 3 chicks sacrificed at day 7 post-infection, total 104 adult flukes were recovered (22, 33, and 49 specimens, respectively), showing the average recovery rate of 17.3%. The overall worm recovery rate from 5 chicks was 13.8%. All of the 5-day-old juvenile flukes were immature without uterine eggs, whereas the 7-day-old adult flukes were fully matured containing a number of uterine eggs.
Description of worms
Family: Echinostomatidae Looss, 1899
Subfamily: Himasthlinae Odhner, 1910
Genus: Acanthoparyphium Dietz, 1909
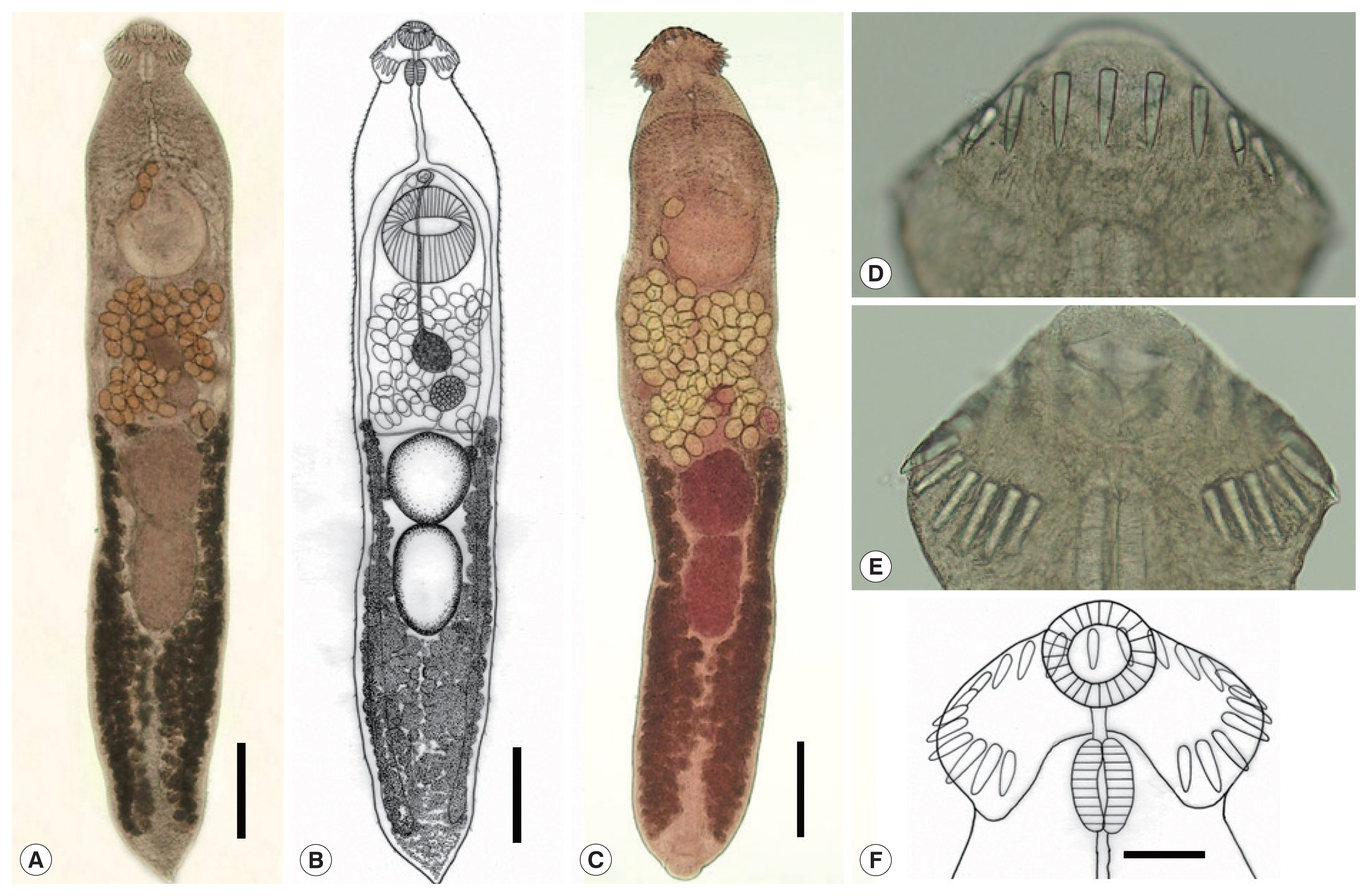
Metacercaria (
Fig. 2C): Metacercariae oval to round, covered with a thin wall, and 0.216 (0.203–0.226) mm in average diameter (n=10). Oral and ventral suckers plainly visible. Head collar equipped with total 23 collar spines uninterrupted dorsally and without corner spines distinctly seen around the oral sucker. Excretory tubules containing small round excretory granules distributed extensively in the anterior and posterior fields of the body.
When the metacercariae were excysted applying gentle pressure on a coverslip, the presence of a head collar with characteristic arrangement of collar spines around the oral sucker was then better visualized.
Adult (
Table 1;
Fig. 3A–F): Body dorsoventrally flattened, elongated leaf-like with slightly attenuated anterior end and more or less tapered posterior end, 3.18 (2.89–3.55) mm in length and 0.68 (0.61–0.85) mm in maximum width at uterine or ovarian level (n=12) (
Fig. 3A–C). Tegument beset with triangular spines, becoming less dense posteriorly, extending to level of uterus or anterior testis. Forebody long representing about 20.4% of whole body length. Anterior end characteristically equipped with an oral sucker and a prominent head collar. Oral sucker subterminal, small and spherical, muscular, about 1/3.5 of the size of the ventral sucker. Head collar small but prominent, reniform, and muscular, armed with a single row of collar spines (
Fig. 3D–F). Collar spines 23 in total, without dorsal interruption, with the formula of 3-3-4-3-4-3-3, including ventral spines 3+3, lateral spines 3+3, and dorsal spines 4+3+4, without corner spines (
Fig. 3F). Collar spines relatively short in length, moderately broad, with sharp-pointed ends (
Fig. 3D, E). The innermost ventral collar spines on each side slightly smaller than the other 21 collar spines. Prepharynx short; pharynx muscular, elongated. Esophagus relatively long; intestinal bifurcation at about 10% of total body length; ceca narrow, blind, overlapped by vitelline follicles, ending nearby the posterior termination of vitelline follicles. Cirrus sac elongated, reaching back far beyond the posterior end of ventral sucker, up to about halfway between ventral sucker and ovary. Terminal part of cirrus armed with tiny spines with a broad base. Seminal vesicle thin-walled, elongated or coiled, containing sperms and connected to muscular and tubular pars prostatica, ejaculatory duct, and a long cirrus armed with small spines on its surface. Genital atrium with genital pore median, just in front of ventral sucker, receiving female (metraterm) and male reproductive (ejaculatory) duct. Metraterm weakly muscular, connected to genital pore. Uterus intercecal, short, between ventral sucker and anterior testis, containing a small number (n=33–75) of eggs. Ovary spherical or transversely elliptical, median or slightly submedian, almost equatorial, between seminal vesicle and Mehlis’ gland. Mehlis’ gland diffuse, dorsal, median, larger than ovary, connected to ovary and uterine tubule. Uterine seminal receptacle present; Laurer’s canal present. Vitelline follicles extensive, extending laterally forming 2 lateral groups, from near posterior extremity up to the level of anterior margin of anterior testis or rarely Mehlis’ gland; the 2 groups of vitellaria not merge near posterior end of body. Two testes post-equatorial, tandem, entire, with smooth surface, almost adjacent to each other. Anterior testis globular, and posterior testis usually slightly elongated. Eggs not numerous, yellowish, oval to ovoid, immature containing a germ cell, with a small, inconspicuous operculum, and a small abopercular thickening or wrinkling at the abopercular end, 0.103 (0.095–0.111) mm long and 66 (62–71) mm wide (n=60). Excretory vesicle Y-shaped, bifurcates just posterior to posterior testis; excretory pore terminal.
Taxonomic summary
- Type host: Gallus domesticus (chick, experimental)
- Site of infection: Small intestine
- Type locality: Daecheon-ri (Village), Aphae-myon (Township; Aphae-do Island), Shinan-gun (County), Jeollanam-do (Province), Republic of Korea
- Deposition of specimens: The specimens are deposited in the Parasite Museum, Institute of Parasitic Diseases, Korea Association of Health Promotion, Seoul, Korea (no. 2021-0011-01, holotype, and no. 2021-0011-02~10, paratypes). Voucher specimens are also deposited in Meguro Parasitological Museum, Tokyo, Japan (MPM Coll. No. 21773).
- Etymology: The specific name refers to the name of the locality of the type specimen, Shinan-gun, where the infected brackish water clams were collected.
Molecular analyses
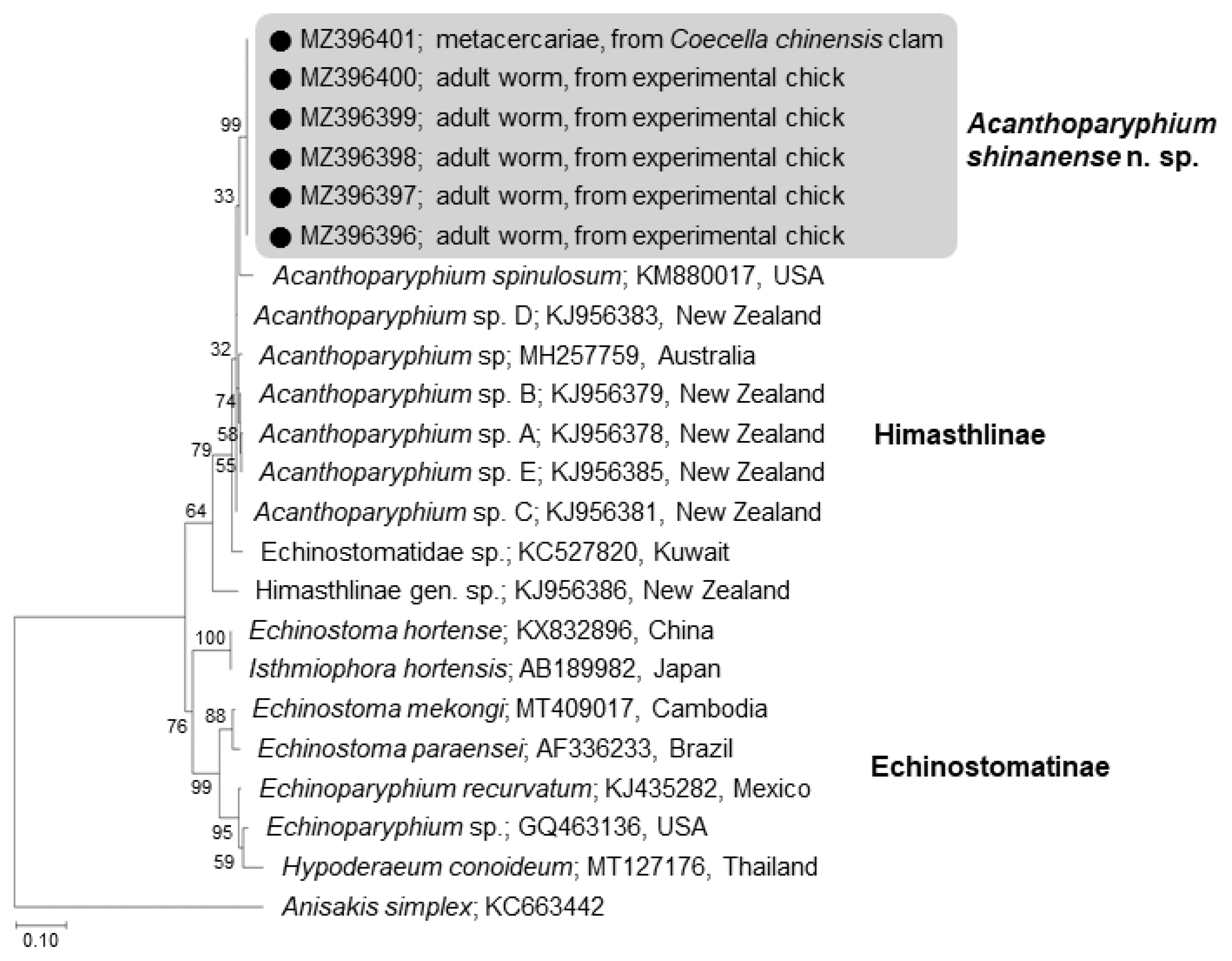
The amplification of 5.8S rRNA, 28S rRNA, and
cox1 genes of our samples produced partial sequences of 459–460 bp, 754–1,134 bp, and 451 bp, respectively. The phylogenetic tree for 5.8S rRNA was established based on sequences of our samples, i.e., 1 isolate (MZ396401) from metacercariae in
C. chinensis clams and 5 (MZ396396–396400) from adult flukes obtained from experimental chicks infected with metacercariae from
R. philippinarum clams, in comparison with other members of the Echinostomatidae, including
A. spinulosum from USA (KM880017) and
Acanthoparyphium spp. from Australia (MH257759) and New Zealand (KJ956378, KJ956379, KJ 956381, KJ956383, KJ956385) (
Fig. 4). The sequences of our samples (n=6) were clustered together (100% homologous) with high bootstrap values and constituted a new lineage distinct from other Echinostomatidae registered in GenBank (
Fig. 4;
Table 2). Our samples revealed low homologies with
A. spinulosum from USA (96.1%),
Acanthoparyphium sp. from Australia (96.6%) and New Zealand (97.0–97.9%) (
Table 2).
The phylogenetic tree for 28S rRNA was established based on our samples, i.e., 1 isolate (MZ146324) from metacercariae in clams and 5 (MZ146319–146323) from experimentally obtained adult flukes in comparison with 24 species of the Echinostomatidae, including
A. spinulosum from Ukraine (KT956939) and
Acanthoparyphium sp. from Australia (MH257769) and USA (KT956940) (
Fig. 5). The sequences of our samples (n=6) were clustered together (100% homologous) with high bootstrap values and constituted a new lineage distinct from other Echinostomatidae registered in GenBank (
Fig. 5;
Table 2). Our samples showed low homologies with
A. spinulosum from Ukraine (97.9%),
Acanthoparyphium n. sp. from USA (98.0%),
Acanthoparyphium sp. from Australia (98.1%), and Himasthla spp. from Ukraine or USA (94.3–95.9%) (
Table 2).
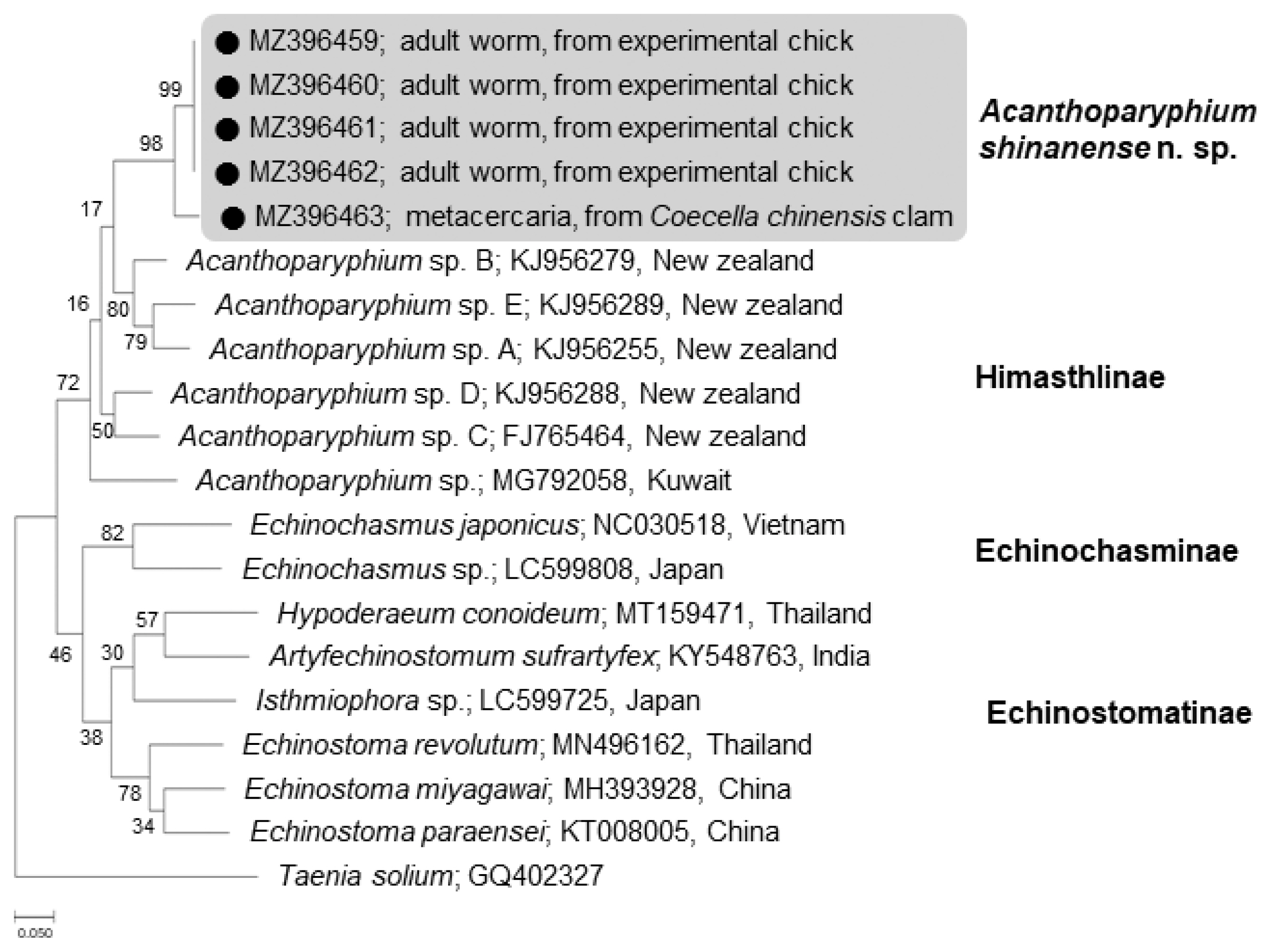
The phylogenetic tree for
cox1 was established based on sequences of our samples, i.e., 1 isolate (MZ396463) from metacercariae in clams and 4 (MZ396459–396462) from adult flukes obtained from experimental chicks, in comparison with other members of the Echinostomatidae, including
Acanthoparyphium spp. from New Zealand (FJ765464, KJ956255, KJ956279, KJ956288, KJ956289) and Kuwait (MG792058) (
Fig. 6). The
cox1 sequences were highly variable depending on different species or isolates of
Acanthoparyphium. The homology between adults (4 isolates) and a metacercaria (1 isolate) in our study was only 94.7%. However, the 4 adult isolates exhibited 100% homology together (
Table 2). The sequence homology between our samples and
Acanthoparyphium sp. from New Zealand was only 84.0–86.9% and that between our samples and
Acanthopariphium sp. from Kuwait was only 83.5–83.7%.
Compared with the 15 valid or ‘need-to-evaluate’ species of
Acanthoparyphium reported so far (
Table 3), the new species differed from most of them [
5,
9,
23,
24] in the body size and shape, sucker ratio, egg size, and the morphology of head collar, collar spines, testes, and the extent of vitellaria.
A. phenicopteri, the type species, is much smaller in body size than the new species, with a broader head collar, smaller sucker ratio (1/2), ventral sucker situated farther back in the body, and smaller number of uterine eggs [
28].
A. haematopi [
25] is differed from the new species in the remarkably smaller egg size, 0.073–0.082 mm in length, and the extent of the vitellaria.
A. loborchis and
Acanthoparyphium ochthodromi Tubangui, 1933 have 2 lobulated testes with irregular margins [
25] unlike the new species.
Acanthoparyphium pagollae Cable et al., 1960 [
29] differs from the new species in the extent of vitellaria, up to the level anterior to the ovary in the former but limited up to the level of anterior border of anterior testis in the latter.
Acanthoparyphium macracanthum Rybakov and Lukomskaya, 1988 has a small, plump or stout body with 2 transversely elongated testes [
30], which is markedly different from the new species.
Acanthoparyphium paracharadrii Velasquez, 1964 has much smaller body and egg sizes, 1.39–1.91 mm and 0.071–0.079 mm in length, respectively, and is unique having a very small cirrus sac [
31].
Acanthoparyphium lobatum Soota et al., 1970 [
32] has 26–28 collar spines that is not consistent with the generic characteristic of
Acanthoparyphium and has 2 deeply branched testes which is a markedly different feature from the new species.
Acanthoparyphium jeetai Chakrabarti, 1972 [
33] reported from a bird in India needs further evaluation.
A. squatarolae Yamaguti, 1934 is differed from the new species in that the former has a less extended vitellaria up to the level of the anterior extremity of the posterior testis and has 2 globular testes [
4]. Also,
A. marilae Yamaguti, 1934 differs from the new species in that the former has a much less extended vitellaria up to the level beyond the posterior margin of the posterior testis and has 2 globular testes [
4].
A. tyosenense Yamaguti, 1939 has a rounded posterior end and 2 globular testes [
5], whereas the new species has a tapering posterior end and 1 globular and 1 longitudinally elongated testes.
A. kurogamo Yamaguti, 1939 is differed from the new species in the position of the ventral sucker and testes (located more posteriorly) and having 2 globular testes [
5].
A. melanittae Yamaguti, 1939 has an extremely anterior extension of the vitellaria up to the level of the ventral sucker and has 2 globular testes [
4].
The new species is morphologically very close to
A. spinulosum Johnston, 1917 in the body size and shape and the longitudinally elongated shape of the posterior testis [
13]. However, the egg size of
A. spinulosum was smaller than the new species, 0.085×0.069 mm [
13], 0.084–0.106×0.056–0.068 mm [
7], or 0.085–0.100×0.066–0.082 mm [
14], compared to 0.095–0.111×0.062–0.071 mm in the new species. However, in another report [
6], the egg size of
A. spinulosum could be 0.092–0.100×0.065–0.076 mm, only slightly smaller than that of the new species. A significant finding was that the new species has vitellaria never extending anteriorly from the level of the anterior margin of the anterior testis or Mehlis’ gland, whereas
A. spinulosum has vitellaria extending up to the level of the anterior margin of the ovary [
6,
13] or the level of Mehlis’ gland [
7].
The phylogenetic trees based on 5.8S rRNA, 28S rRNA, and cox1 sequences revealed a unique lineage of our samples. The sequence homology based on 5.8S rRNA between our samples and A. spinulosum from USA (KM880017) was 96.1% and that between our samples and Acanthoparyphium sp. from Australia (MH257759) was 96.6%, relatively low and not enough for them to be identical. Similarly, the sequence homology based on 28S rRNA between our samples and A. spinulosum from Ukraine (KT956939) was 97.9%, that between our samples and Acanthoparyphium n. sp. from USA was 98.0%, and that between our samples and Acanthoparyphium sp. from Australia (MH257769) was 98.1%, all of these figures are considered not enough for them to be identical. The sequence homology based on cox1 gene between our samples and Acanthoparyphium sp. from Kuwait (MG792058) was 83.5–83.7% and that between our samples and Acanthoparyphium sp. from New Zealand (FJ765464, KJ956255, KJ956279, KJ956288, KJ956289) was 84.0–86.9%, indicating them to be far from identical. Nucleotide sequences of other Acanthoparyphium spp. reported morphologically are not yet available in GenBank.
DISCUSSION
A. phoenicopteri, the type species, was first reported in Europe (Tunisia) in 1898 (described under the name
Echinostomum phoenicopteri) [
28]. Subsequently,
A. spinulosum was discovered from the golden plover
Charadrius dominicus near Sydney, Australia [
13]. Thereafter, a third species,
A. ochthodromi, was described from the Philippines [
34]. Yamaguti [
4,
5] described 7 new species in Japan (6 species) and Korea (1 species), which included
A. charadrii Yamaguti, 1939,
A. kurogamo,
A. marilae,
A. melanittae,
A. spinulosum suzugamo Yamaguti, 1939,
A. squatarolae, and
A. tyosenense. Later,
Acanthoparyphium longivitellatum Oschmarin, 1956 was reported in Russia. However, Skrjabin and Bashkirova [
24] synonymized
A. spinosulum suzugamo and
A. longivitellatum as synonyms of
A. spinulosum. Soota et al. [
23] synonymized
A. charadrii as a synonym of
A. spinulosum. Skrjabin and Bashkirova [
24] and Chen [
25] synonymized
A. tyosenense with
A. kurogamo; however, this synonymy was denied by Chai et al. [
9] because of their different body shape and position of the acetabulum and testes [
5]. In Puerto Rico and the Philippines,
A. pagollae and
A. paracharadrii were described, respectively [
29,
31]. In India,
Acanthoparyphium cambellense Soota et al., 1970 [
23],
A. lobatum Soota et al., 1970 [
32], and
A. jeetai Chakrabarti, 1972 [
33] were described. However, Fischthal and Kuntz [
14] synonymized
A. cambellense with
A. spinulosum. In China,
A. haematopi and
A. loborchis were described in 1970 and 1979, respectively [
25]. The most lately reported species was
A. macracanthum from Russia [
30]. Thus, in the genus
Acanthoparyphium, 19 species have been described, and among them 15 species are now recognized as valid or ‘need-to-evaluate’ species (
Table 3). Geographically,
A. spinulosum is the most widely distributed species.
The new species is morphologically very close to
A. spinulosum. The drawing of
A. spinulosum adult in the original report [
13] showed that the 2 testes are both globular being not consistent with the feature of the new species. However, among the worm description within the text of the original report the posterior testis was described as a longitudinally elongate form [
13]. In another report which described the adult worm of
A. spinulosum, the 2 testes were drawn as globular (anterior one) and almost globular (posterior one) with no description of the testes morphology within the text [
6]. However, a third drawing of an adult
A. spinulosum from California, USA showed that the posterior testis is longitudinally elongated just like in our specimens, and in the text, it is written as ‘The anterior testis is usually wider and shorter than the posterior.’ [
7]. Therefore, we considered that the morphology of the posterior testis is not a differential character between the new species and
A. spinulosum. Rather, the larger egg size and the more limited distribution of vitelline follicles in the new species (see Remarks) could be considered as significant differential characters between adults of the new species and
A. spinulosum. The size of the metacercariae was also different between the 2 species. In the new species, the diameter of the metacercariae was 0.203–0.226 mm but the diameter of
A. spinulosum metacercariae was 0.18–0.20 mm, smaller than the new species.
The new species is different biologically from
A. spinulosum. In our study, the natural second intermediate host was 2 species of brackish water bivalves,
R. philippinarum and
C. chinensis. However, in Australia, the first intermediate host was 2 species of brackish water gastropods,
Pyrazus australis and
Salinator fragilis, and the latter gastropod species was experimentally used to obtain the metacercariae [
6]. In USA [
7,
35] and Kuwait [
18], brackish water gastropod species, namely,
Cerithidea californica (syn.
Cerithidea hegewischi californica) and
Cerithidea cingulata, respectively, were confirmed to be the first and second intermediate host. In USA, a bivalve species,
Crassostrea virginica (a species of oysters), was also found to be a second intermediate host for
A. spinulosum [
15,
36]. Information obtained so far indicates that the new species takes bivalve snails as the second (possibly also the first) intermediate host, whereas
A. spinulosum takes gastropod and bivalve snails as the first and/or second intermediate host.
Nevertheless, morphological and biological differences alone seem to be not enough for a definite identification of
Acanthoparyphium spp. Thus, we used molecular analysis of our specimens (adults and metacercariae) based on 5.8S rRNA, 28S rRNA, and
cox1 genes. Some difficulties were also met in molecular analysis largely because of the lack of enough information on
Acanthoparyphium spp. in GenBank to compare with our specimens. Under the diagnosis of
A. spinulosum, there are only 2 depositions, one from USA (KM880017) (5.8S rRNA) and the other from Ukraine (KT956939) (28S rRNA). Another isolate (assumed as
Cercaria queenslandae I Canon, 1978) of
A. spinulosum based on 28S rRNA from a cerithiid gastropod
Clypeomorus batillariaeformis in southern Great Barrier Reef, Queensland, Australia has been deposited under the name
Acanthoparyphium sp. (MH257769) [
22]. This isolate was molecularly almost identical (99.6–100% identical) with
A. spinulosum from Ukraine (KT956939) and it is strongly suggested that these cercariae from Australia might be those of
A. spinulosum. However, it is mentioned in the paper that the
Cercaria queenslandae I Cannon, 1978 had 25 collar spines, which is inconsistent with the diagnosis of
Acanthoparyphium [
22]. Thus, it is possible that
C. queenslandae I might have been a different species belonging to the Himasthlinae.
The homologies between our samples (metacercariae and adults) and
A. spinulosum from USA (KM880017), our samples and
A. spinulosum from Ukraine (KT956939), and our samples and
Acanthoparyphium sp. from Queensland (MH257769; cercaria) were 96.1%, 97.9%, and 98.1%, not high enough for them to be identical. The sequences of these nuclear genes seem to be not so highly variable within the same species of the Echinostomatidae, for example, 97.8% between
Echinostoma mekongi (MT409017 in 5.8S rRNA) [
37] and
Echinostoma paraensei (AF336233 in 5.8S rRNA), 2 different species of
Echinostoma. It is also referable that, using a molecular study, Tkach et al. [
19] obtained 2
Acanthoparyphium spp., including
A. spinulosum from Ukraine (KT956939) and
Acanthoparyphium n. sp. from USA (KT956940); their genetic identity based on 28S rRNA was 98.0%, but the 2 isolates were treated as different species.
In
cox1 gene, there is no nucleotide deposition of
A. spinulosum in GenBank. Thus, we could not compare our samples directly with
A. spinulosum. Instead, we could compare the sequences of our samples with those reported under the name
Acanthoparyphium sp. Within the genus
Acanthoparyphium,
cox1 sequences of 6 isolates or types, i.e., 1 isolate from Kuwait (MG792058; unpublished) and 5 types from New Zealand [
38], including
Acanthoparyphium sp. A (KJ956255), B (KJ 956279), C (FJ765464), D (KJ956288), and E (KJ956289), are available. Our samples revealed low homologies with these isolates or types, 83.5–86.9%, and it seems that the new species is considered not homologous with these 2 groups. However, it was surprising to see that
cox1 sequences between different types (A–E) of
Acanthoparyphium sp. from New Zealand also showed marked variation from 84.0% to 86.9%. The 5 types (A–E) reported from New Zealand may constitute 2 or more species. In our samples, the
cox1 sequences between metacercariae and adults were only 94.7%, considerably lower than expected. Further study is needed to confirm whether this low homology in
cox1 sequences was due to an intraspecific variation or reflects distinct genetic lineages.
Notes
-
We declare that there is no conflict of interest related to this study.
ACKNOWLEDGMENTS
We appreciate the staff of Institute of Parasitic Diseases, Korea Association of Health Promotion, Seoul, Korea who helped surveys on parasites in marine clams on Aphae-do Island, Shinan-gun, Jeollanam-do, Republic of Korea during 2018–2020.
Fig. 1Map showing the surveyed area (arrow) on Aphae-do (Island), Shinan-gun (County), Jeollanam-do (Province), Republic of Korea where the 2 species of brackish water clams were collected.
Fig. 2Intermediate host and a metacercaria of Acanthoparyphium shinanense n. sp. (A) Ruditapes philippinarum (the Manila clam) with various morphologies. (B) Coecella chinensis (the Chinese wedge clam) showing minor variation in morphology. (C) Metacercaria encysted with a thin shell, round, showing an oral sucker armed with collar spines (n=23) and excretory bladder with excretory granules. Bar=0.05 mm.

Fig. 3Whole adult worms and the head collar of Acanthoparyphium shinanense n. sp. Ventral views. (A) Unstained specimen recovered from an experimental chick infected with the metacercariae at day 7 post-infection. Note the level of vitellaria distribution, longitudinally elongated posterior testis, and tapering posterior extremity. Bar=0.35 mm. (B) Line drawing of the worm in Fig. 3A. Bar=0.35 mm. (C) Another adult specimen stained with Semichon’s acetocarmine followed by clearing in glycerin alcohol and mounting in glycerin jelly to maintain the morphology of organs and structures, especially the uterine eggs. Bar=0.35 mm. (D) Head collar with dorsal and lateral collar spines (unstained). (E) Head collar with lateral and ventral spines (unstained). (F) Line drawing of the head collar and collar spines. Note that the spines are in a single row without end group spines and dorsally uninterrupted. The terminal spine on each side is the smallest. Bar=0.05 mm.
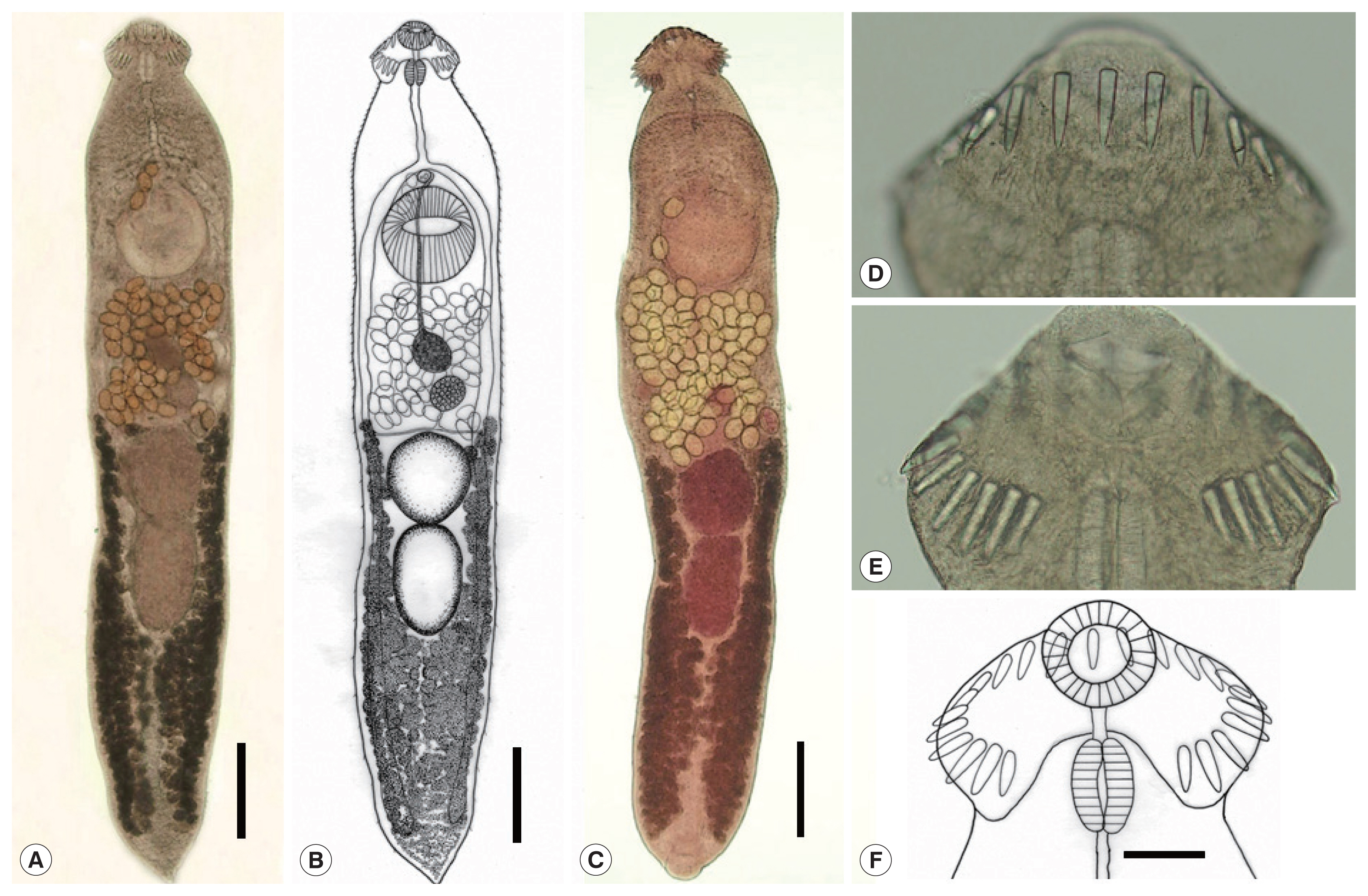
Fig. 4A phylogenetic tree inferred from 5.8S rRNA sequences of Acanthoparyphium shinanense n. sp. in relation with A. spinulosum, Acanthoparyphium sp., and other members of the Himasthlinae and Echinostomatinae constructed using the maximum-likelihood method. ●: our specimens.
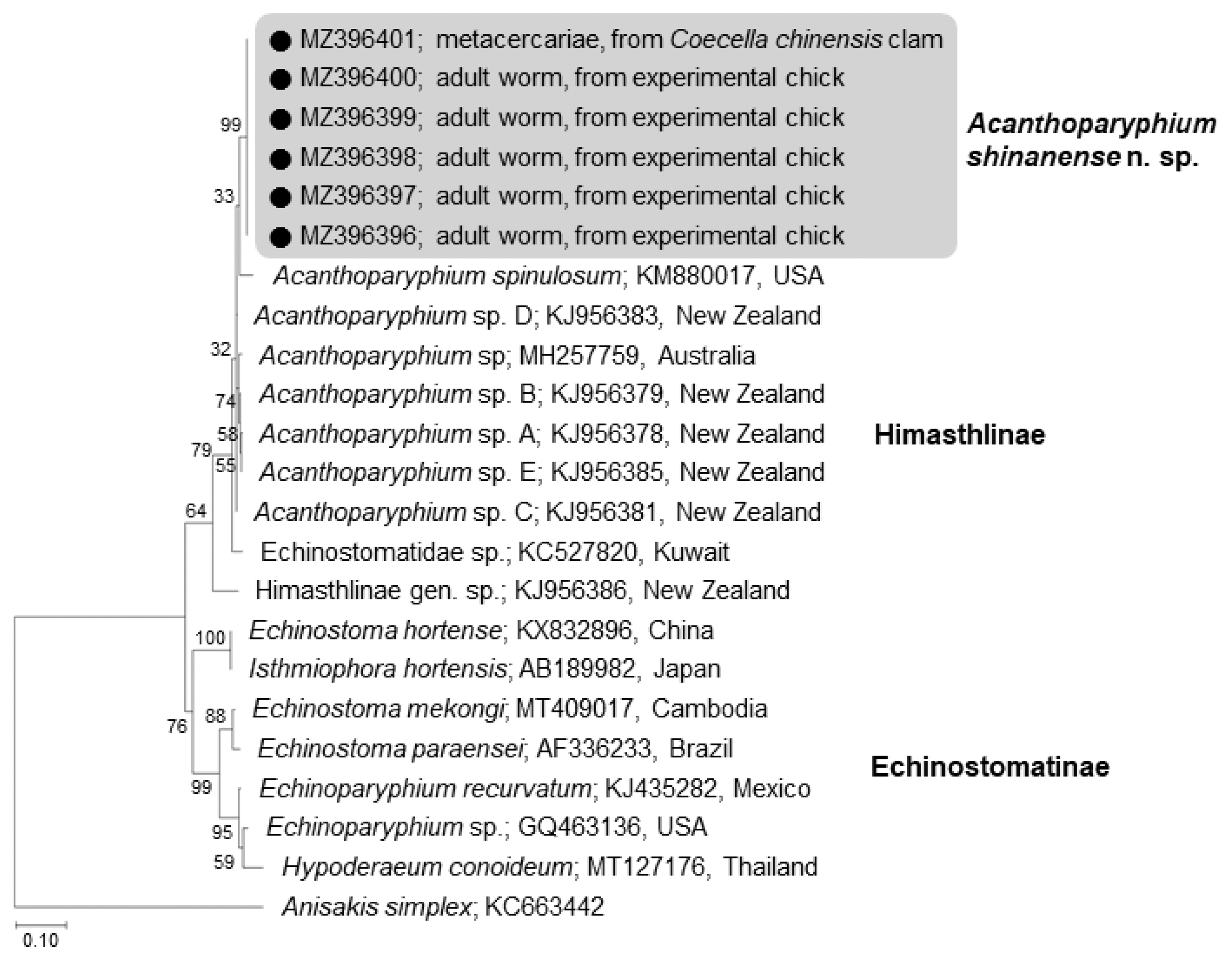
Fig. 5A phylogenetic tree inferred from 28S rRNA sequences of Acanthoparyphium shinanense n. sp. in relation with A. spinulosum, Acanthoparyphium sp., Acanthoparyphium n. sp., and other members of the Himasthlinae, Echinochasminae, and Echinostomatinae constructed using the maximum-likelihood method. ●: our specimens.

Fig. 6A phylogenetic tree inferred from mitochondrial cytochrome c oxidase 1 (cox1) gene sequences of Acanthoparyphium shinanense n. sp. in relation with Acanthoparyphium sp. (Himasthlinae) and members of the Echinochasminae and Echinostomatinae constructed using the maximum-likelihood method. ●: our specimens.
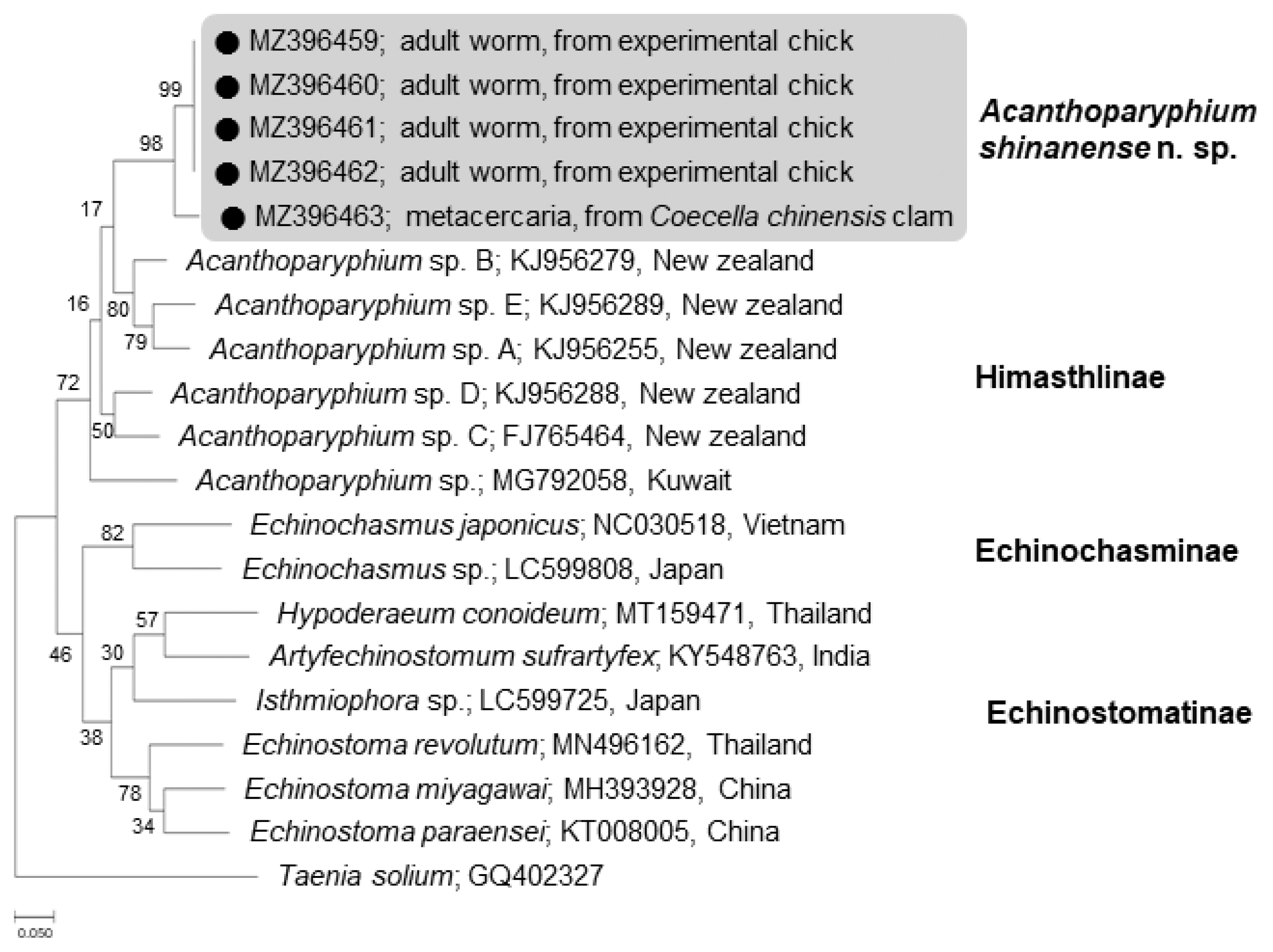
Table 1Measurements of Acanthoparyphium shinanense n. sp. (adults) in comparison with other Acanthoparyphium species (unit: μm)
Table 1
|
Species |
Acanthoparyphium shinanense n. sp. (Present study) |
Acanthoparyphium spinulosum
|
Acanthoparyphium tyosenense
|
|
|
|
Johnston [13] |
Martin and Adams [7] |
Yamaguti [5] |
Chai et al. [9] |
|
|
|
|
|
|
|
No. of specimens measured |
(n=12) Range (Mean) |
(n=2) Range |
(n=20) Range (Mean) |
(n=10) Range |
(n=10) Range (Mean) |
|
Body length (BL) |
2,885–3,551 (3,176) |
5,550 |
3,320–5,530 (4,090) |
2,450–3,850 |
2,480–3,130 (2,830) |
|
|
Body width (BW) |
606–847 (683) |
800 |
600–1,220 (830) |
500–750 |
520–730 (630) |
|
|
Ratio of BL/BW |
4.65:1 |
6.94:1 |
4.93:1 |
- |
4.49:1 |
|
|
Head collar length (CL) |
199–252 (228) |
252a
|
220a
|
164a
|
164a
|
|
|
Head collar width (CW) |
332–434 (388) |
407 |
366a
|
230–300 |
250–300 (270) |
|
|
Oral sucker length (OSL) |
94–141 (117) |
155 |
121a
|
93–110 |
90–110 (100) |
|
|
Oral sucker width (OSW) |
93–171 (133) |
145 |
103–165 (127) |
109a
|
93a
|
|
|
Angle spine length (ASL) |
54–72 (64) |
47a
|
50–72 (63) |
39–54 |
50a
|
|
|
Angle spine width (ASW) |
15–20 (18) |
19a
|
12–22 (17) |
9–12 |
11a
|
|
|
Lateral spine length (LSL) |
67–78 (72) |
75 |
- |
- |
54a
|
|
|
Lateral spine width (LSW) |
16–21 (18) |
16 |
- |
- |
11a
|
|
|
Dorsal spine length (DSL) |
53–73 (65) |
- |
- |
- |
- |
|
|
Dorsal spine width (DSW) |
15–21 (19) |
- |
- |
- |
- |
|
|
Prepharynx length (PL) |
117–159 (137) |
81a
|
0–90 |
- |
- |
|
|
Pharynx length (PHL) |
97–150 (121) |
133 |
93–137 (113) |
84–108 |
80–100 (90) |
|
|
Pharynx width (PHW) |
61–104 (76) |
107 |
59–131 (92) |
57–80 |
60–90 (70) |
|
|
Esophagus length (EL) |
228–440 (344) |
388 |
373–700 (493) |
300–450 |
170–280 (220) |
|
|
Cirrus sac length (CSL) |
812–1,217 (965) |
- |
558–1134 (825) |
550–880 |
780a
|
|
|
Cirrus sac width (CSW) |
83–180 (114) |
- |
70–196 (127) |
120–190 |
140a
|
|
|
Seminal vesicle length (SVL) |
96–234 (161) |
168a
|
122a
|
- |
- |
|
|
Seminal vesicle width (SVW) |
61–153 (82) |
168a
|
97a
|
- |
- |
|
|
Ventral sucker length (VSL) |
383–458 (407) |
582 |
342–473 (388) |
300–375 |
240–320 (290) |
|
|
Ventral sucker width (VSW) |
353–424 (390) |
543 |
351–535 (413) |
355a
|
291a
|
|
|
Ovary length (OVL) |
92–153 (123) |
194 |
115–230 (164) |
110–150 |
110–140 (120) |
|
|
Ovary width (OVW) |
111–155 (133) |
136 |
109–205 (153) |
150–200 |
130–180 (150) |
|
|
Mehlis’ gland length (MEL) |
63–132 (93) |
- |
122a
|
- |
185a
|
|
|
Mehlis’ gland width (MEW) |
189–326 (265) |
- |
170a
|
- |
344a
|
|
|
Ant. testis length (ATL) |
226–375 (315) |
582 |
340–644 (410) |
270–400 |
260–330 (290) |
|
|
Ant. testis width (ATW) |
275–377 (322) |
542 |
240–532 (360) |
275–450 |
310–390 (350) |
|
|
Ratio of ATL/ATW |
0.98:1 |
1.07:1 |
1.13:1 |
- |
0.83:1 |
|
|
Post. testis length (PTL) |
303–483 (380) |
776 |
460–756 (535) |
368a
|
320–370 (340) |
|
|
Post. testis width (PTW) |
200–290 (252) |
542 |
196–560 (294) |
355a
|
290–380 (340) |
|
|
Ratio of PTL/PTW |
1.51:1 |
1.43:1 |
1.81:1 |
1.04:1a
|
1:01 |
|
|
Forebody length (FORE) |
503–733 (646) |
883a
|
952a
|
681a
|
450a
|
|
|
ODIV |
482–687 (601) |
799a
|
726a
|
586a
|
371a
|
|
|
OVAR |
115–271 (180) |
420a
|
369a
|
368a
|
476a
|
|
|
TEND |
850–1,110 (969) |
2,186a
|
1,262a
|
927a
|
768a
|
|
|
OSW/PHW |
1.2–2.3 (1.8) |
1.4a
|
1.4a
|
- |
1.3a
|
|
|
BW/BL (%) |
19–26 (22) |
14a
|
20a
|
- |
22a
|
|
|
FORE/BL (%) |
17–24 (20) |
16a
|
23a
|
- |
16a
|
|
|
CW/BW (%) |
699–966 (822) |
1,364a
|
1,118a
|
- |
1,048a
|
|
|
ODIV/BL (%) |
16–23 (19) |
14a
|
18a
|
- |
13a
|
|
|
OVAR/BL (%) |
4–8 (6) |
8a
|
9a
|
- |
17a
|
|
|
TEND/BL (%) |
27–34 (31) |
39.4a
|
31a
|
- |
27a
|
|
|
Egg length |
95–111 (103) |
85 |
84–106 (96) |
84–110 |
105–111 (108) |
|
|
Egg width |
62–71 (66) |
69 |
56–68 (64) |
60–69 |
55–63 (59) |
Table 2Sequence comparison of our samples with other members of the Himasthlinae in GenBank based on 5.8S rRNA, 28S rRNA, and cox1 genes
Table 2
|
5.8S rRNA region |
28S rRNA region |
cox1 |
|
Between our isolates (A. shinanense n. sp.) |
100 |
|
100 |
|
94.7–100 |
|
Acanthoparyphium sp. C (KJ956381, New Zealand) |
97.9 |
Acanthoparyphium sp. (MH257769, Australia) |
98.1 |
Acanthoparyphium sp. B (KJ956279, New Zealand) |
86.7–86.9 |
|
Acanthoparyphium sp. D (KJ956383, New Zealand) |
97.7 |
Acanthoparyphium n. sp. (KT956940, USA) |
98.0 |
Acanthoparyphium sp. D (KJ956288, New Zealand) |
86.7 |
|
Acanthoparyphium sp. A (KJ956378, New Zealand) |
97.0 |
Acanthoparyphium spinulosum (KT956939, Ukraine) |
97.9 |
Acanthoparyphium sp. A (KJ956255, New Zealand) |
85.6 |
|
Acanthoparyphium sp. B (KJ956379, New Zealand) |
97.0 |
Himasthla limnodromi (KT956943, USA) |
95.9 |
Acanthoparyphium sp. C (FJ765464, New Zealand) |
85.1–85.3 |
|
Acanthoparyphium sp. E (KJ956385, New Zealand) |
97.0 |
Himasthla leptosoma (KT956942, Ukraine) |
94.3 |
Acanthoparyphium sp. E (KJ956289, New Zealand) |
84.0–84.8 |
|
Acanthoparyphium sp. (MH257759, Australia) |
96.6 |
Himasthla militaris (KT956944, Ukraine) |
94.3 |
Acanthoparyphium sp. (MG792058, Kuwait) |
83.5–83.7 |
|
Acanthoparyphium spinulosum (KM880017, USA) |
96.1 |
|
|
|
|
Table 3Species of Acanthoparyphium reported among the literature
Table 3
|
Species, nominator, year |
Countries reporteda
|
Taxonomic validity |
|
Valid or need-to-evaluate species |
|
A. phoenicopteri (Lühe, 1898) Dietz, 1909 |
Tunisia, China |
Valid (type species) |
|
A. haematopi Ku and Chiu, 1979
|
China |
Valid |
|
A. jeetai Chakrabarti, 1972 |
India |
Need to study |
|
A. kurogamo Yamaguti, 1939 |
Japan, China |
Valid |
|
A. lobatum Soota et al. 1970 |
India |
Need to study |
|
A. loborchis Wang, 1977 |
China |
Valid |
|
A. macracanthum Rybakov and Lukomskaya, 1988 |
Russia |
Valid |
|
A. marilae Yamaguti, 1934 |
Japan, Australia, China, Korea |
Valid |
|
A. melanittae Yamaguti, 1939 |
Japan |
Valid |
|
A. ochthodromi Tubangui, 1933 |
The Philippines, China |
Valid |
|
A. pagollae Cable et al. 1960 |
Puerto Rico |
Valid |
|
A. paracharadrii Velasquez, 1964
|
The Philippines |
Valid |
|
A. spinulosum Johnston, 1917 |
Australia, Japan, Taiwan, USA, Ukraine, Kuwait, New Zealand |
Valid |
|
A. squatarolae Yamaguti, 1934 |
Japan, Australia, China |
Valid |
|
A. tyosenense Yamaguti, 1939b,c
|
Korea, Japan |
Valid |
|
|
Species synonymized with A. spinulosum
|
|
A. cambellense Soota et al. 1970 |
India |
Syn. by Fischthal and Kuntz [14] |
|
A. charadrii Yamaguti, 1939 |
Japan, China, Europe |
Syn. by Soota et al. [23] |
|
A. longivitellatum Oschmarin, 1956 |
Russia |
Syn. by Skrjabin and Bashkirova [24] |
|
A. spinulosum suzugamo Yamaguti, 1939 |
Japan, China |
Syn. by Skrjabin and Bashkirova [24] |
References
- 1. Chai JY. Echinostomes in humans. In Fried B, Toledo R eds, The Biology of Echinostomes. New York, USA. Springer; 2009, pp 147-183.
- 2. Chai JY. Chapter 19. Echinostomes. In Xiao L, Ryan U, Feng Y eds, Biology of Foodborne Parasites. Boca Raton, USA. CRC Press, Taylor & Francis Group; 2015, pp 427-443.
- 3. Chai JY. Human Intestinal Fluke: From Discovery to Treatment and Control. Dordrecht, The Netherlands. Springer Nature; 2019, pp 1-549.
- 4. Yamaguti S. Studies on the helminth fauna of Japan. Part 3. Avian trematodes, II. Jpn J Zool 1934;5:543-583.
- 5. Yamaguti S. Studies on the helminth fauna of Japan. Part 25. Trematodes of birds, IV. Jpn J Zool 1939;8:129-210.
- 6. Bearup AJ. Life history of Acanthoparyphium spinulosum Johnston, 1917 (Trematoda: Echinostomatidae). Austral J Zool 1960;8:217-225. https://doi.org/10.1071/ZO9600217
- 7. Martin WE, Adams JE. Life cycle of Acanthoparyphium spinulosum Johnston, 1917 (Echinostomatidae: Trematoda). J Parasitol 1961;47:777-782. https://doi.org/10.2307/3275470
- 8. Velasquez CC. Life history of Acanthoparyphium paracharadrii sp. n. (Trematoda: Echinostomatidae). J Parasitol 1964;50:261-265. https://doi.org/10.2307/3276282
- 9. Chai JY, Han ET, Park YK, Guk SM, Lee SH. Acanthoparyphium tyosenense: the discovery of human infection and identification of its source. J Parasitol 2001;87:794-800. https://doi.org/10.1645/0022-3395(2001)087[0794:attdoh]2.0.CO;2
- 10. Martorelli SR, Poulin R, Mouritsen KN. A new cercaria and metacercaria of Acanthoparyphium (Echinostomatidae) found in an intertidal snail Zeacumantus subcarinatus (Batillaridae) from New Zealand. Parasitol Int 2006;55:163-167. https://doi.org/10.1016/j.parint.2006.02.001
- 11. Han ET, Chai JY. Mactra veneriformis, an intertidal clam, as a new second intermediate host for Acanthoparyphium marilae (Digenea: Echinostomatidae). Korean J Parasitol 2008;2:101-104. https://doi.org/10.3347/kjp.2008.46.2.101
- 12. Leung TLF, Keeney DB, Poulin R. Cryptic species complexes in manipulative echinostomatid trematodes: when two become six. Parasitology 2009;136:241-252. https://doi.org/10.1017/S0031182008005374
- 13. Johnston SJ. On the trematodes of Australian birds. J Royal Soc New South Wales 1917;50:187-261.
- 14. Fischthal JH, Kuntz RE. Some digenetic trematodes of mammals from Taiwan. Proc Helminthol Soc Wash 1976;42:149-157.
- 15. Little JW, Hopkins SH, Schlicht FG. Acanthoparyphium spinulosum (Trematoda: Echinostomatidae) in oysters of Port Isabel, Texas. J Parasitol 1966;52:663.
- 16. Bass HS, LeFlore WB. In vitro excystment of the metacercaria of Acanthoparyphium spinulosum (Trematoda: Echinostomatidae). Proc Helminthol Soc Wash 1984;51:149-153.
- 17. Nguyen AT, Kuwata C, Kuris AM. A synthetic workflow for a coordinated direct observation and genetic tagging applied to a complex host-parasite interaction. Parasitol Res 2015;114:2015-2021. https://doi.org/10.1007/s00436-015-4437-8
- 18. Abdul-Salam J, Al-Taqi M, Sreelatha BS. Ultrastructure of metacercarial cyst wall of Acanthoparyphium spinulosum (Digenea: Echinostomatidae). J Parasit Dis 2007;31:108-113.
- 19. Tkach VV, Kudlai O, Kostadinova A. Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). Int J Parasitol 2016;46:171-185. https://doi.org/10.1016/j.ijpara.2015.11.001
- 20. Yamaguti S. Systema Helminthum. I(Part I):The Digenetic Trematodes of Vertebrates. New York, USA. Interscience Publishers Inc; 1958, pp 1-979.
- 21. Yamaguti S. Synopsis of Digenetic Trematodes of Vertebrates. Tokyo, Japan. Keigaku Publishing Co; 1971, pp 1-1073.
- 22. Huston DC, Cutmore SC, Cribb TH. Molecular systematics of the digenean community parasitizing the cerithiid gastropod Clypeomorus batillariaeformis Habe & Kusage on the Great Barrier Reef. Parasitol Int 2018;67:722-735. https://doi.org/10.1016/j.parint.2018.07.008
- 23. Soota TD, Srivastava CB, Ghosh RK. Studies on the helminth fauna of the Great Nicobar Island. Part I. Trematoda. Proc Indian Acad Sci (Section B) 1970;72:241-250.
- 24. Skrjabin KI, Bashkirova EI. Family Echinostomatidae Dietz. 1909, In Skrjabin KI ed, Trematodes of Animals and Man. Moscow-Leningrad. Russia Izdatelstvo Akademii Nauk SSSR; 1956, pp 51-930 (in Russian).
- 25. Chen HT. Fauna Sinica. Platyhelminthes. Trematoda. Digenea (I). Beijing, China. Science Press; 1985, pp 1-697 (in Chinese).
- 26. Boyce K, Hide G, Craig PS, Harris PD, Reynolds C, Pickles A, Rogan MT. Identification of a new species of digenean Notocotylus malhamensis n. sp. (Digenea: Notocotylidae) from the bank vole (Myodes glareolus) and the field vole (Microtus agrestis). Parasitology 2012;139:1630-1639. https://doi.org/10.1017/S0031182012000911
- 27. Leung TLF, Donald KM, Keeney DB, Koehler AV, Peoples RC, Poulin R. Trematode parasites of Otago Harbour (New Zealand) soft-sediment intertidal ecosystems: life cycles, ecological roles and DNA barcodes. NZ J Marine Freshwat Res 2009;43:857-865. https://doi.org/10.1080/00288330909510044
- 28. Dietz E. Die Echinostomiden der Vögel. Zool Jarb 1910;12(suppl):265-512. (in German).
- 29. Cable RM, Conner RS, Balling JW. Digenetic trematodes of Puerto Rican shore birds. Sci Surv Puerto Rico Virgin Island (by New York Acad Sci) 1960;17:187-255.
- 30. Rybakov AV, Lukomskaya OG. On the life cycle of Acanthoparyphium macracanthum sp. n. (Trematoda: Echinostomatidae). Parazitologiya 1988;22:224-229. (in Russian).
- 31. Velasquez CC. Life history of Acanthoparyphium paracharadrii sp. n. (Trematoda: Echinostomatidae). J Parasitol 1964;50:261-265. https://doi.org/10.2307/3276282
- 32. Soota TD, Srivastava CB, Ghosh RK. The helminth fauna of Andaman and Nicobar Islands, Trematoda. Rec Zool Surv India 1972;67:281-285.
- 33. Chakrabarti S. On a trematode parasite Acanthoparyphium jeetai n. sp. (Echinostomatidae: Himasthlinae) from little stint, Calidrus (Calidrus) minuta (Leiser) from Chennal Coast, India. Rec Zool Surv India 1972;103:71-78.
- 34. Tubangui MA. Trematode parasites of Philippine vertebrates, VI. Description of new species and classification. Phil J Sci 1933;52:167-197.
- 35. Torchin ME, Byers JE, Huspeni TC. Differential parasitism of naïve and introduced snails: replacement of a parasite fauna. Biol Invas 2005;7:885-894.35. https://doi.org/10.1007/s10530-004-2967-6
- 36. Little JW. The excystment, growth and reproduction of Acanthoparyphium spinulosum Johnston, 1917 (Trematoda) in chicks fed various diets. Parasitology 1970;60:61-78. https://doi.org/10.1017/S0031182000077258
- 37. Cho J, Jung BK, Chang T, Sohn WM, Sinuon M, Chai JY. Echinostoma mekongi n. sp. (Digenea: Echinostomatidae) from riparian people along the Mekong River in Cambodia. Korean J Parasitol 2020;58:431-443. https://doi.org/10.3347/kjp.2020.58.4.431
- 38. Keeney DB, Palladino J, Poulin R. Broad geographic analyses reveal varying patterns of genetic diversity and host specificity among echinostome trematodes in New Zealand snails. Parasitology 2015;142:406-415. https://doi.org/10.1017/S0031182014001279
- 39. Ito J. Studies on the brackish water cercariae in Japan. III. Three new echinostome cercariae in Tokyo Bay, with a list of Japanese echinostome cercariae. Jpn J Med Sci Biol 1957;10:439-453.
- 40. Nguyen AT. Genetic diversity and its relationship to host-specificity of a trematode parasite (Acanthoparyphium spinulosum). PhD Thesis. University of California (Santa Barbara); USA: 2012. 1-87.
- 41. Richard J. Acanthoparyphium charadrii Yamaguti, 1939 chez Squatarola squatarola. Mem Mus Nat Hist Natur s A Zool 1966;41:125-126. (in French).