Abstract
Felids are the unique definitive host of Toxoplasma gondii. The intestine of felid is the only site for initiating Toxoplasma gondii sexual reproduction. T. gondii excretes millions of infectious oocysts from the intestine, which are the primary source of infection. There are many difficulties in developing vaccines and drugs to control oocyst excretion due to the lack of an appropriate experimental model. Here, we established an in vitro feline intestinal epithelial cell (IEC) infection system and an efficient animal model of T. gondii Chinese 1 genotype, Wh6 strain (TgCtwh6). The Kunming mice brain tissues containing TgCtwh6 cysts were harvested 42-day post-infection. The bradyzoites were co-cultured with cat IECs in vitro at a ratio of 1:10. Five 3-month-old domestic cats were orally inoculated with 600 cysts each. The oocysts were detected by daily observation of cat feces by microscopy and polymerase chain reaction. We found that the parasite adhered and invaded cat IECs in vitro, transformed into tachyzoites, and then divided to form rose-like structures. These parasites eventually destroyed host cells, escaped, and finished the asexual reproduction process. Schizonts associated with sexual reproduction have not been observed during development in vitro cultured cells. However, schizonts were detected in all infected cat intestinal epithelial cells, and oocysts were presented in all cat feces. Our study provides a feasible cell model and an efficient infection system for the following studies of T. gondii sexual reproduction, and also lays a foundation to develop drugs and vaccines for blocking excretion and transmission of oocysts.
-
Key words: Toxoplasma gondii, cat intestinal epithelial cell, development, oocyst excretion, Chinese 1 genotype Wh6 strain
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite infecting almost all kinds of warm-blooded animals that pose a threat to the public human health and livestock production [
1]. Human infection rate of
T. gondii has been rising from 7.94% in 2010 to 9.69% in 2017 in China [
2]. Toxoplasmosis is crippling to immunodeficiency population, organ transplantation recipients, pregnant women and the developing fetus [
3]. Moreover,
T. gondii infection is also associated with increased risk of psychosis [
4–
6].
T. gondii has a complex life cycle. Feline is the only definitive host and can excrete millions of environmentally resistant oocysts which can spread among many other hosts. Cats play a crucial role in the epidemiology of toxoplasmosis. In China, the number of cats ranked the world’s second largest, and the number of domestic cats was approximately to 53 million (
http://www.mapsofworld.com/world-top-ten/countries-with-most-pet-cat-population.html). Simultaneously,
T. gondii infections are also common in cats in China, the seropositive rate was up to 79.4% in some areas [
7,
8], much higher than the worldwide average (30 to 40%) [
9–
13]. Further, Chinese I (ToxoDB#9) is the major epidemic
T. gondii strain prevalent in humans and animals in China, it shows a unique genotype, and has distinct difference from the classical classification clonal lineages I, II, and III, which are mainly prevalent in Europe, Africa, and North America [
14,
15]. Thus, the transmission of Chinese I strain probably more easily occurs among cats and humans. Dong et al. [
16] also confirmed that Chinese I oocysts had led to outbreaks of clinical toxoplasmosis in pigs and humans. However, there is a lack of vaccines, drugs and effective strategies to block oocysts excretion due to the lack of appropriate experimental models.
TgCtwh6 strain was isolated from cats in Wuhan city in 2011 and identified to be Chinese 1 genotype [
17]. The virulence of
TgCtwh6 is moderate between the RH strain and Pru strain. It is able to form brain cysts and cause latent infection in mice [
18]. Up to date, the development process of
TgCtwh6 in cats and the efficiency of oocysts excretions are unknown. There is little information about the initial mechanism of sexual reproduction of Chinese I strain in cat IECs.
This study established 2 infection systems of IECs infected with TgCtwh6 strain in vitro and in vivo, respectively, under laboratory conditions. Growth and development of TgCtwh6 in cat IECs were observed and the oocyst excretion efficiency was detected. Our study provides a feasible cell model and an efficient infection system serving for molecular mechanism studies of T. gondii sexual reproduction and could be applied to establish an oocyst detection method to control feline toxoplasmosis.
MATERIALS AND METHODS
Ethics
All animal experimentation were carried out according to the International Guiding Principles for Biomedical Research Involving Animals, as issued by the Council for the International Organizations of Medical Sciences and the guidelines set by the Institutional Animal Care and Use Committee of Shandong First Medical University (approval number: w202103030088, w202103030118). Cats were narcotized by Lidocaine Hydrochloride injection, and then intestinal tissue of 2 cm long was removed by surgery, and cats were well taken care of and treated.
Animals and Toxoplasma strains
Five 3-month-old and one new born cats and Kunming (KM) mice (4 to 5 weeks old) were purchased from Pengyue Experimental Animal Breeding Co. (Jinan, Shandong, China) with the animal license number SCXK(Lu)20190003. All cats were verified to be seronegative for T. gondii by using the modified agglutination test (MAT) with free of gastrointestinal disease, feline immunodeficiency virus, and feline leukemia virus. All cat intestinal tissues in this study were acquired by surgery.
T. gondii Chinese 1 genotype, Wh6 strain (TgCtwh6), was kindly provided by Prof. JL Shen (Anhui Medical University, Hefei, China).
Resuscitation of cat IECs
Primary cat IECs were isolated and cultured as previously described [
19]. The IECs stored in liquid nitrogen were immediately placed in a 37°C water bath for 2 to 3 min until it was completely melted. After thawing, the contents were aseptically removed into 10 ml of fresh IECs-DMEM medium supplemented with 2.5% fetal bovine serum (FBS), 5 μg/ml of insulin, 10 ng/ml of epidermal growth factor, 100 U/ml of penicillin, and 100 mg/ml of streptomycin, and then centrifuged at 1,200 rpm for 3 min. Cell pellets were resuspended with 10 ml fresh IECs-DMEM medium, and transferred into a T-25 tissue culture flask, incubated in a 37°C incubator with 5% CO
2. The fresh cultural medium was changed every 2–3 days. Identification of cat IECs was conducted by using immunohistochemistry.
KM mouse was orally infected with 30 cysts to prepare brain tissue cysts. After 42 days, the brain tissue cysts detected under the microscope were removed, washed in cold PBS, and were purified using Percoll (Sigma, St. Louis, Missouri, USA) gradients according to previously described [
20]. The purified brain tissue cysts were digested by 0.25% tryptase for 1 min at 37°C. Bradyzoites were centrifuged at 3,000 rpm for 10 min and resuspended in DMEM medium with 3% FBS.
Cat IECs were inoculated into 6 well plates. After cells reached 80% or more confluency, the prepared-bradyzoites of TgCtwh6 were seeded to cells at ratios of 1:10 and allowed to grow for 8 days. The co-cultured cat IECs were washed with PBS, fixed with 4% paraformaldehyde, and stained with Wright-Giemsa at different time points of infection.
Identification of parasites in vitro-cultured cat IECs
The parasites released from cat IECs were collected for RNA extraction with TRIzol, and cDNA was synthesized using the Takara PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Japan) with random 6 primers. Bradyzoite antigen1 (TgBAG1,
TGME49_259020) was used as a marker for detecting bradyzoites, dense granule protein 11B (TgGRA11B,
TGME49_ 237800) was used as a marker for detecting merozoites, and surface antigen 1 (TgSAG1, GenBank:
AAO61460.1) was used as a marker for tachyzoites. Equal amounts of cDNA were used as a template for each PCR reaction, and the PCR products were examined on 1.1% agarose gel. Primer sequences were listed as
Table 1.
The mice brain tissue cysts were fed to 5 cats (600 cysts per cat). All cat feces were collected daily and the production of oocysts was monitored by microscopic examination and PCR using
T. gondii-specific target genes (529 bp repetitive sequence and B1 gene). Oocyst DNA was extracted from cat feces using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) as previously described [
21]. Two sets of
T. gondii specific primers (
Supplementary Table S1) were used in separate PCR reactions [
22]. PCR products were detected by 1.1% agarose gels with ethidium bromide staining. The PCR products were sequenced, and the results were subjected to nucleotide BLAST analysis.
The 2 cm-long intestinal tissues were separated from cats, once oocysts appeared in their faeces. The schizonts within separated feline intestinal tissues were detected by IHC and IFA.
For IHC evaluations, the intestinal tissues were treated as following: fixation, paraffin embedding, sections, deparaffinization, peroxidase blocking, and antigen retrieval, deparaffinized, peroxidase blocked, antigen retrieval. The sections were incubated with the mouse serum infected with T. gondii at 1:10 dilution. Subsequently, sections were incubated with horse reddish peroxidase-conjugated anti-mouse IgG (1:200 dilution) at 25°C for 50 min. Sections were stained with 4′, 6-diamidino-2-phenylindole dihydrochloride (DAB) chromogen. Finally, sections were counterstained with hematoxylin. Sections were observed under microscope.
For IFA, slides were incubated with the serum of mice infected with T. gondii (1:10 dilution), after which sections were incubated with secondary antibody cyanine dye CY3 (1:200 diluted). The slides were counterstained with 2-(4-Amidinoph enyl)-6-indolecarbamidine dihydrochloride (DAPI) solution in a dark place, and added spontaneous fluorescence quenching reagent to terminate reactions after washing. Microscopy images were obtained by fluorescent microscopy.
RESULTS
TgCtwh6 invades in vitro cultured cat IECs
The cat IECs derived from our primary culture system [
19] were successfully resuscitated, with reliable morphology and clear cell boundaries (
Supplementary Fig. S1A-C). The cells attached to the wall of cell culture flasks within 24 h and then grow rapidly, exhibited a typical fusiform shape of epithelial cells, thus could be characterized by cytokeratin 18-IHC (
Supplementary Fig. S1A-C). The purified-parasites from infected KM mice brain tissue (
Supplementary Fig. S1D-F) were seeded to the IECs. After 36 h, the parasites entered into IECs and were bordered by vesicle structure with strong light transmittance that looked like parasitophorous vacuoles (PV) (
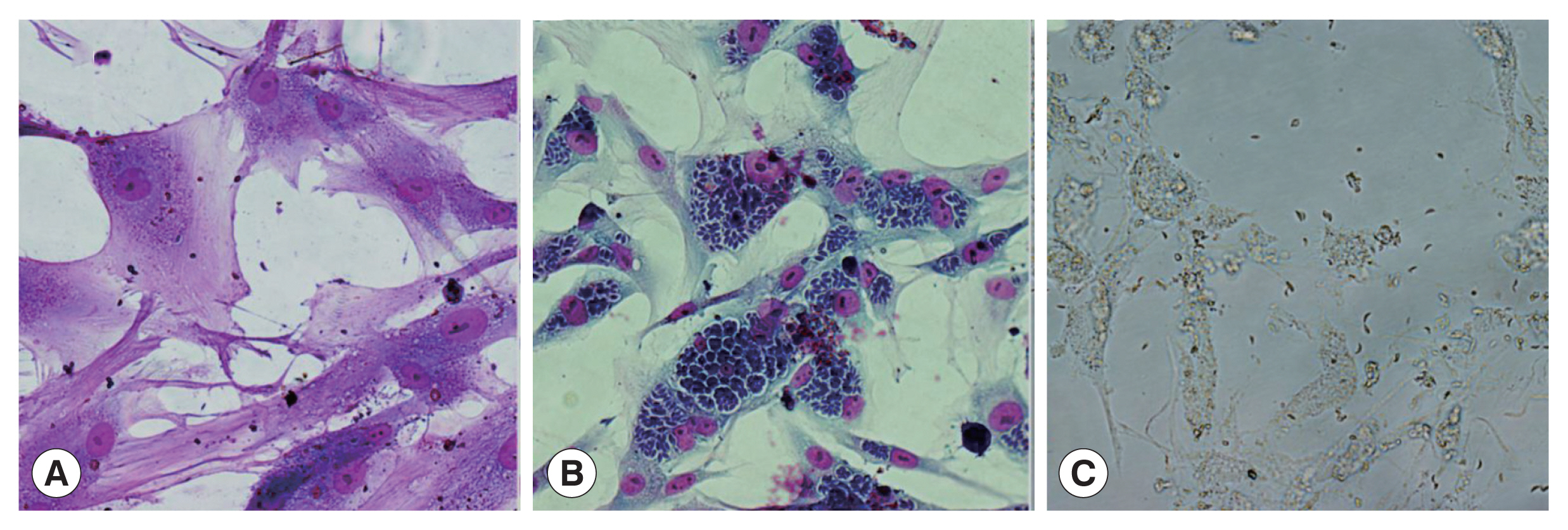
Fig. 1A). After 5 days of infection, a large number of parasites formed rose-like structures within cat IECs (
Fig. 1B), and began to escape from host cells. After 8 days of infection, almost all of the host cells burst and the parasites dissociated (
Fig. 1C). However, none of schizonts and gametocytes were observed in this system.
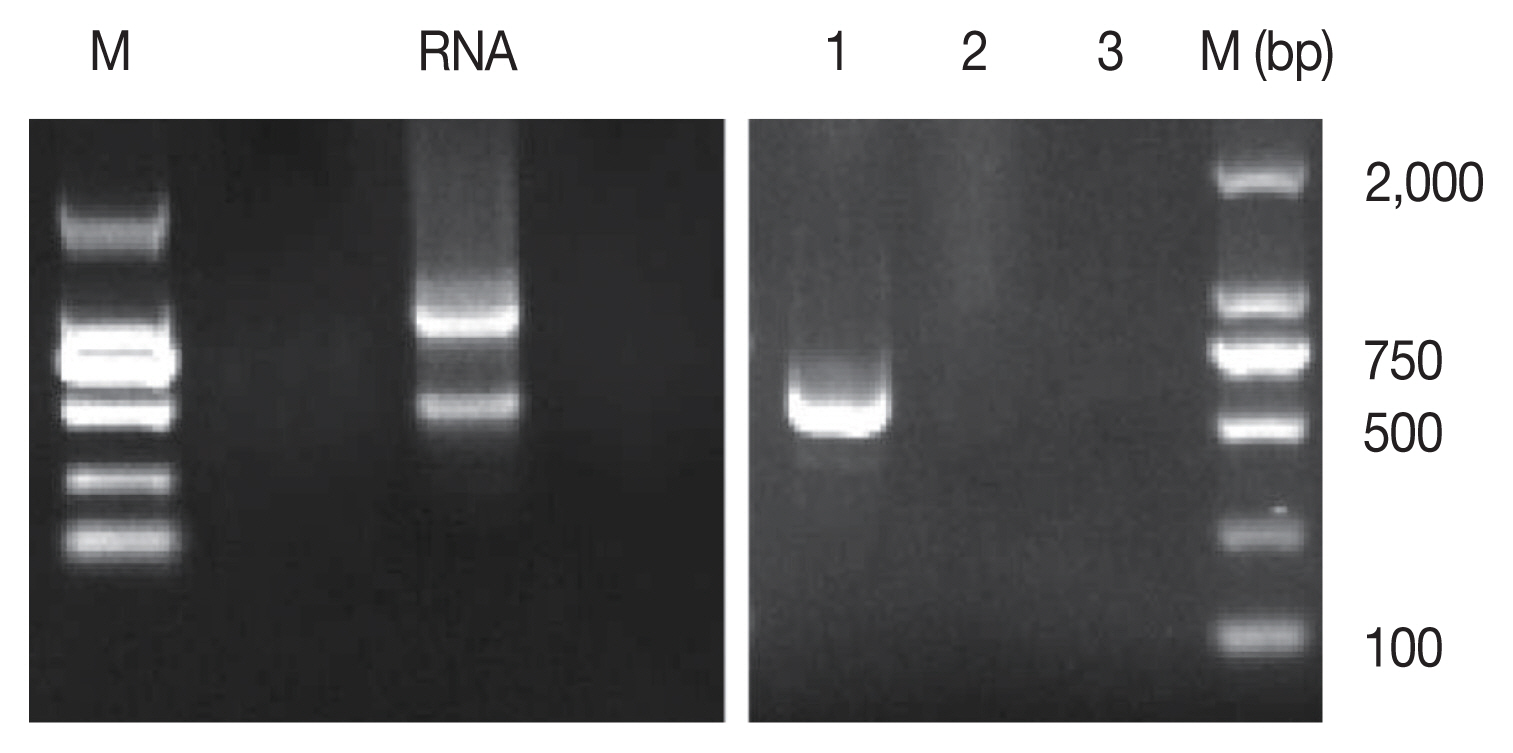
The total RNA of parasites harvested from the above culture system was extracted and cDNA was synthesized. Using the cDNA as a template, PCR was done. PCR results showed that the bands of bradyzoite-specific gene (TgBAG1) and merozoite-specific gene (TgGRA11B) could not be amplified, but the band of tachyzoite-specific gene (TgSAG1) was clearly detected (
Fig. 2), indicating that the intracellular parasites were tachyzoites.
Three to five days after infection,
T. gondii oocysts were identified in the feces of infected cats (
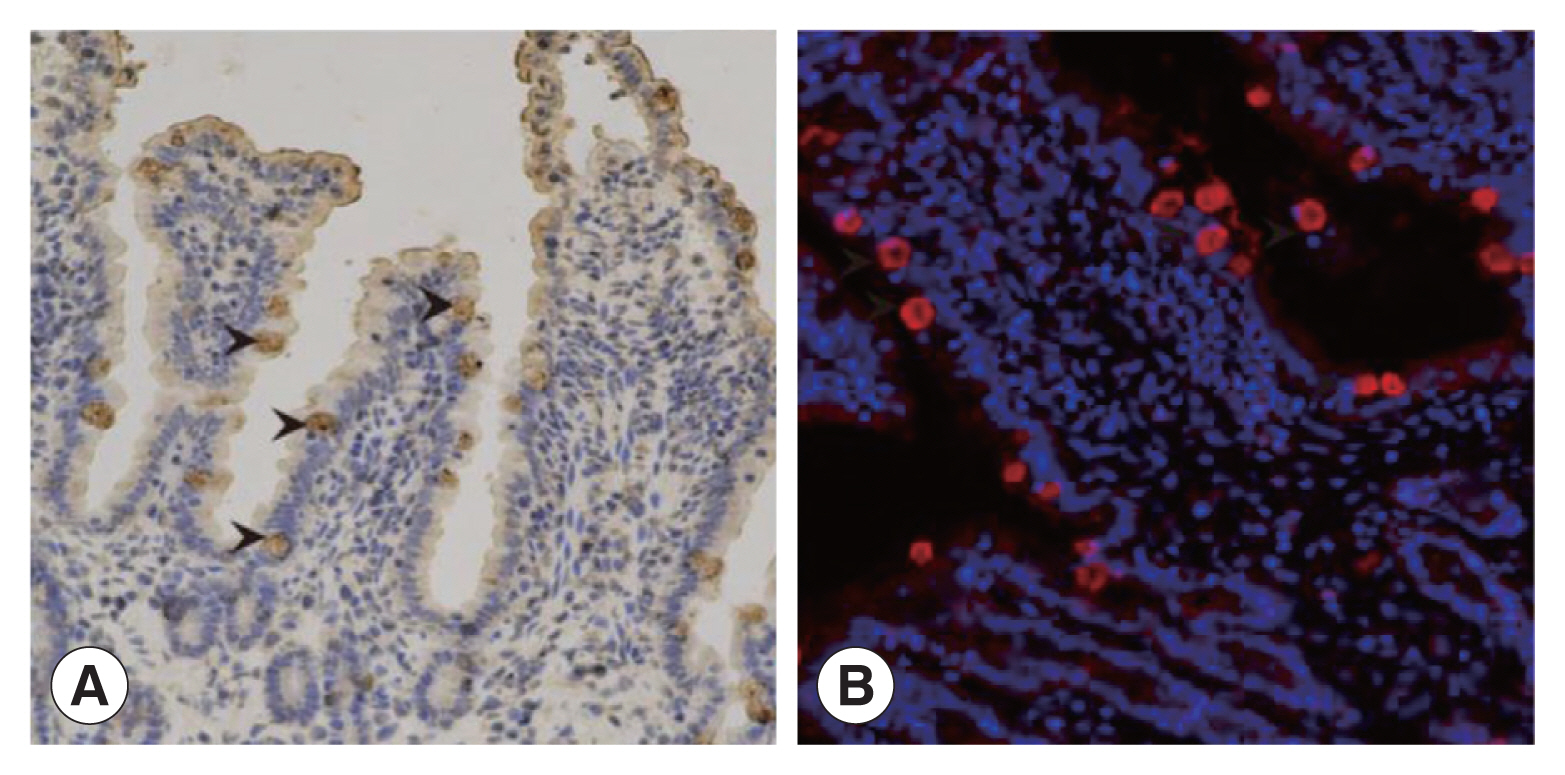
Supplementary Fig. S2). IHC results showed that intestinal villus separated from the infected cats were intact. The enterocytes close to the intestinal cavity were transparent with intact integrity and contained brownish yellow schizotypal structures (
Fig. 3A). In addition, many round or oval schizotypal structures were detected in cat enterocyte cell layers (
Fig. 3B), indicating that sexual reproduction of
T. gondii could be successfully initiated in cat IECs in vivo.
DISCUSSION
Our results showed that
TgCtwh6 was able to invade cat IECs under the experimental conditions. We had successfully observed the entire intracellular asexual reproduction process of
TgCtwh6 tachyzoites in host cells in vitro. The parasite formed rose-like structures within cat IECs, which is consistent with a previous report [
23]. This result indicated that cat IEC system is stable and suitable to be an experimental model. However, the sexual reproduction of
TgCtwh6 was not observed, because schizonts and gametocytes associated with sexual reproduction did not emerge in our vitro cultured cells. Our result is also consistent with a previous study, which suggested that
T. gondii sexual development did not occur unless the
in vitro cultured cat IECs were supplemented with linoleic acid [
24]. Our result demonstrated that the parasites might accomplish the whole intracellular asexual reproduction process without linoleic acid, and that cat IECs are not the only determinant that leading cats to be the definitive host of
T. gondii.
Our in vivo results showed that
TgCtwh6 easily entered into sexual reproduction in all orally infected cats. It successfully generated schizonts in IECs of infected cats. Moreover,
TgCtwh6 oocysts were excreted with higher efficiency rate than naturally infected cats. A previous epidemiological study [
15] suggested that no more than 1% cats naturally infected with
T. gondii could excrete oocysts. The efficiency of oocysts excretion was only 4% for naturally infected cats from Kunming Province and no more than 1% from Henan Province [
24,
25]. Some naturally exposed cats may be orally infected with parasites which can induce protective immunity to oocyst excretion [
26].
Our results indicated that
TgCtwh6 was able to conduct sexual reproduction in cats, therefore, more attention should be paid to its pathogenesis and transmission. We provided an efficient in vivo infection system to easily conduct and identify sexual reproduction of
T. gondii. There are many interesting unanswered questions concerning sexual reproduction of
T. gondii in the definitive host, especially how often cats excreted oocysts in their lifetime and whether they had protection to re-excretion of
T. gondii oocysts [
27,
28]. We believe that our study provides a feasible cell model and an efficient infection system serving for molecular mechanism studies of
T. gondii sexual reproduction and can be applied to establish an oocyst detection method to control feline toxoplasmosis.
Notes
-
The authors declare no conflict of interest related to this study.
Supplementary Information
ACKNOWLEDGMENTS
We are very grateful to Dr. Jilong Shen (Anhui, Anhui Medical University, Hefei, China) for the provision of Chinese I T. gondii strain-TgCtwh6.
This work was supported by the National Natural Science Foundation of China (No. 81702026), Medicine and Health Science Technology Development Plan of Shandong Province (No. 202101050261, No. 202101050270), Taishan Scholars Project of Shandong Province (tsqn202103186), Academic promotion programme of Shandong First Medical University (No. 2019QL005), and The Innovation Project of Shandong Academy of Medical Sciences.
Fig. 1The development of TgCtwh6 bradyzoites in cat IECs in vitro. Wright-Giemsa staining (×400). (A) At 36 h after infection, bradyzoites began to invade into cat IECs. (B) 5 days after infection, the parasites in cat IECs form rose-like structures. (C) Almost cat IECs burst and the parasites dissociate 8 days after infection.
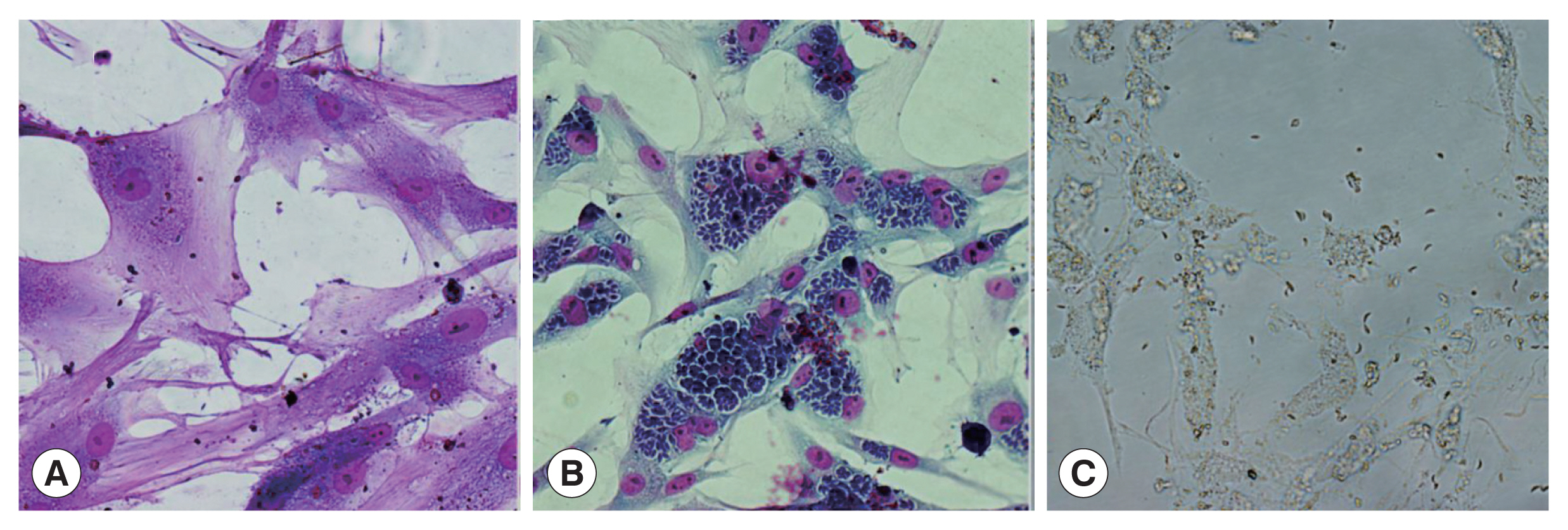
Fig. 2Identification of Toxoplasma stages in cultured cat IECs in vitro. M, DNA Marker; RNA, RNA of parasites and co-cultured cat IECs. 1, The tachyzoite-specific surface antigen 1 (TgSAG1) gene; 2, The bradyzoite-specific protein, bradyzoite antigen1 (TgBAG1) gene; 3, The merozoite-specific protein, dense granule protein 11B (TgGRA11B) gene.
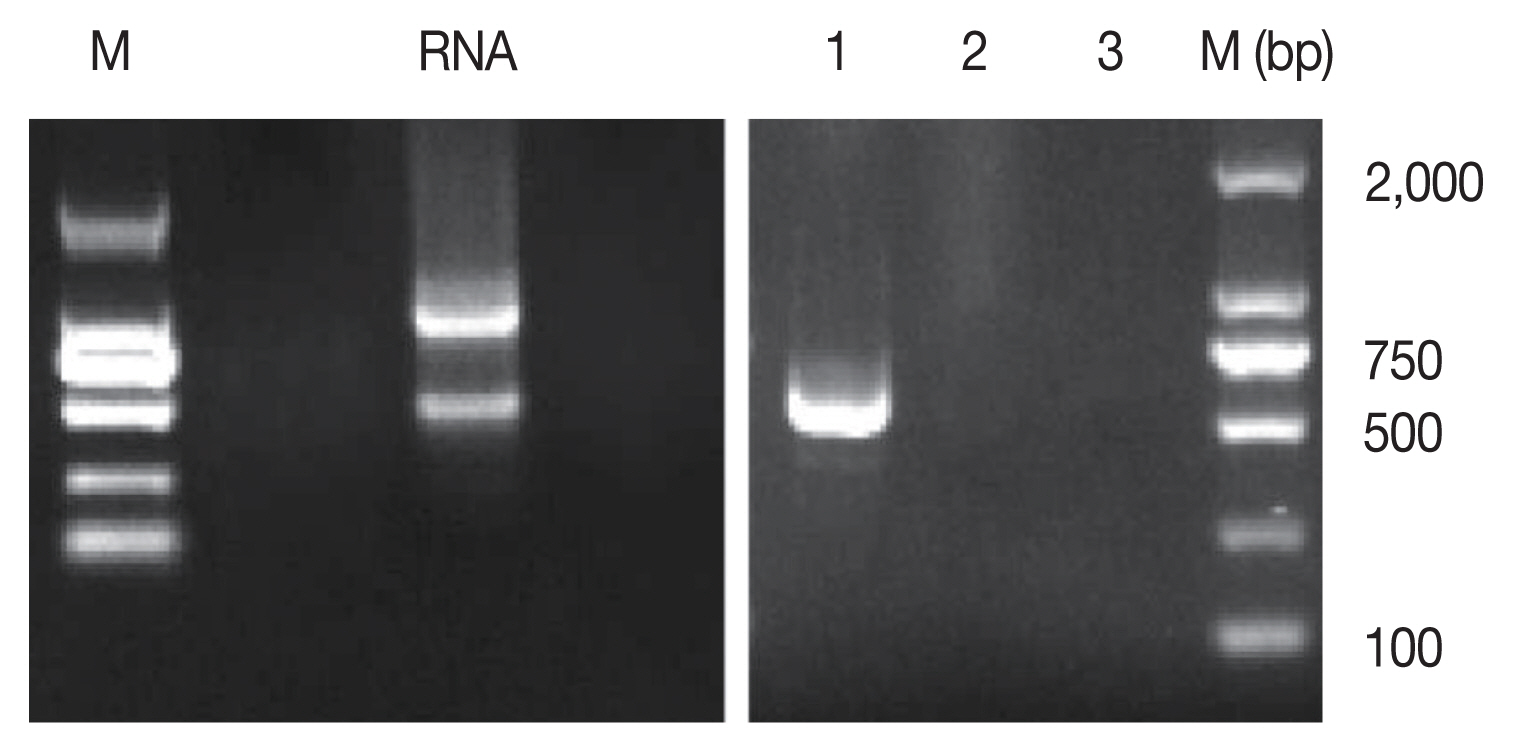
Fig. 3The discovery of Toxoplasma schizonts in cat IECs in vivo. (A) The schizonts of T. gondii detected by IHC (×200). (B) The schizonts of T. gondii detected by IFA (×200).
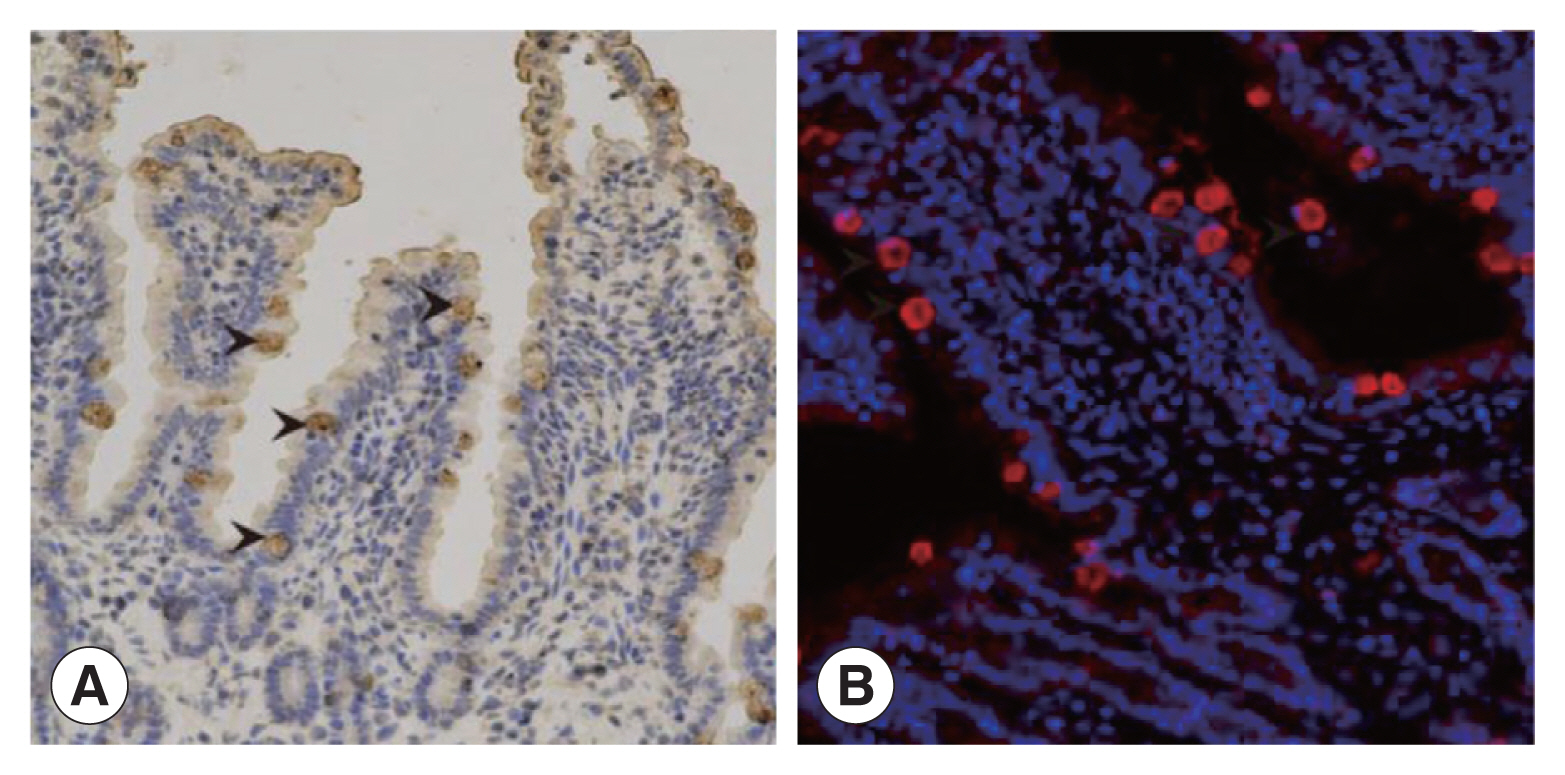
Table 1Primers for detecting Toxoplasma gondii stages
Table 1
|
Target gene |
Primer sequence |
Amplicon size |
|
TgBAG1 |
5′-CTAGACTATTTGGAT-3′
5′-CTGTCGAACTCCACG-3′ |
398 bp |
|
TgGRA11B |
5′-ATGTCCCACCGCATGGCAT-3′
5′-TGGCTTCAACTCGTCCTCTTCC-3′ |
1,413 bp |
|
TgSAG1 |
5′-TTCACTCTCAAGTGCCCT-3′
5′-TCAATGCTTCTCAGGCGATC-3′ |
650 bp |
References
- 1. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000;30:1217-1258. https://doi.org/10.1016/S0020-7519(00)00124-7
- 2. Pan M, Lyu C, Zhao J, Shen B. Sixty years (1957–2017) of research on Toxoplasmosis in China an overview. Front Microbiol 2017;8:1825. https://doi.org/10.3389/fmicb.2017.01825
- 3. Achaw B, Tesfa H, Zeleke AJ, Work L, Addisu A, Yigzaw N, Tegegne Y. Seroprevalence of Toxoplasma gondii and associated risk factors among psychiatric outpatients attending University of Gondar Hospital, Northwest Ethiopia. BMC Infect Dis 2019;19:581. https://doi.org/10.1186/s12879-019-4234-6
- 4. Lindgren M, Torniainen-Holm M, Härkänen T, Dickerson F, Yolken RH, Suvisaari J. The association between Toxoplasma and the psychosis continuum in a general population setting. Schizophr Res 2018;193:329-335. https://doi.org/10.1016/j.schres.2017.06.052
- 5. Frye MA, Coombes BJ, McElroy SL, Jones-Brando L, Bond DJ, Veldic M, Romo-Nava F, Bobo WV, Singh B, Colby C, Skime MK, Biernacka JM, Yolken R. Association of cytomegalovirus and Toxoplasma gondii antibody titers with bipolar disorder. JAMA Psychiatry 2019;76:1285-1293. https://doi.org/10.1001/jamapsychiatry.2019.2499
- 6. Stepanova EV, Kondrashin AV, Sergiev VP, Morozova LF, Turbabina NA, Maksimova MS, Romanov DV, Kinkulkina MA, Lazareva AV, Morozov EN. Toxoplasmosis and mental disorders in the Russian Federation (with special reference to schizophrenia). PLoS One 2019;14:e0219454. https://doi.org/10.1371/journal.pone.0219454
- 7. Ding H, Gao YM, Deng Y, Lamberton PHL, Lu DB. A systematic review and meta-analysis of the seroprevalence of Toxoplasma gondii in cats in mainland China. Parasit Vectors 2017;10:27. https://doi.org/10.1186/s13071-017-1970-6
- 8. Qiu HY, Zhang XX, Jiang J, Cai Y, Xu P, Zhao Q, Wang CR. Toxoplasma gondii seropositivity and associated risk factors in cats (Felis catus) in three provinces in northeastern China from 2013 to 2019. Vector Borne Zoonotic Dis 2020;20:723-727. https://doi.org/10.1089/vbz.2019.2583
- 9. Kolören Z, Dubey JP. A review of toxoplasmosis in humans and animals in Turkey. Parasitology 2020;147:12-28. https://doi.org/10.1017/S0031182019001318
- 10. Sroka J, Karamon J, Dutkiewicz J, Wójcik Fatla A, Zając V, Cencek T. Prevalence of Toxoplasma gondii infection in cats in southwestern Poland. Ann Agric Environ Med 2018;25:576-580. https://doi.org/10.26444/aaem/94675
- 11. Gebremedhin EZ, Tadesse G. A meta-analysis of the prevalence of Toxoplasma gondii in animals and humans in Ethiopia. Parasit Vectors 2015;8:1-9. https://doi.org/10.1186/s13071-015-0901-7
- 12. Salman D, Pumidonming W, Oohashi E, Igarashi M. Prevalence of Toxoplasma gondii and other intestinal parasites in cats in Tokachi sub-prefecture, Japan. J Vet Med Sci 2018;80:960-967. https://doi.org/10.1292/jvms.17-0713
- 13. Taetzsch SJ, Gruszynski KR, Bertke AS, Dubey JP, Monti KA, Zajac AM, Lindsay DS. Prevalence of zoonotic parasites in feral cats of Central Virginia, USA. Zoonoses Public Health 2018;65:728-735. https://doi.org/10.1111/zph.12488
- 14. Su C, Dubey JP. Isolation and genotyping of Toxoplasma gondii strains. Methods Mol Biol 2020;2071:49-80. https://doi.org/10.1007/978-1-4939-9857-9_3
- 15. Dubey JP, Cerqueira-Cézar CK, Murata FHA, Kwok OCH, Yang YR, Su C. All about toxoplasmosis in cats: the last decade. Vet Parasitol 2020;283:1091. https://doi.org/10.1016/j.vetpar.2020.109145
- 16. Dong H, Su R, Lu Y, Wang M, Liu J, Jian F, Yang Y. Prevalence, risk factors, and genotypes of Toxoplasma gondii in food animals and humans (2000–2017) from China. Front. Microbiol 2018;9:2108. https://doi.org/10.3389/fmicb.2018.02108
- 17. Chen ZW, Gao JM, Huo XX, Wang L, Yu L, Halm-Lai F, Xu YH, Song WJ, Hide G, Shen JL, Lun ZR. Genotyping of Toxoplasma gondii isolates from cats in different geographic regions of China. Vet Parasitol 2011;183:166-170. https://doi.org/10.1016/j.vetpar.2011.06.013
- 18. Zhang AM, Shen Q, Li M, Xu XC, Chen H, Cai YH, Luo QL, Chu DY, Yu L, Du J, Lun ZR, Wang Y, Sha Q, Shen JL. Comparative studies of macrophage-biased responses in mice to infection with Toxoplasma gondii ToxoDB #9 strains of different virulence isolated from China. Parasit Vectors 2013;6:308. https://doi.org/10.1186/1756-3305-6-308
- 19. Zhao GH, Liu Y, Cheng YT, Zhao QS, Qiu X, Xu C, Xiao T, Zhu S, Liu GZ, Yin K. Primary culture of cat intestinal epithelial cells in vitro and the cDNA library construction. Acta Parasitol 2018;63:360-367. https://doi.org/10.1515/ap-2018-0041
- 20. Watts EA, Dhara A, Sinai AP. Purification Toxoplasma gondii Tissue Cysts Using Percoll Gradients. Curr Protoc Microbiol 2017;45:20C.2.1-20C.2.19. https://doi.org/10.1002/cpmc.30
- 21. Nasiru Wana M, Mohd Moklas MA, Watanabe M, Zasmy Unyah N, Alhassan Abdullahi S, Ahmad Issa Alapid A, Nordin N, Basir R, Abd Majid R. Molecular detection and genetic diversity of Toxoplasma gondii oocysts in cat faeces from Klang Valley, Malaysia, using B1 and REP genes in 2018. Pathogens 2020;9:576. https://doi.org/10.3390/pathogens9070576
- 22. Homan WL, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200-to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic andquantitive PCR. Int J Parasitol 2000;30:69-75. https://doi.org/10.1016/s0020-7519(99)00170-8
- 23. Moura Mde A, Amendoeira MR, Barbosa HS. Primary culture of intestinal epithelial cells as a potential model for Toxoplasma gondii enteric cycle studies. Mem Inst Oswaldo Cruz 2009;104:862-864. https://doi.org/10.1590/s0074-02762009000600007
- 24. Martorelli Di Genova B, Wilson SK, Dubey JP, Knoll LJ. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol 2019;17:e3000364. https://doi.org/10.1371/journal.pbio.3000364
- 25. Jelińska M, Białek A, Gielecińska I, Mojska H, Tokarz A. Impact of conjugated linoleic acid administered to rats prior and after carcinogenic agent on arachidonic and linoleic acid metabolites in serum and tumors. Prostaglandins Leukot Essent Fatty Acids 2017;126:1-8. https://doi.org/10.1016/j.plefa.2017.08.013
- 26. Freyre A, Choromanski L, Fishback JL, Popiel I. Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J Parasitol 1993;79:716-719.
- 27. Dubey JP. Duration of immunity to shedding of Toxoplasma gondii oocysts by cats. J Parasitol 1995;81:410-415.
- 28. Zulpo DL, Sammi AS, Dos Santos JR, Sasse JP, Martins TA, Minutti AF, Cardim ST, de Barros LD, Navarro IT, Garcia JL. Toxoplasma gondii: a study of oocyst re-shedding in domestic cats. Vet Parasitol 2018;249:17-20. https://doi.org/10.1016/j.vetpar.2017.10.021
 , Lixin Zhang1, Lisha Dai1, Haozhi Xu1, Chao Xu1, Ting Xiao1, Jin Li1, Hui Sun1, Beibei Zhou2, Kun Yin1,*
, Lixin Zhang1, Lisha Dai1, Haozhi Xu1, Chao Xu1, Ting Xiao1, Jin Li1, Hui Sun1, Beibei Zhou2, Kun Yin1,*