Abstract
Trichomoniasis is caused by a sexually transmitted flagellate protozoan parasite Trichomonas vaginalis. T. vaginalis-derived secretory products (TvSP) contain lipid mediators such as leukotriene B4 (LTB4) and various cysteinyl leukotrienes (CysLTs) which included LTC4, LTD4, and LTE4. However, the signaling mechanisms by which T. vaginalis-induced CysLTs stimulate interleukin (IL)-8 production in human mast cells remain unclear. In this study, we investigated these mechanisms in human mast cells (HMC-1). Stimulation with TvSP resulted in increased intracellular reactive oxygen species (ROS) generation and NADPH oxidase 2 (NOX2) activation compared to unstimulated cells. Pre-treatment with NOX2 inhibitors such as diphenyleneiodonium chloride (DPI) or apocynin significantly reduced ROS production in TvSP-stimulated HMC-1 cells. Additionally, TvSP stimulation increased NOX2 protein expression and the translocation of p47phox from the cytosol to the membrane. Pretreatment of HMC-1 cells with PI3K or PKC inhibitors reduced TvSP-induced p47phox translocation and ROS generation. Furthermore, NOX2 inhibitors or NOX2 siRNA prevented CREB phosphorylation and IL-8 gene expression or protein secretion induced by TvSP. Pretreatment with a CysLTR antagonist significantly inhibited TvSP-induced ROS production, CREB phosphorylation, and IL-8 production. These results indicate that CysLT-mediated activation of NOX2 plays a crucial role in ROS-dependent IL-8 production in human mast cells stimulated by T. vaginalis-secreted CysLTs. These findings enhance our understanding of the inflammatory response in trichomoniasis and may inform the development of targeted therapies to mitigate this response.
-
Key words: Trichomonas vaginalis, reactive oxygen species, NADPH oxidase 2, human mast cell, cysteinyl leukotrienes, interleukin-8
Introduction
Tissue-resident mast cells play a crucial role in allergic and parasitic infections [
1,
2]. When activated by extracellular stimuli, mast cells rapidly degranulate and secrete chemokines that activate other immune cells, promoting their migration to sites of inflammation [
2]. Chemokines such as interleukin (IL)-8 (CXCL8), monocyte chemotactic protein-1 (MCP-1; CCL2), and RANTES (CCL5) secreted by mast cells facilitate immune cell migration to inflammation sites [
2,
3]. IL-8, a key proinflammatory chemokine, is crucial in acute inflammation and immune responses and is implicated in the pathogenesis of various inflammatory diseases [
3,
4]. Produced by various cell types, IL-8 plays a vital role in the early stages of inflammation, regulating the recruitment and activation of immune cells essential for an effective immune response [
4]. In patients with
Trichomonas vaginalis infection, mast cells are the predominant inflammatory cells found in vaginal discharge, commonly alongside neutrophils [
1]. During
T. vaginalis infection, mast cells produce various chemokines that promote the migration of other immune cells to the infection site.
Trichomonas vaginalis is a common sexually transmitted protozoan parasite that infects the human genitourinary tract [
5]. Annually, more than 180 million people worldwide are infected with this parasite [
5].
T. vaginalis infection causes vaginitis and cervicitis in women and urethritis or prostatitis in men [
1,
5,
6]. When trichomonas infects humans, it secretes various substances that directly damage the vaginal or cervical epithelium and elicit immune responses.
T. vaginalis-derived secretory products (TvSPs) include various pathogenic mediators such as lipid mediators, proteins, carbohydrates, and proteolytic enzymes [
7,
8]. Our previous study showed that
T. vaginalis secretes lipid mediators such as leukotriene B
4 (LTB
4) and cysteinyl leukotrienes (CysLTs), including leukotriene C
4 (LTC
4), leukotriene D
4 (LTD
4), and leukotriene E
4 (LTB
4) [
9–
11].
T. vaginalis-derived LTB
4 induces chemotaxis of human neutrophils and activates mast cells, leading to degranulation, migration, Ractive Oxygen Species (ROS) production, and IL-8 secretion [
9–
14]. These responses occur through a signaling axis involving
T. vaginalis-derived LTB
4 and BLT (LTB
4 receptor) in human mast cells.
CysLTs are lipid mediators derived from arachidonic acid metabolism via the 5-lipoxygenase pathway [
15]. These potent inflammatory mediators are involved in various physiological and pathological processes. CysLTs are primarily synthesized and released by activated immune cells, such as mast cells, eosinophils, and basophils, in response to allergens, infections, or other stimuli [
15,
16]. They exert their effects by binding to specific receptors, primarily the CysLT receptors (CysLTRs), which are G-protein-coupled receptors expressed on various cell types, including immune, epithelial, and endothelial cells [
16]. Previously, we reported that
T. vaginalis-secreted CysLTs induced MCP-1 secretion through interaction with the CysLTRs of mast cells [
11]. However, the signaling mechanism involved in IL-8 production in mast cells by
T. vaginalis-derived CysLTs is not well understood. Therefore, in this study, we investigated whether CysLTR-mediated signaling mechanisms are involved in IL-8 production in human mast cells stimulated with
T. vaginalis-secreted CysLTs.
Materials and Methods
Ethics statement
Not-applicable.
Reagents
Diphenyleneiodonium chloride (DPI) and apocynin were obtained from Calbiochem (Gibbstown, NJ, USA). Bay u-9773 cells were purchased from Enzo Life Sciences (Farmingdale, NY, USA). LTC4 was sourced from Biomol (Plymouth Junction, PA, USA). Anti-phospho-AKT, anti-AKT, anti-phospho-CREB, and anti-β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Anti-NOX2, anti-ATPase, anti-phospho-p47phox, and anti-p47phox antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cultivation of T. vaginalis and preparation of T. vaginalis-derived secretory products (TvSP)
The T016 strain of
T. vaginalis [
17] was kindly donated by Prof. Jae-Sook Ryu in Hanyang University College of Medicine.
T. vaginalis was axenically sub-cultured at 37°C in Diamond’s trypticase-yeast extract-maltose medium supplemented with 10% heat-inactivated horse serum (Gibco/Invitrogen, Gaithersburg, MD, USA) and 0.5% penicillin/streptomycin (Gibco/Invitrogen). To obtain TvSP for HMC-1 stimulation, the trichomonads (1×10
7 cells) were washed once with Hank’s balanced salt solution (HBSS) (Gibco/Invitrogen), resuspended in 1 ml of HBSS, and incubated for 1 h at 37°C. The culture supernatant was centrifuged at 12,000 g for 10 min, and the supernatant was filtered through a 0.22-μm filter to obtain TvSP. Protein concentrations were measured using the BCA protein assay, with bovine serum albumin as the standard.
HMC-1 cells were cultured in Iscove’s modified Dulbecco’s medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Biomeda, Foster City, CA, USA) and 1% penicillin/streptomycin. The cells were maintained in a 5% CO2 incubator. Cell viability, assessed by the trypan blue exclusion assay, was consistently 99%.
HMC-1 cell stimulation and pretreatment
HMC-1 cells (1×105) were seeded in 48-well plates and incubated for specified durations with or without 100 μl TvSP (collected from 1×107 trichomonads/ml) at 37°C in a 5% CO2 incubator. The cells were pretreated with various pharmacological inhibitors and the CysLTR antagonist Bay-u9773 before stimulation with or without TvSPs for 30 min at 37°C.
Measurement of intracellular ROS generation and p47phox phosphorylation in HMC-1 cells
Intracellular ROS accumulation in HMC-1 cells was measured using the red fluorescent probe hydroethidium (HE) and the green fluorescent probe DCF-DA. HMC-1 cells (1×105/well) were prestained at 37°C for 10 min with 1 μm HE, which is rapidly oxidized in the presence of O2−, or 5 μm DCF-DA, which is rapidly oxidized to highly fluorescent DCF in the presence of intracellular H2O2. The cells were cultured for 1 h with or without TvSP or LTB4 in 24-well tissue culture plates in a CO2 incubator. After incubation, cells were washed twice with wash buffer before measuring DCF fluorescence using a FACSCalibur TM (BD Bioscience). At least 10,000 gated events were analyzed for each sample. HMC-1 cells (1×105) were seeded in 48-well plates and incubated for specified durations with or without TvSP or LTC4 for 1 h at 37°C in a 5% CO2 incubator. Cytosolic and membrane fractions were prepared using a Cytosol/Membrane Fraction Isolation Kit (BioVision) according to the manufacturer’s instructions.
RT-PCR for IL-8 gene expression and protein secretion in HMC-1 cells
HMC-1 cells (1×106 cell/sample) were incubated for 30 min in the absence or presence of TvSP (100 μg/ml). RNA from cells was extracted using QIAGEN RNeasy Plus Min Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Isolated RNA was reverse transcribed into single-strand cDNA with AccuPower RT PreMix (Bioneer, Deajon, Korea) and an oligo (dT) primer. Single-strand cDNA was then amplified with a gene-specific antisense primer. The primers were as follows: hIL-8 (293 bp), 5′-ATTTGAAAA TGGCATTCCCC-3′ (sense) and 5′-TCATCAGCAAAAGGGATGGA-3′ (antisense); and β-actin (273 bp), 5′-CAAGAGATGGCCACGGCTGCT-3′ (sense) and 5′-TCCTTCTGCA TCCTGTCGGCA-3′ (antisense). The amplification protocol for hIL-8 was 95°C for 30 sec (30 cycles), 58°C for 30 sec, and 72°C for 30 sec. β-actin was amplified at 95°C for 30 sec (30 cycles), 55°C for 30 sec, and 72°C for 30 sec. Protocols were completed with a 7-min extension at 72°C. The final PCR products were resolved on a 2% agarose gel containing ethidium bromide and visualized under ultraviolet light. For IL-8 protein secretion analysis, HMC-1 cells (5×105) pretreated with or without Bay u-9773 (1–10 μm) for 30 min, were incubated with or without TvSP or LTC4 for 1 h at 37°C in a 5% CO2 incubator. After incubation, culture supernatants and cell lysates were collected for MCP-1 ELISA (Thermo Scientific, Waltham, MA, USA).
Short interfering RNA (siRNA)-mediated NADPH oxidase (NOX2) knockdown in HMC-1 cells
ON-target, SMARTpool NOX2 siRNA (L-011021-00-0005) and scrambled siRNA (D-001810-01-05) were purchased from Dhamarcon (Lafayette, CO, USA). siRNA transfection was performed using Lipofectamine (Thermo Fisher Scientific, MA, USA), following the manufacturer’s instructions. At 72 h post-transfection, the efficiency of NOX2 knockdown was confirmed by western blotting using specific antibodies with β-actin as the loading control. Transfected HMC-1 cells were washed, placed in fresh cell culture medium, and incubated with TvSP for subsequent experiments.
Immunoblotting
HMC-1 cells (5×105) were pretreated for various durations with or without pharmacological inhibitors or receptor antagonists, then stimulated for the indicated time periods with or without TvSP. Cells were lysed in a lysis buffer (50 mm Tris-HCl, pH 8.0, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mm ethylenediaminetetraacetic acid (EDTA), 1 mm phenylmethylsulfonyl fluoride (PMSF), 1 mM sodium orthovanadate, and a proteinase inhibitor cocktail) on ice for 30 min. Lysates were centrifuged at 15,000 g for 5 min, separated by SDS-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane (Millipore), and blocked with 5% skim milk. The blot was probed with primary antibodies at 4°C overnight, followed by incubation with secondary horseradish peroxidase (HRP)-conjugated antibodies. Immunoreactivity was detected using LumiGLO (Cell Signaling Technology).
Statistical analysis
Data are represented as the mean±standard deviation (SD) from 3–4 independent experiments. Statistical analysis was performed using Student’s t-test. Differences were considered significant at P<0.05.
Results
NADPH oxidase involvement in ROS production in HMC-1 cell induced by TvSP
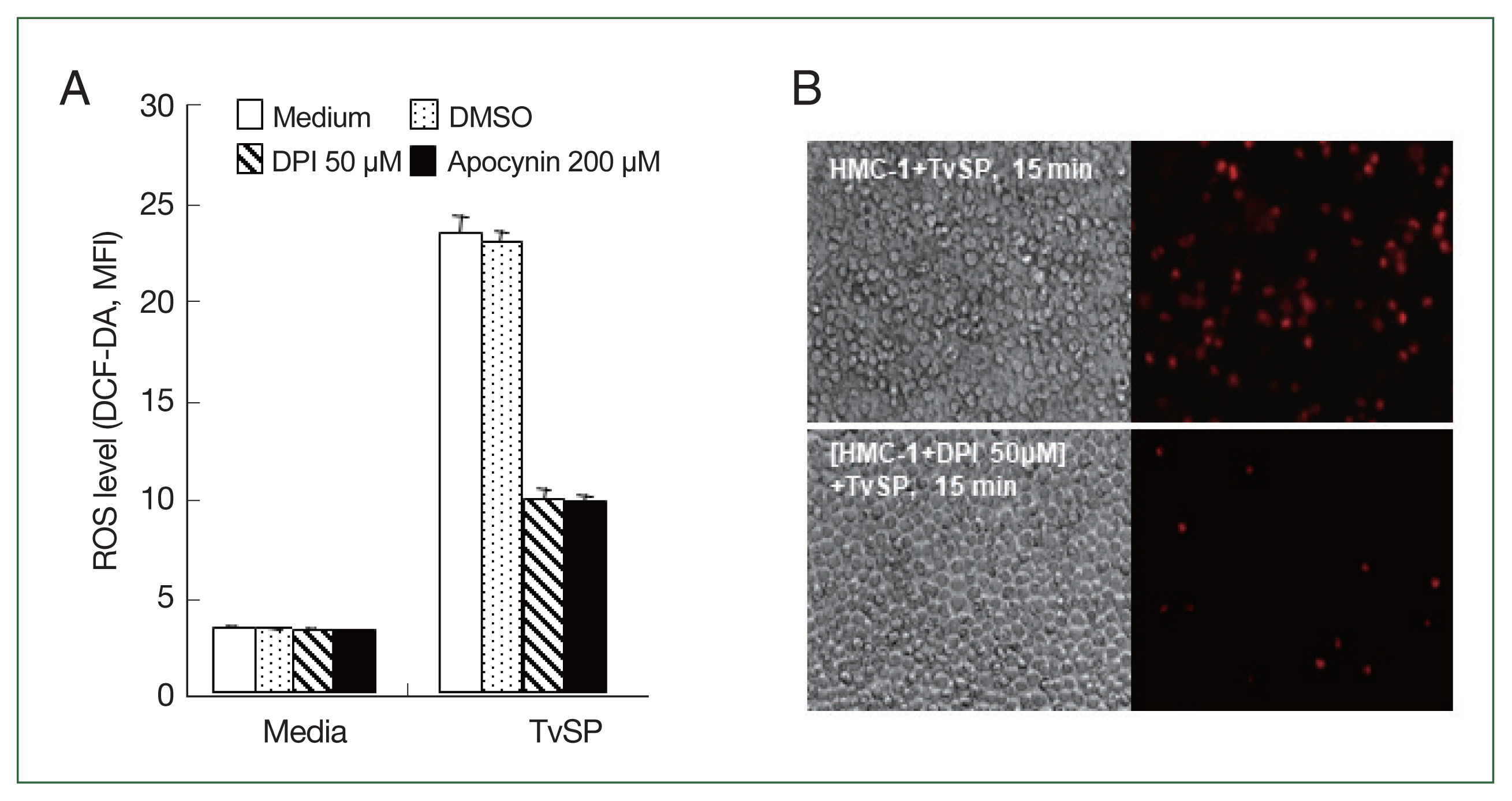
We investigated whether NADPH oxidases are involved in ROS generation in HMC-1 cells stimulated by TvSP. As shown in
Fig. 1A, pretreatment of HMC-1 cells with the general NOX inhibitors DPI or apocynin significantly reduced TvSP-induced ROS generation compared to the vehicle control group. TvSP-induced ROS generation in HMC-1 cells was observed using fluorescence microscopy. Intracellular ROS accumulation was measured using the red fluorescent probe HE. Pretreatment with DPI inhibited TvSP-induced ROS generation in HMC-1 cells compared to the untreated control group (
Fig. 1B).
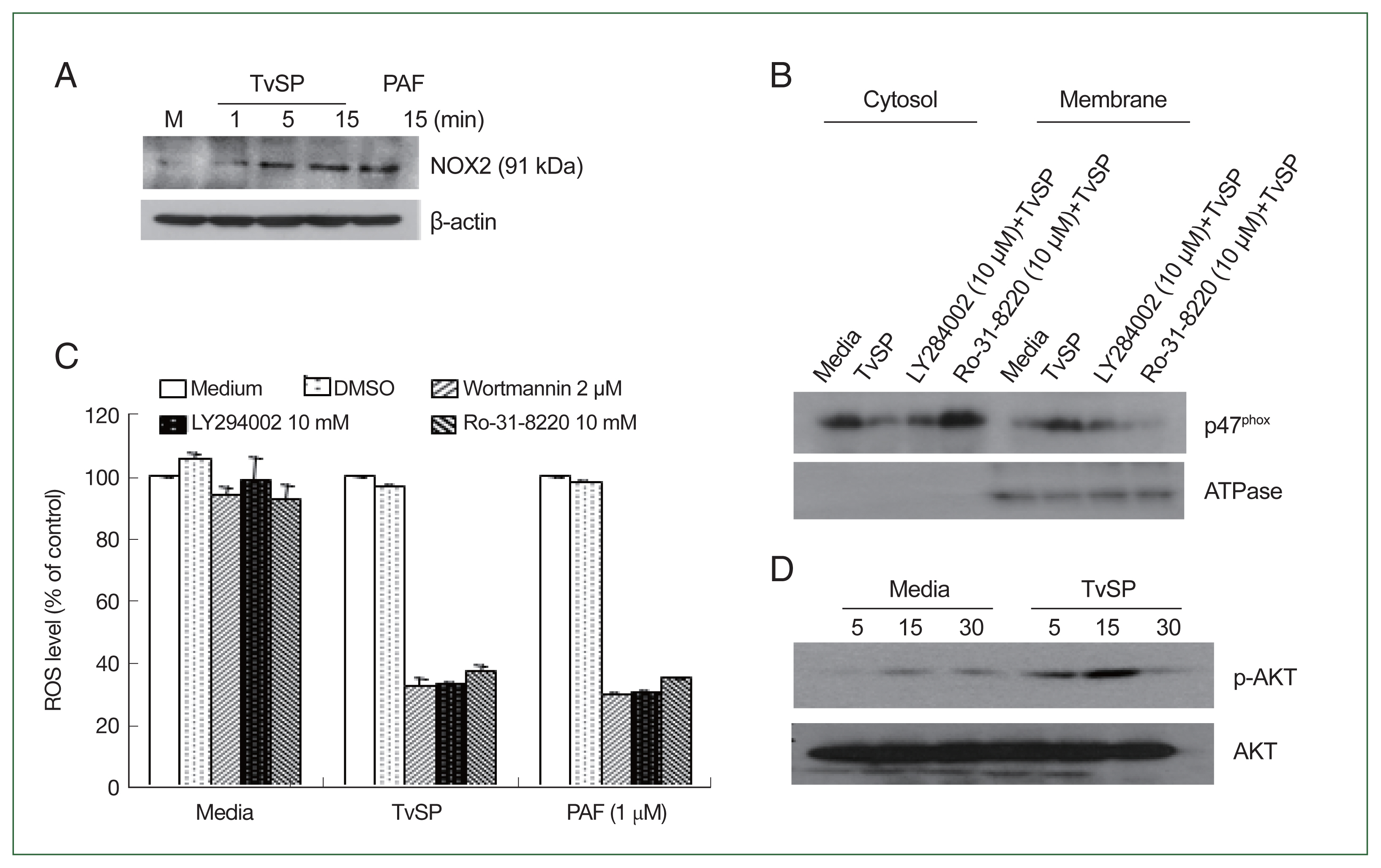
We examined whether TvSP induces NOX2 activation in HMC-1 cells. NOX2 expression in TvSP-stimulated HMC-1 cells increased in a time-dependent manner (
Fig. 2A). PAF was used as a positive control, showing similar NOX2 activation. We also investigated the intracellular distribution of p47
phox in HMC-1 cells. In untreated cells, p47
phox was predominantly in the cytosol, whereas in TvSP-stimulated HMC-1 cells, p47
phox translocated to the membrane. Pretreatment with the PI3K inhibitor LY264002 or the PKC inhibitor Ro-31-8220 reduced TvSP-induced p47
phox membrane translocation (
Fig. 2B). Additionally, pretreatment with PI3K inhibitors (wortmannin or LY294002) or PKC inhibitors significantly reduced NOX2-derived ROS generation in TvSP-stimulated HMC-1 cells (
Fig. 2C). A similar phenomenon was observed in PAF. TvSP also induced AKT phosphorylation in HMC-1 cells in a time-dependent manner (
Fig. 2D).
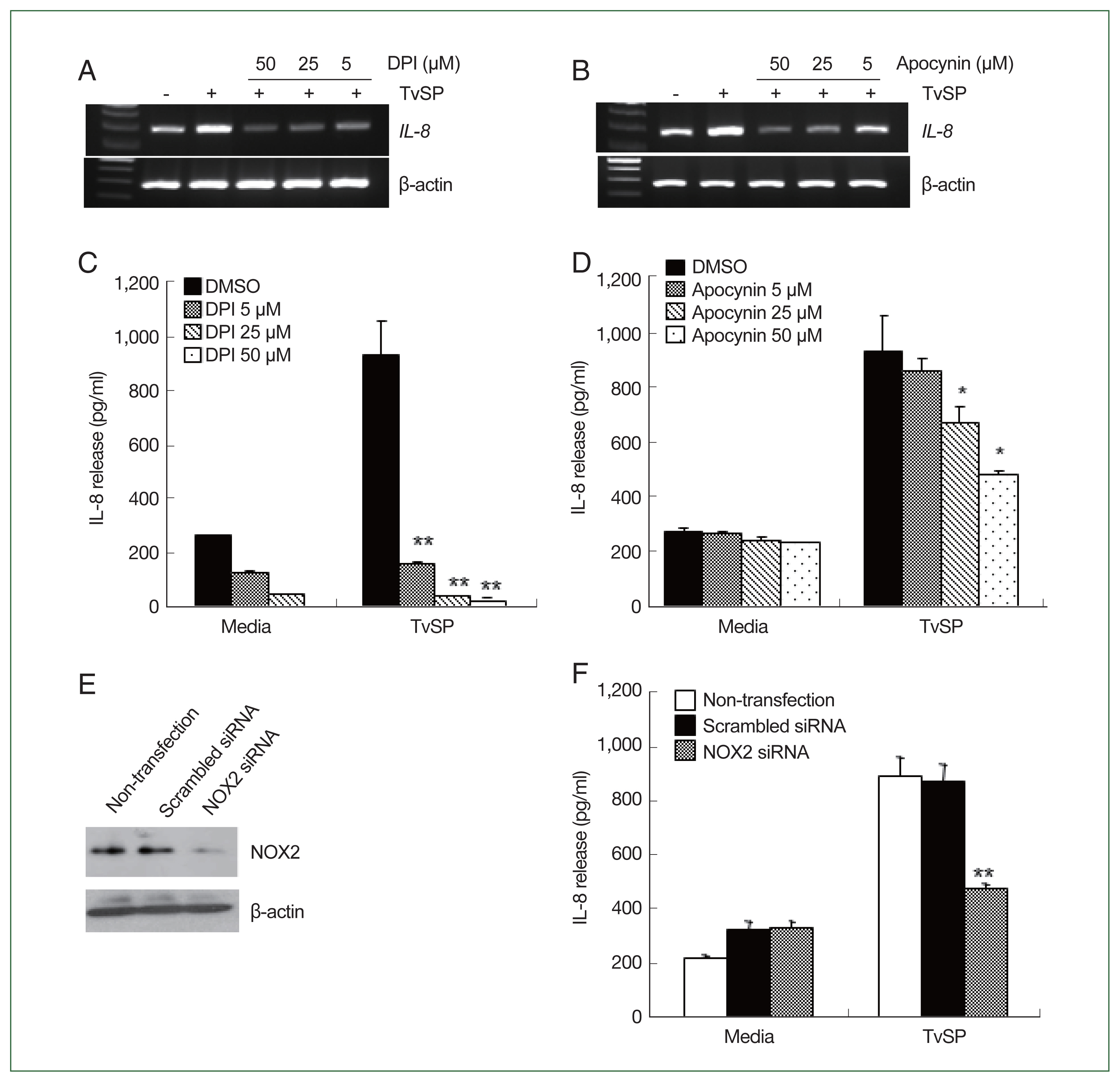
We examined whether NOX2 was involved in TvSP-induced IL-8 production in HMC-1 cells. As shown in
Fig. 3A and B, TvSP stimulation increased IL-8 mRNA levels in HMC-1 cells. Pretreatment with DPI or apocynin inhibited the TvSP-induced
il-8 mRNA expression in HMC-1 cells (
Fig. 3A and B). We also examined the role of NOX2-derived ROS in TvSP-induced IL-8 secretion in HMC-1 cells. Pretreatment with NOX2 inhibitors decreased TvSP-induced IL-8 secretion in HMC-1 cells in a dose-dependent manner (
Fig. 3C, D). Finally, we evaluated the role of NOX2-derived ROS in TvSP-induced IL-8 secretion by transfecting HMC-1 cells with NOX2 siRNA (
Fig. 3E). NOX2 siRNA suppressed TvSP-induced IL-8 secretion in HMC-1 cells (
Fig. 3F).
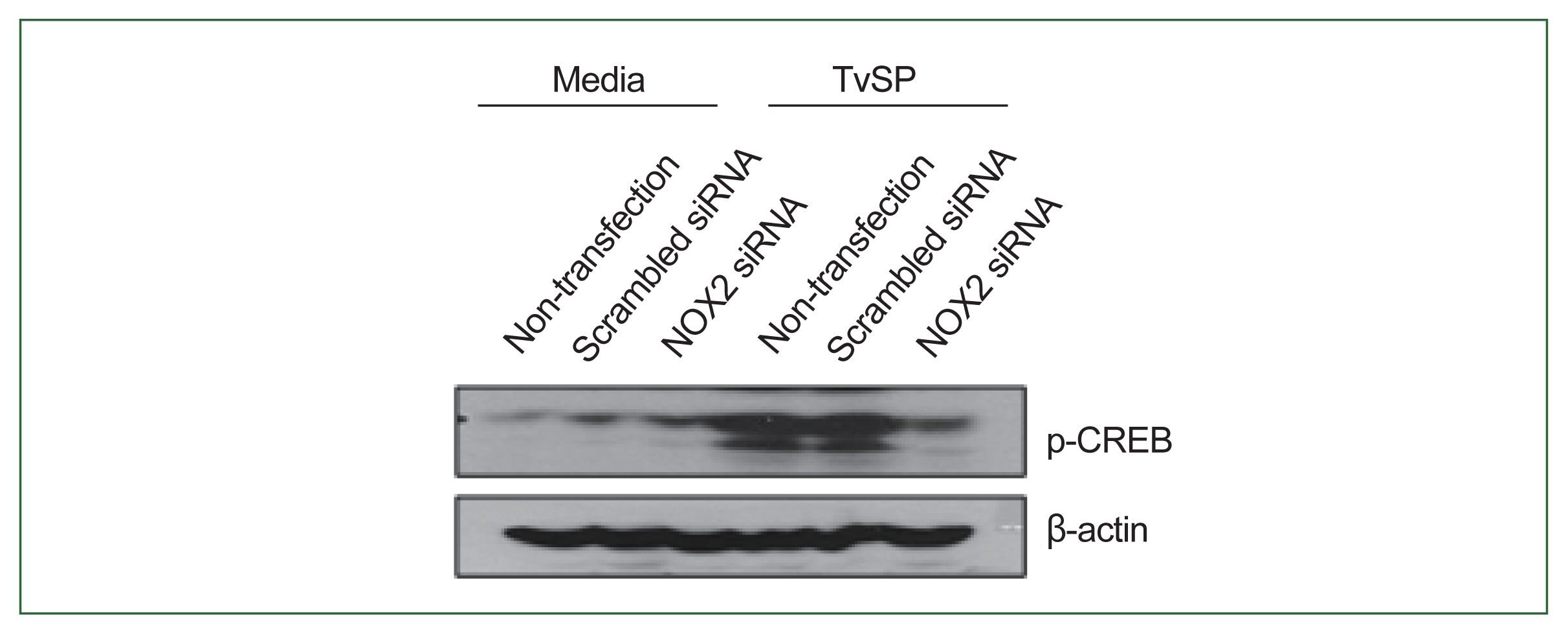
We investigated the role of NOX2 in TvSP-induced CREB phosphorylation in HMC-1 cells. As shown in
Fig. 4, CREB phosphorylation was lower in NOX2-silenced HMC-1 cells compared to non-transfected or scramble siRNA-transfected cells. These results indicate that NOX2 is essential for CREB activation in response to TvSP (
Fig. 4).
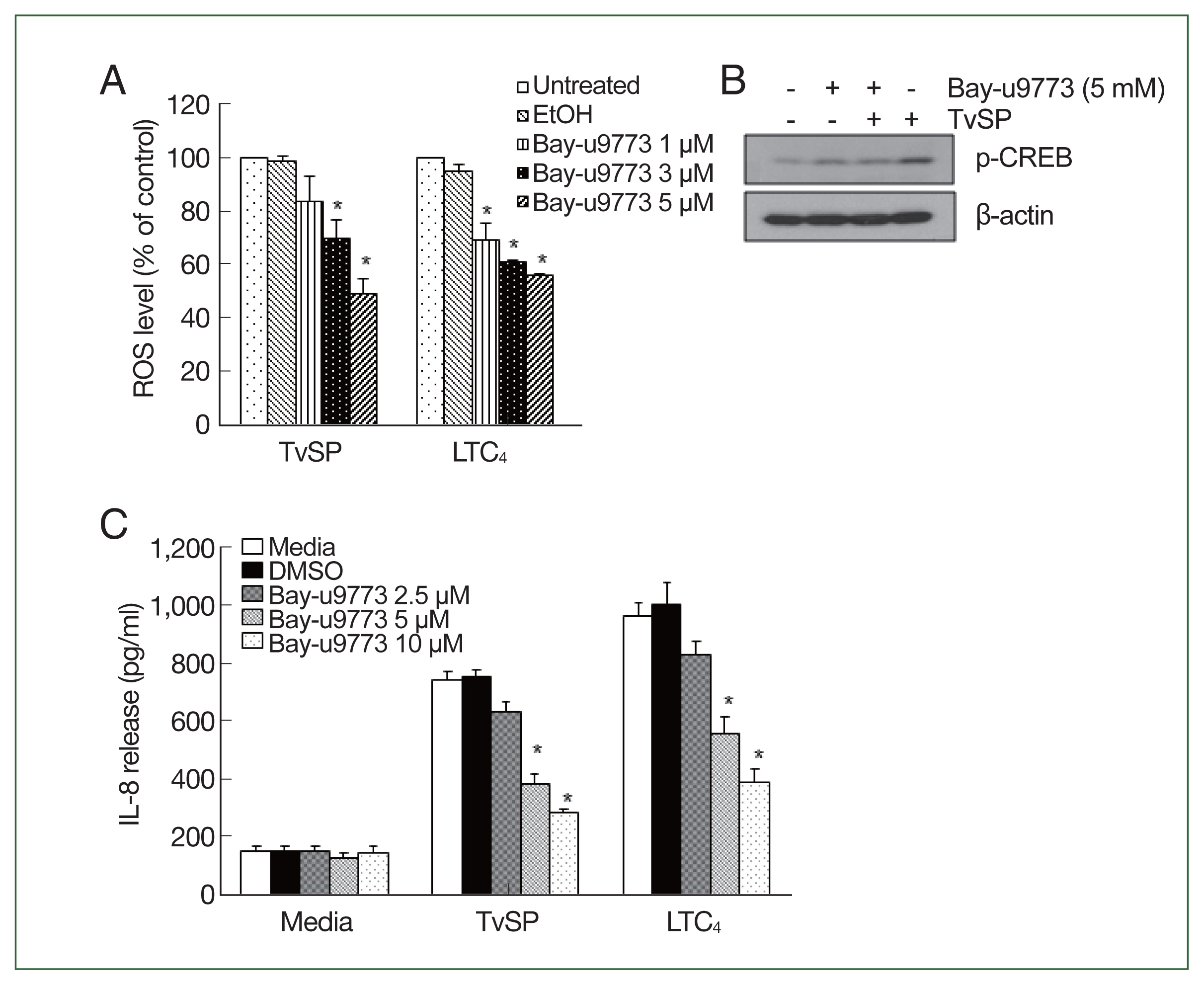
We examined the involvement of the CysLT receptor in TvSP-induced ROS production and IL-8 production. Pretreatment with the CysLT antagonist Bay-u9773 significantly reduced TvSP-induced ROS production in HMC-1 cells in a dose-dependent manner (
Fig. 5A). The effects of LTC4 stimulation were similar to those of TvSP. Bay-u9773 also significantly reduced TvSP-induced CREB phosphorylation (
Fig. 5B) and IL-8 production (
Fig. 5C) in mast cells. The effects of LTC
4 stimulation were consistent with those of TvSP.
Discussion
This study shows that T. vaginalis-secreted CysLTs might induce IL-8 production in HMC-1 cells via CysLTR-mediated NOX2 activation. We found that TvSP stimulation rapidly induced NOX2-derived ROS through p47phox translocation to the membrane in HMC-1 cells, which was required for CREB phosphorylation and IL-8 production. Blocking of TvSP signaling by treatment with a CysLTR antagonist suppressed TvSP-induced ROS production, CREB phosphorylation, and IL-8 production in HMC-1 cells. These findings suggest that ROS production via CysLTR-mediated NOX2 activation plays a crucial role in IL-8 production during mast cell-mediated tissue inflammation caused by T. vaginalis infection.
The signaling interactions between parasites and immune cells are critical for immune cell activation against parasites. Immune cells express various surface receptors, including lipid mediator receptors [
15,
18]. Parasite-derived lipid mediators transmit signals to host cells through these receptors. For instance,
Entamoeba histolytica-secreted PEG2 promotes IL-8 production via EP4 receptors in colon epithelial cells, inducing immune cell migration [
19]. Conversely,
Schistosoma mansoni-secreted PED2 inhibits the migration of Langerhans cells through the adenylate cyclase-coupled PGD2 receptor [
20]. In
T. vaginalis infection,
T. vaginalis-secreted LTB
4 induces IL-8 production through BLT1-mediated activation of CREB or NF-κB in human neutrophils and mast cells [
10,
21]. Similar to the LTB
4-BLT axis,
T. vaginalis-secreted CysLTs signal through host cell CysLTRs. Our study found that
T. vaginalis-secreted CysLTs induced ROS-dependent IL-8 production in human mast cells via CysLTR-mediated signaling. These results suggest that parasite-host crosstalk is very important for tissue inflammatory responses during innate immunity.
In mast cells, CysLTR-mediated signaling is closely related to IL-8 secretion, which is essential for the recruitment and infiltration of neutrophils and other granulocytes to infection sites [
4,
22]. In this study, we observed that TvSP-stimulated HMC-1 cells pretreated with a CysLTR antagonist showed significantly reduced IL-8 production compared to untreated TvSP-stimulated HMC-1 cells. This suggests that the interaction between
T. vaginalis-secreted CysLTs and mast cell CysLTRs is crucial for the signaling cascade that mediates IL-8 production.
T. vaginalis-secreted CysLTs induce IL-8 production via CREB activation in mast cells. Similar results were obtained using LTC
4 as a positive control. Our findings align with previous reports showing that CysLT binding to CysLTR activates transcription factors to induce IL-8 production. For instance, LTD
4 induces AP-1- and NF-κB-dependent IL-8 expression through CysLT1 receptor engagement [
23]. Treatment with LTC
4 or LTD
4 induces CysLT2 receptor-mediated IL-8 production in HEK293 cells [
22,
24]. Treatment with pranlukast, a CysLTR1 antagonist, inhibits TNF-α-induced NF-κB activation in U937 and Jurkat cells [
25]. Furthermore,
T. vaginalis-secreted CysLTs promote migration, degranulation and MCP-1 production and NF-κB phosphorylation in mast cells [
11]. These results suggest that the CysLT-CysLTR pathway is crucial for mast cell activation and immune response during
T. vaginalis infection.
NOX2-derived ROS in immune cells are crucial for host immune defense [
26,
27]. Specifically, the phosphorylation and membrane translocation of p47
phox are required for NOX2 activation in immune cells [
26]. PI3K/PKC signaling is essential for NOX2-mediated signal transduction [
27]. Recent reports have shown that NOX2-derived ROS is essential for signaling involved in cytokine secretion, immune cell migration, and immune cell death induced by protozoan parasites such as
E. histolytica and
T. vaginalis [
11,
13,
14,
18,
28]. Our study confirmed that upon TvSP stimulation, phosphorylated p47
phox translocates from the cytoplasm to the membrane in a PI3K/PKC-mediated manner in mast cells (
Fig. 2B). In addition, TvSP-induced IL-8 secretion and CREB phosphorylation were significantly reduced in HMC-1 cells pretreated with NOX inhibitors or transfected with NOX2 siRNA, compared to control cells. These findings indicate that NOX2-derived ROS are essential for TvSP-induced IL-8 secretion by HMC-1 cells. Previously, we reported that the interaction between
T. vaginalis-secreted CysLTs and CysLTR is crucial for mast cell degranulation, MCP-1 secretion, and NF-κB phosphorylation [
11]. In this study, we demonstrated that NOX2-derived ROS generated through the interaction between
T. vaginalis-secreted CysLTs and CysLTRs are involved in CREB phosphorylation and IL-8 production in human mast cells.
In conclusion, we have demonstrated that CysLTs from T. vaginalis contribute to ROS production, CREB phosphorylation, thereby increase IL-8 secretion by human mast cells via NOX2 activation. Understanding the host-parasite cross-talk between T. vaginalis CysLTs and host cell CysLTRs may provide insights into the pathogenesis of human trichomoniasis.
Notes
-
Author contributions
Conceptualization: Lee YA, Shin MH
Formal analysis: Lee YA
Funding acquisition: Lee YA, Shin MH
Investigation: Lee YA
Methodology: Lee YA
Project administration: Shin MH
Supervision: Shin MH
Visualization: Shin MH
Writing – original draft: Lee YA
Writing – review & editing: Lee YA, Shin MH
-
The authors declare no conflict of interest related to this study.
Acknowledgment
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (NRF-2020R1I1A1A01064838) to YA Lee and by a faculty research grant from the Yonsei University College of Medicine (6-2021-0238) to MH Shin. Authors are grateful to Prof. JS Ryu, Hanyang University Colleage of Medicine for her kind clonation of the T. vaginalis T016 strain.
Fig. 1Effect of NADPH oxidase inhibitors, diphenyleneiodonium chloride (DPI) or apocynin, on ROS production in HMC-1 cells stimulated with TvSP. HMC-1 (5×105/well) cells were pretreated with or without DPI or apocynin, then cultured with TvSP for 15 min. (A) Intracellular ROS accumulation in HMC-1 cells was measured using the green fluorescence probe 5 μm DCF-DA. After incubation, cells were washed twice with wash buffer before measuring DCF fluorescence using a FACS Calibur TM (BD Bioscience). At least 10,000 gated events were analyzed for each sample. Data are representative of 3 independent experiments performed in duplicate. (B) TvSP-induced ROS generation in HMC-1 cells was observed using fluorescent microscopy. Intracellular ROS accumulation was measured using the red fluorescence probe 1 μm HE. Increased red fluorescence was observed. Magnification ×200.

Fig. 2TvSP induces the NOX2 activation and ROS generation via PKC or PI3 kinase-mediated signaling in HMC-1 cells. (A) NOX2 expression in TvSP-stimulated HMC-1 cells. (B) Effects of inhibitors on p47phox protein translocation in HMC-1 cells induced by TvSP. HMC-1 cells were seeded in 6-well plate at 1×107 cells/well, then pretreated with or without the PI3 kinase inhibitor LY294002 or the PKC inhibitor Ro-31-8220 for 30 min before the addition of TvSP using transwell for 15 min. After incubation, cells were harvested, proteins were analyzed by SDS-PAGE and immunoblotting with anti-p47phox or anti-ATPase antibodies. (C) Effect of PI3K or PKC inhibitors on TvSP-induced ROS generation in HMC-1 cells. HMC-1 cells (3×105/well) were pretreated with wortmannin (2 μm), or LY294002 (10 μm), or Ro-31-8220 (10 μm) without T. vagianlis for 30 min at 37°C in a CO2 incubator, then incubated with or without TvSP in transwells (24 wells, pore size 0.2 μm) for 2 h at 37°C in a CO2 incubator. PAF was used as a positive control at 1 μm for 1 h. DMSO (0.5%) was used as the vehicle control for inhibitors. After incubation, ROS generation was measured by FACS analysis. Data were normalized to the MFI of ROS levels in HMC-1 cells stimulated with TvSP and treated with wortmannin (2 μm), LY294002 (10 μm), or Ro-31-8220 (10 μm). Values are expressed as percentages against HMC-1 (white bar, % of control) of each group. Basal activity (100% values, MFI) of DCF for each group was 3.4 (medium), 36.6 (TvSP), and 44.59 (PAF). Data are presented as the mean±SD from 4 independent experiments. (D) AKT phosphorylation in TvSP-stimulated HMC-1 cells. After incubation, cells were harvested, proteins were analyzed by SDS-PAGE and immunoblotting with anti-phospho-AKT, AKT or anti-β actin antibodies. The figure is representative of 3 separate experiments showing similar results.

Fig. 3NOX2 is involved in IL-8 production in HMC-1 cells induced by TvSP. Effect of DPI (A) and apocynin (B) on TvSP-induced IL-8 mRNA expression in HMC-1 cells. HMC-1 cells pretreated with or without DPI or apocynin were stimulated for 60 min with or without TvSP. Effect of DPI (C) and apocynin (D) on TvSP-induced IL-8 production in HMC-1 cells. HMC-1 cells pretreated with or without DPI or apocynin were stimulated for 16 h with or without TvSP. After stimulation, IL-8 protein in culture supernatants from HMC-1 cells was measured by ELISA. Data are expressed as the mean±SD from 3 independent experiments. *P<0.05, **P<0.005 compared to the value for the control. (E) Expression of NOX2 protein levels in NOX2 siRNA-transfected HMC-1 cells. β-actin was used as a control. (F) Effect of NOX2 siRNA on TvSP-induced IL-8 production in HMC-1 cells. Data are expressed as the mean±SD from 3 independent experiments. **P<0.01 compared to the value for the control.

Fig. 4NOX2 is required for CREB phosphorylation in HMC-1 cells induced by TvSP. Effect of NOX2 siRNA on TvSP-induced CREB phosphorylation in HMC-1 cells. β-actin was used as a control. The figure is representative of 3 separate experiments showing similar results.

Fig. 5CysLT receptor is involved in ROS generation and IL-8 production in HMC-1 cells stimulated with TvSP. (A) Effect of CysLT antagonist on TvSP or LTC4-stimulated ROS generation in HMC-1 cells. Data are expressed as the mean±SD from 3 independent experiments. *P<0.05 compared to the value for the control. (B) Effect of CysLT antagonist on TvSP-induced CREB phosphorylation in HMC-1 cells. β-actin was used as a control. The figure is representative of 3 separate experiments showing similar results. (C) Effect of the CysLT antagonist on TvSP or LTC4-stimulated IL-8 production in HMC-1 cells. Data are expressed as the mean±SD from 3 independent experiments. *P<0.05 compared to the value for the control.

References
- 1. Kobayashi TK, Fujimoto T, Okamoto H, Yuasa M, Sawaragi I. Association of mast cells with vaginal trichomoniasis in endocervical smears. Acta Cytol 1983;27(2):133-137.
- 2. Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Res 2020;9:F1000 Faculty Rev-196https://doi.org/10.12688/f1000research.22037.1
- 3. Mukai K, Tsai M, Saito H, Galli SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev 2018;282:121-150.
https://doi.org/10.1111/imr.12634
- 4. Matsushima K, Yang D, Oppenheim JJ. Interleukin-8: an evolving chemokine. Cytokine 2022;153:155828.
https://doi.org/10.1016/j.cyto.2022.155828
- 5. Mercer F, Johnson PJ.
Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol 2018;34(8):683-693.
https://doi.org/10.1016/j.pt.2018.05.006
- 6. Han IH, Kim JH, Ryu JS. Inflammatory response to Trichomonas vaginalis in the pathogenesis of prostatitis and benign prostatic hyperplasia. Parasites Hosts Dis 2023;61(1):2-14.
https://doi.org/10.3347/PHD.22160
- 7. Kummer S, Hayes GR, Gilbert RO, Beach DH, Lucas JJ, et al. Induction of human host cell apoptosis by Trichomonas vaginalis cysteine proteases is modulated by parasite exposure to iron. Microb Pathog 2008;44(3):197-203.
https://doi.org/10.1016/j.micpath.2007.09.004
- 8. Szempruch AJ, Dennison L, Kieft R, Harrington JM, Hajduk SL. Sending a message: extracellular vesicles of pathogenic protozoan parasites. Nat Rev Microbiol 2016;14(11):669-675.
https://doi.org/10.1038/nrmicro.2016.110
- 9. Shaio MF, Lin PR, Lee CS, Hou SC, Tang P, et al. A novel neutrophil-activating factor released by Trichomonas vaginalis
. Infect Immun 1992;60(11):4475-4482.
https://doi.org/10.1128/iai.60.11.4475-4482.1992
- 10. Nam YH, Min D, Kim HP, Song KJ, Kim KA, et al. Leukotriene B4 receptor BLT-mediated phosphorylation of NF-κB and CREB is involved in IL-8 production in human mast cells induced by Trichomonas vaginalis-derived secretory products. Microbes Infect 2011;13(14–15):1211-1220.
https://doi.org/10.1016/j.micinf.2011.07.006
- 11. Lee YA, Nam YH, Min A, Shin MH.
Trichomonas vaginalis-secreted cysteinyl leukotrienes promote migration, degranulation and MCP-1 production in mast cells. Parasite Immunol 2020;42(12):e12789.
https://doi.org/10.1111/pim.12789
- 12. Nam YH, Min A, Kim SH, Lee YA, Kim KA, et al. Leukotriene B (4) receptors BLT1 and BLT2 are involved in interleukin-8 production in human neutrophils induced by Trichomonas vaginalis-derived secretory products. Inflamm Res 2012;61(2):97-102.
https://doi.org/10.1007/s00011-011-0425-3
- 13. Min A, Lee YA, Kim KA, El-Benna J, Shin MH. SNAP23-dependent surface translocation of leukotriene B4 (LTB4) receptor 1 is essential for NOX2-mediated exocytotic degranulation in human mast cells induced by Trichomonas vaginalis-secreted LTB4
. Infect Immun 2016;85(1):e00526-16.
https://doi.org/10.1128/IAI.00526-16
- 14. Min A, Lee YA, Kim KA, Shin MH. BLT1-mediated O-GlcNAcylation is required for NOX2-dependent migration, exocytotic degranulation and IL-8 release of human mast cell induced by Trichomonas vaginalis-secreted LTB(4). Microbes Infect 2018;20(6):376-384.
https://doi.org/10.1016/j.micinf.2018.05.005
- 15. Liu M, Yokomizo T. The role of leukotrienes in allergic diseases. Allergol Int 2015;64(1):17-26.
https://doi.org/10.1016/j.alit.2014.09.001
- 16. Kanaoka Y, Austen KF. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv Immunol 2019;142:65-84.
https://doi.org/10.1016/bs.ai.2019.04.002
- 17. Kim KS, Moon HS, Kim SS, Ryu JS. Involvement of macrophages in proliferation of prostate cancer cells infected with Trichomonas vaginalis
. Korean J Parasitol 2021;59(6):557-564.
https://doi.org/10.3347/kjp.2021.59.6.557
- 18. Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett 2016;178:10-14.
https://doi.org/10.1016/j.imlet.2016.07.003
- 19. Lejeune M, Moreau F, Chadee K. Prostaglandin E2 produced by Entamoeba histolytica signals via EP4 receptor and alters claudin-4 to increase ion permeability of tight junctions. Am J Pathol 2011;179(2):807-818.
https://doi.org/10.1016/j.ajpath.2011.05.001
- 20. Angeli V, Faveeuw C, Roye O, Fontaine J, Teissier E, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med 2001;193(10):1135-1147.
https://doi.org/10.1084/jem.193.10.1135
- 21. Nam YH, Min D, Park SJ, Kim KA, Lee YA, et al. NF-κB and CREB are involved in IL-8 production of human neutrophils induced by Trichomonas vaginalis-derived secretory products. Korean J Parasitol 2011;49(3):291-294.
https://doi.org/10.3347/kjp.2011.49.3.291
- 22. Thompson C, Cloutier A, Bossé Y, Poisson C, Larivée P, et al. Signaling by the cysteinyl-leukotriene receptor 2. Involvement in chemokine gene transcription. J Biol Chem 2008;283(4):1974-1984.
https://doi.org/10.1074/jbc.M608197200
- 23. Thompson C, Cloutier A, Bossé Y, Thivierge M, Gouill CL, et al. CysLT1 receptor engagement induces activator protein-1- and NF-kappaB-dependent IL-8 expression. Am J Respir Cell Mol Biol 2006;35(6):697-704.
https://doi.org/10.1165/rcmb.2005-0407OC
- 24. Lin K, Fang S, Cai B, Huang X, Zhang X, et al. ERK/Egr-1 signaling pathway is involved in CysLT2 receptor-mediated IL-8 production in HEK293 cells. Eur J Cell Biol 2014;93(7):278-288.
https://doi.org/10.1016/j.ejcb.2014.05.001
- 25. Ichiyama T, Hasegawa S, Umeda M, Terai K, Matsubara T, et al. Pranlukast inhibits NF-kappa B activation in human monocytes/macrophages and T cells. Clin Exp Allergy 2003;33(6):802-827.
https://doi.org/10.1046/j.1365-2222.2003.01673.x
- 26. El-Benna J, Dang PM, Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 2008;30(3):279-289.
https://doi.org/10.1007/s00281-008-0118-3
- 27. Vermot A, Petit-Härtlein I, Smith SME, Fieschi F. NADPH Oxidases (NOX): an overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants (Basel) 2021;10(6):890.
https://doi.org/10.3390/antiox10060890
- 28. Lee YA, Shin MH. Involvement of NOX2-derived ROS in human hepatoma HepG2 cell death induced by Entamoeba histolytica
. Parasites Hosts Dis 2023;61(4):388-396.
https://doi.org/10.3347/PHD.23094