Abstract
We previously reported that leukotriene B4 (LTB4) contained in Trichomonas vaginalis-derived secretory products (TvSP) play an essential role in interleukin-8 (IL-8) production in human mast cell line (HMC-1 cells) via LTB4 receptor (BLT)-mediated Nuclear Factor-kappa B (NF-κB) activation. Dynamin, a GTPase, has been known to be involved in endocytosis of receptors for signaling of production of cytokine or chemokines. In the present study, we investigated the role of dynamin-mediated BLT1 endocytosis in TvSP-induced IL-8 production. When HMC-1 cells were transfected with BLT1 or BLT2 siRNA, TvSP-induced IL-8 production was significantly inhibited compared with that in cells transfected with control siRNA. In addition, pretreatment of HMC-1 cells with a dynamin inhibitor (Dynasore) reduced IL-8 production induced by TvSP or LTB4. TvSP- or LTB4-induced phosphorylation of NF-κB was also attenuated by pretreatment with Dynasore. After exposing HMC-1 cells to TvSP or LTB4, BLT1 was translocated from the intracellular compartments to the plasma membrane within 30 min. At 60 min after stimulation with TvSP or LTB4, BLT1 remigrated from the cell surface to intracellular areas. Pretreatment of HMC-1 cells with dynamin-2 siRNA blocked internalization of BLT1 induced by TvSP or LTB4. Co-immunoprecipitation experiments revealed that dynamin-2 strongly interacted with BLT1 60 min after stimulation with TvSP or LTB4. These results suggest that T. vaginalis-secreted LTB4 induces IL-8 production in HMC-1 cells via dynamin 2-mediated endocytosis of BLT1 and phosphorylation of NF-κB.
-
Key words: Trichomonas vaginalis, leukotrienes B4, leukotriene B4 receptor 1, interleukin-8, endocytosis, dynamin, mast cells
Introduction
Trichomonas vaginalis is a flagellated protozoan parasite that is transmitted sexually and causes the most common non-viral sexual infections worldwide [
1]. This parasite infects the human vagina, cervix, prostate, and urethra [
2].
T. vaginalis infection is clinically important because of its association with preterm delivery, low birth weight, cervical or prostate cancer, and the transmission of human immunodeficiency virus type 1 transmission [
1–
3].
Trichomonas vaginalis-derived secretory products (TvSP) contain various substances such as proteins, carbohydrates, proteases, and lipid mediators [
4,
5]. Our previous study showed that
T. vaginalis secretes lipid mediators, such as leukotriene B
4 (LTB
4) and cysteinyl leukotrienes (CysLTs) [
5,
6]. In particular,
T. vaginalis-secreted LTB
4 promotes immune responses such as cell migration, secretion of pro-inflammatory cytokines and chemokines, and activation of transcription factors in immune cells, thereby facilitating the activation of other immune cells [
7–
9].
Mast cells, along with neutrophils, are the immune cells primarily observed during
Trichomonas infection [
2,
10]. Engagement with
T. vaginalis-secreted LTB
4 and the G protein-coupled receptors (GPCRs) BLT1 or BLT2 (LTB
4 receptors) on neutrophils and human mast cell lines (HMC-1 cells) trigger the production of interleukin-8 (IL-8) through phosphorylation of NF-κB and CREB [
8]. Similarly, signaling interactions between
T. vaginalis-secreted LTB
4 and BLT1 in mast cells result in degranulation and migration of HMC-1 cells [
9].
T. vaginalis-derived LTB
4 induces NADPH oxidases 2 (NOX2)-derived reactive oxygen species (ROS) generation, which is responsible for Mitogen-Activated Protein Kinase (MAPK) activation and exocytosis via BLT1-meduated signaling in HMC-1 [
11]. In addition, mast cells are equipped with numerous plasma membrane receptors that sense and react rapidly to external stimuli [
6,
12]. Correct localization of cell surface receptors and appropriate protein levels are important for signal activation and regulation of the defense against pathogens. GPCR-mediated signaling induced by both host- and pathogen-derived ligands is critical for the initiation and maintenance of the inflammatory response, promoting cytokine production and migration [
13]. However, the detailed signaling mechanisms by which
T. vaginalis-secreted LTB
4 induces immune responses via BLT1 in mast cells are not fully understood.
Dynamin is a GTPase that plays a critical role in receptor internalization, a process by which membrane-bound receptors are removed from the cell surface and transported to the interior of the cell for degradation or recycling [
5,
14]. Studies have shown that dynamin is required for the internalization of a variety of receptors, including the epidermal growth factor receptor, the transferrin receptor, and the β-adrenergic receptor [
15–
18]. The regulation of dynamin-mediated receptor internalization has been implicated in the regulation of cellular signaling and maintenance of proper cellular functions [
16–
18]. Many studies have shown that dynamin plays a key role in the endocytosis of lipid rafts via fission between the plasma membrane and vesicles containing molecules or receptors [
17–
19]. In addition, BLT1 endocytosis is dynamin-dependent [
20]. However, the role of dynamin-mediated BLT1 endocytosis in the cellular responses of HMC-1 cells stimulated with
T. vaginalis-secreted LTB
4 has not yet been reported.
In this study, we investigated whether dynamin-mediated BLT1 internalization is required for IL-8 production in HMC-1 cells induced by T. vaginalis-secreted LTB4.
Materials and Methods
Ethics statement
Not-applicable.
Reagents
Dynasore (dynamin inhibitor I) was purchased from Calbiochem (Gibbstown, NJ, USA). LTB4 was purchased from Biomol (Plymouth Junction, PA, USA). Anti-phospho-NF-κB and anti-β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Rabbit anti-human P2Y7 (LTB4R1) (H-165), anti-LTB4R2 (H-86), anti-dynamin II (C-18), and anti-dynamin-2 (G-4) antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit anti-BLT1 antibody (NBP2-27422) was purchased from Novus Biologicals (Centennial, CO, USA). Methyl-β-cyclodextrin (MβCD) and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). ON-target Plus SMARTpool BLT1 siRNA (L-005653-00-0005), BLT2 siRNA (L-005654-00-0010), dynamin-2 siRNA (L-004007-00-0010), and scrambled siRNA (D-001810-01-05) were purchased from Dhamarcon (Lafayette, CO, USA).
Cultivation of T. vaginalis and preparation of TvSP
The T016 strain of T. vaginalis was axenically sub-cultured at 37°C in Diamond’s trypticase-yeast extract-maltose medium supplemented with 10% heat-inactivated horse serum (Gibco/Invitrogen, Gaithersburg, MD, USA) and 0.5% penicillin/streptomycin (Gibco/Invitrogen). Log-phase cells of T. vaginalis were used for TvSP preparation. To obtain various doses of TvSP for HMC-1 stimulation, the trichomonads (1×107 cells) were washed once with Hank’s balanced salt solution (HBSS) (Gibco/Invitrogen), resuspended in 1 ml of HBSS, and incubated for 1 h at 37°C. The culture supernatant was centrifuged at 15,000 g for 10 min. The supernatant was filtered through a 0.22-μm filter to obtain TvSP.
Human mast cell lines (HMC-1 cells) culture and stimulation
HMC-1 cells were grown in Iscove’s modified Dulbecco’s medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Biomeda, Foster City, CA, USA) and 1% penicillin/streptomycin in a 5% CO2 incubator. Cell viability, as assessed by trypan blue exclusion assay, was consistently at 99%. HMC-1 cells (1×105) were seeded in a 48-well plate and incubated for the indicated durations in the presence or absence of 100 μl TvSP collected from 1×107 trichomonads/mL at 37°C in a 5% CO2 incubator. The cells were pretreated in the presence or absence of a dynamin inhibitor (Dynasore) and stimulated with or without TvSP.
IL-8 ELISA in HMC-1 cells
HMC-1 cells (5×105) pretreated with or without dynasore (1–10 μm) for 30 min were incubated in the presence or absence of TvSP or LTB4 for 6 h or 16 h at 37°C in a 5% CO2 incubator. After incubation, culture supernatants and cell lysates were collected and analyzed using a specific human IL-8 screening set (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
Short interfering RNA (siRNA)-mediated BLT knockdown in HMC-1 cells
Short interfering RNA (siRNA) transfection was performed using Lipofectamine, following the manufacturer’s instructions. All reagents, except siRNA, were used for mock transfection. At 72-h post-transfection, the transfected HMC-1 cells were washed, placed in fresh cell culture medium, and co-incubated with TvSP. Cell lysates were extracted from all transfectants. The efficiency of the siRNA knockdown of BLT1 and BLT2 was confirmed by western blotting using specific antibodies with β-actin as the loading control. At 72 h post-transfection, cell viability was monitored using trypan blue exclusion assay (data not shown). Cell viability was maintained above 96% in all experimental samples.
Immunoblotting and co-immunoprecipitation (co-IP)
HMC-1 cells (5×105) were pretreated for various durations in the presence or absence of pharmacological inhibitors, and stimulated for the indicated time periods in the presence or absence of TvSP. Cells were lysed in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, and a proteinase inhibitor cocktail) on ice for 30 min. Cell lysates were centrifuged at 15,000 g for 5 min, separated by SDS-PAGE, transferred onto a polyvinylidene fluoride membrane (Millipore), and blocked with 5% skim milk. The blot was probed with primary antibodies at 4°C overnight, followed by incubation with the appropriate secondary horseradish peroxidase-conjugated antibodies. The immunoreactivity was detected using LumiGLO (Cell Signaling). For co-IP, cells were incubated for 30 or 60 min with or without LTB4 or TvSP. The cells were harvested, washed twice with ice-cold phosphate buffer saline (PBS), and lysed in Pierce lysis buffer containing a protease inhibitor cocktail (Sigma-Aldrich). Lysates were precleared with protein A/G agarose (Santa cruz) for 30 min at 4°C. Subsequently, precleared lysates were incubated with following antibodies at 4°C overnight. The following antibodies were used: anti-dynamin II (C-18), anti-dynamin-2 (G-4), anti-human P2Y7 (LTB4R1) (H-165), and anti-human BLT1 (NBP2-27422). The beads were washed twice with co-IP washing buffer (50 mM Tris-HCl, 150 mM NaCl, and 1% Triton X-100; pH 7.4) for 10 min and resuspended in 2×SDS sample buffer. The precipitates were analyzed by western blot using anti-dynamin (G-4) or anti-human BLT1 (NBP2-27422).
Flow cytometric measurement of BLT1 or BLT2 expression in HMC-1 cells
HMC-1 cells (1×105) were seeded in a 48-well plate and incubated in the presence or absence of TvSP or LTB4 for up to 1 h at 37°C in a 5% CO2 incubator. After incubation, cells were washed twice with PBS. For surface staining for BLT1 or BLT2 expression, cells were incubated for 30 min at 4°C with fluorescein isothiocyanate-conjugated anti-BLT1 or anti-BLT2 antibody or isotype control (10 μg/μl) and washed 3 times with FACS buffer. For intracellular BLT1 staining, cells were fixed and permeabilized with 0.3% Triton X-100 for 5 min at room temperature, washed twice with FACS buffer, and blocked with 1% bovine serum albumin in PBS. After washing, the cells were incubated with anti-BLT1 antibody for 30 min at 4°C, washed twice, and analyzed using flow cytometry (FACSCalibur, BD Biosciences). Surface and intracellular expression of BLT1 or BLT2 was analyzed using flow cytometry and counted as mean fluorescence intensity. An appropriate irrelevant isotype control antibody was used to measure the extent of nonspecific binding.
Statistical analysis
The data are represented as the mean±standard deviation (SD) from 3–4 independent experiments. Data were analyzed using Student’s t-test. Differences were considered significant when the P-value was less than 0.05.
Results
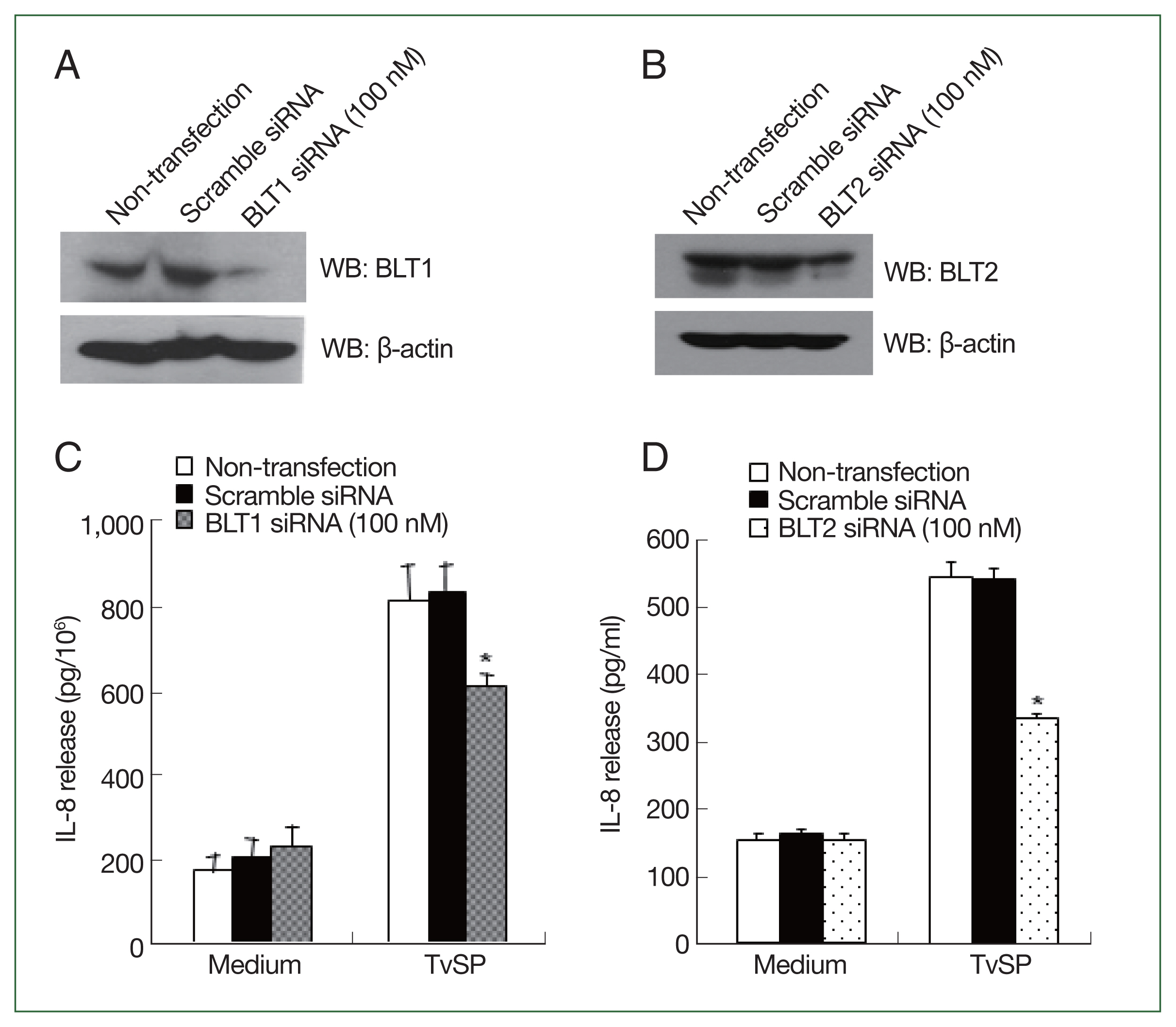
BLTs knockdown inhibits IL-8 production in HMC-1 cells induced by T. vaginalis-derived secretory products (TvSP)
To investigate the involvement of BLTs in TvSP-stimulated IL-8 production in HMC-1 cells, we used specific siRNAs against BLT1 and BLT2 to knockdown the LTB
4 receptors.
Fig. 1A and B show successful knockdown of BLT1 or BLT2 in HMC-1 cells using siRNA, respectively. TvSP-stimulated IL-8 production in BLT1 silenced HMC-1 cells was lower than in non-transfected or scrambled siRNA-treated HMC-1 cells (
Fig. 1C). BLT2 knockdown also resulted in a decrease in TvSP-induced IL-8 production in HMC-1 cells (
Fig. 1D).
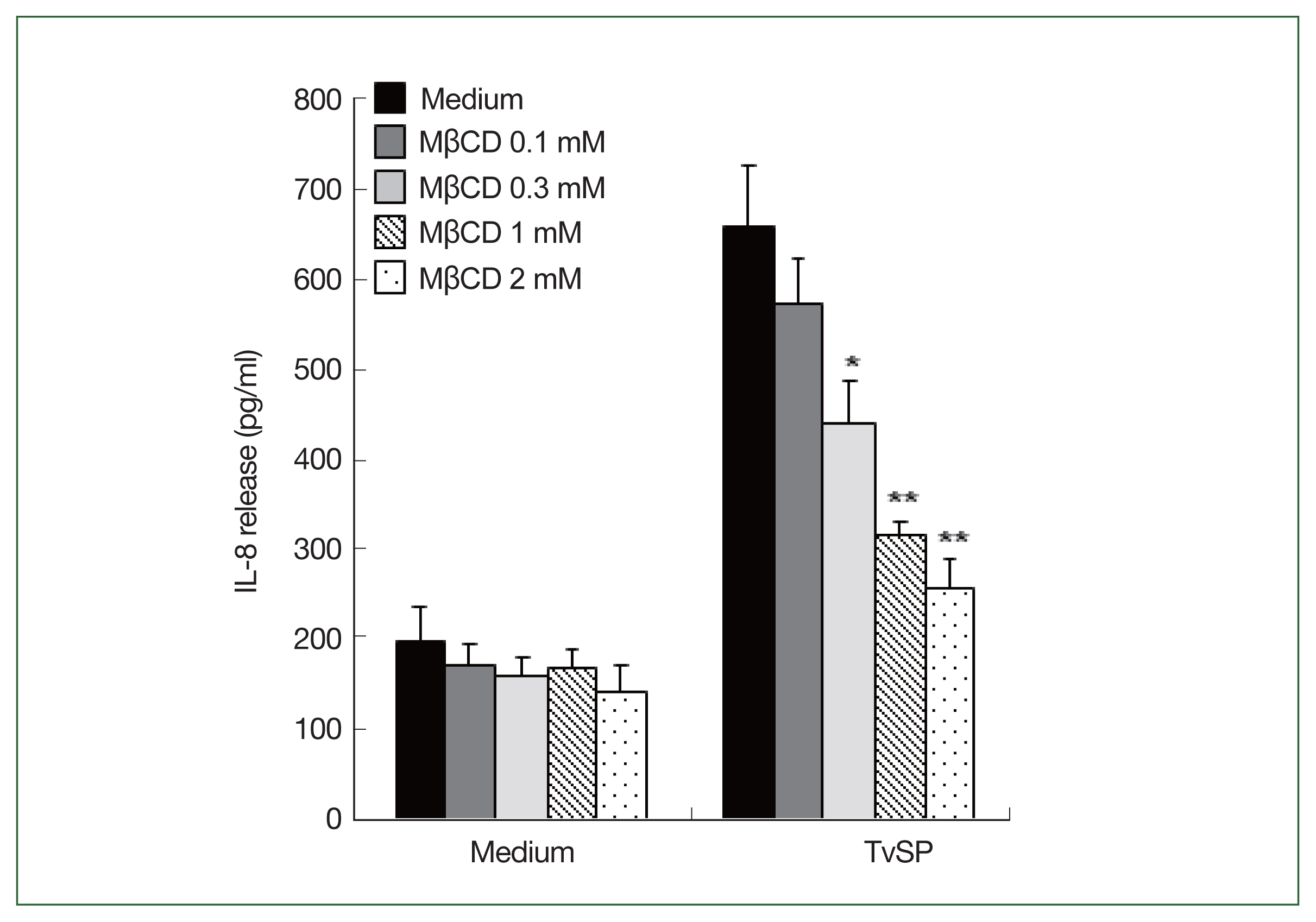
Lipid rafts are generally considered nanodomains on cell membranes that play important roles in signaling, infection, and membrane trafficking [
19]. To examine the effect of lipid raft formation on TvSP-stimulated IL-8 production in HMC-1 cells, we pretreated HMC-1 cells with lipid raft inhibitor MβCD before TvSP stimulation. MβCD is also extensively used as a cholesterol-depleting reagent and reduces clathrin-dependent endocytosis [
19]. Pretreatment of HMC-1 cells with MβCD dramatically diminished TvSP-induced IL-8 production in dose-dependent manner compared to the result of vehicle control (
Fig. 2).
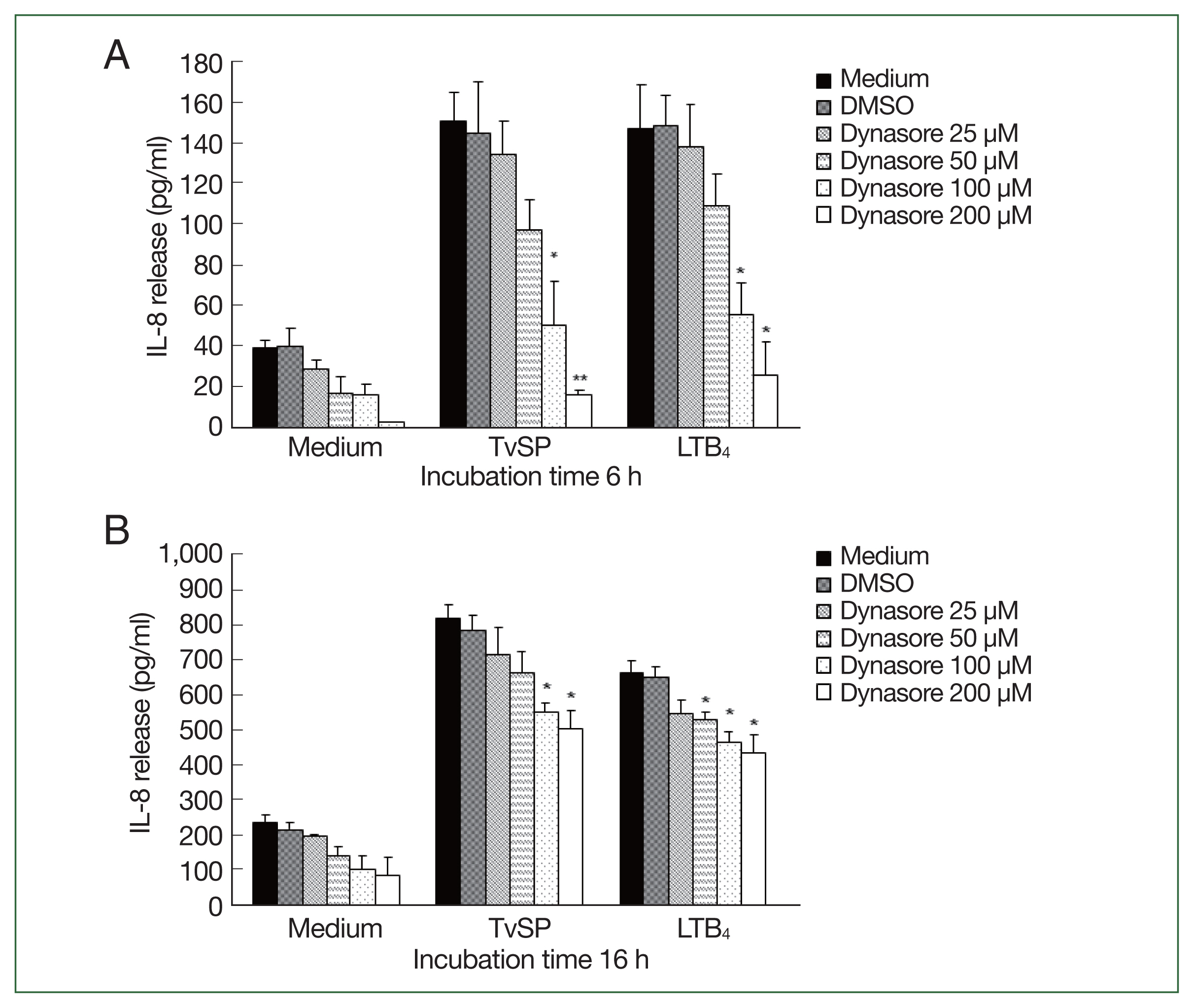
Dynamin is an essential GTPase for membrane fission during clathrin-mediated endocytosis in eukaryotic cells [
14]. Dynasore rapidly and reversibly inhibit dynamin-dependent endocytosis [
5]. We investigated the role of dynamin in the TvSP-induced IL-8 production in HMC-1 cells. When HMC-1 cells were stimulated with TvSP for 6 h or 16 h, the total amount of IL-8 production in TvSP-induced HMC-1 cells was increased in a time-dependent manner. When HMC-1 cells were pretreated with Dynasore for 6 h, IL-8 production induced by TvSP was significantly reduced in a dose-dependent manner (
Fig. 3A). Similar results were obtained in HMC-1 cells treated with LTB
4, which were used as a positive control. In HMC-1 cells for 16 h, pretreatment of HMC-1 cells with Dynasore reduced the IL-8 production induced by TvSP, but the inhibitory effect of Dynasore was less than that of HMC-1 cells stimulated with TvSP for 6 h. Similar results were obtained in HMC-1 cells treated with LTB
4, which was used as a positive control.
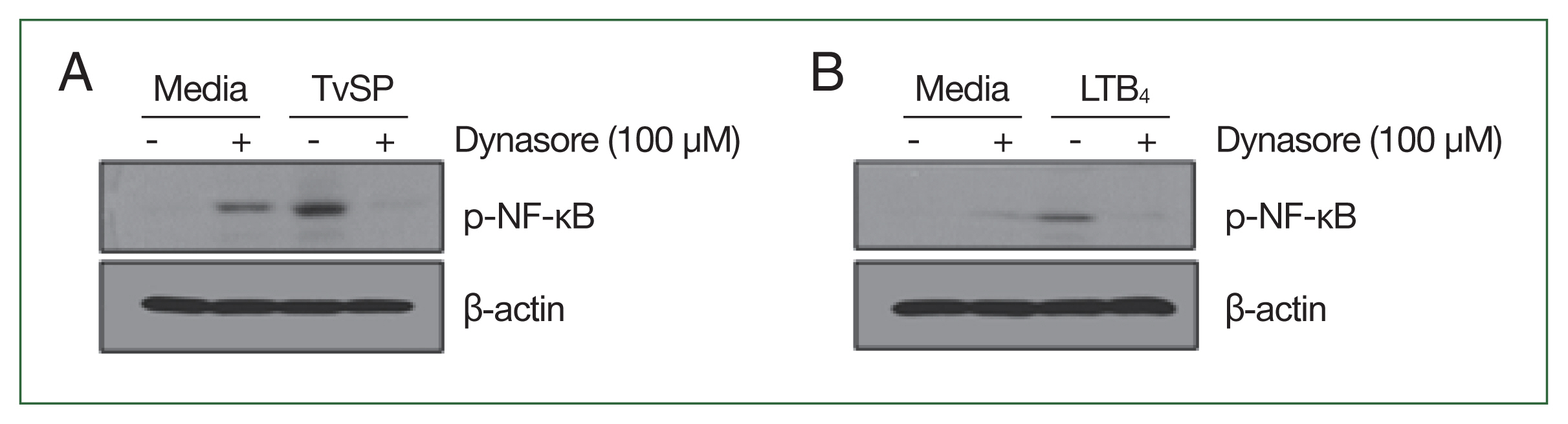
We examined the effects of dynamin-mediated endocytosis on NF-κB phosphorylation in TvSP-stimulated HMC-1 cells. Although dynasore treatment alone slightly increased NF-κB phosphorylation in HMC-1 cells, stimulation with TvSP strongly induced NF-κB phosphorylation (
Fig. 4A). In contrast, TvSP-induced NF-κB phosphorylation was blocked by pretreatment of HMC-1 cells with dynasore (
Fig. 4A). The results of LTB
4 stimulation were consistent with those of TvSP stimulation (
Fig. 4B).
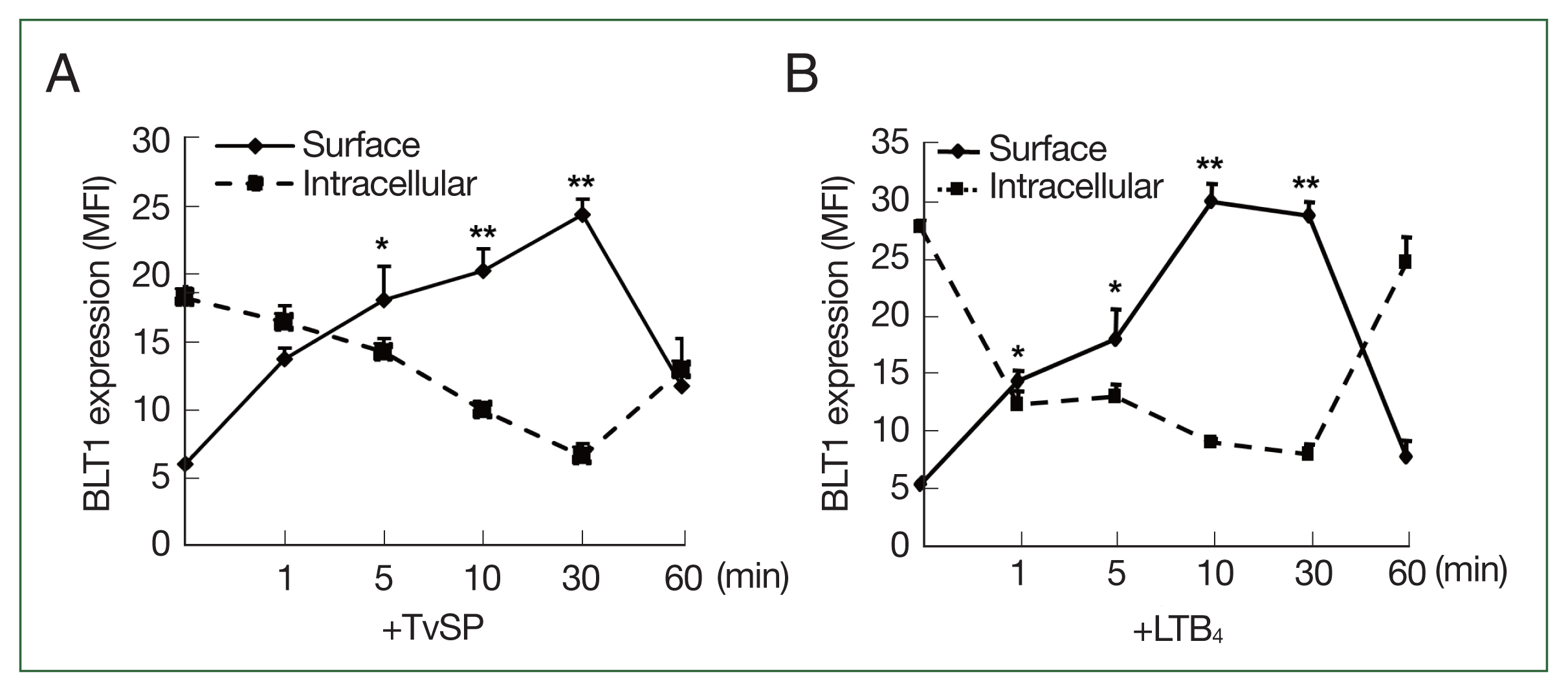
We investigated the kinetics of the surface/intracellular expression of BLT1 in TvSP-stimulated HMC-1 cells for up to 60 min. In resting HMC-1 cells, intracellular BLT1 was more abundantly distributed in the cytosol than on the cell surface. As shown in
Fig. 5A, TvSP treatment gradually increased BLT1 surface expression in HMC-1 cells for up to 10 min. The surface expression of BLT1 persisted for up to 30 min, decreased rapidly after 30 min, and returned to the initial levels before TvSP stimulation at 60 min. In contrast, the intracellular expression of BLT1 was the highest before TvSP stimulation, and its expression level rapidly decreased within 1 min of TvSP stimulation. The intracellular expression of BLT1 gradually decreased until 30 min, and then recovered to its initial expression level at 60 min. In LTB
4-treated HMC-1 cells, BLT1 surface/intracellular expression was similar to that in HMC-1 cells treated with TvSP (
Fig. 5B).
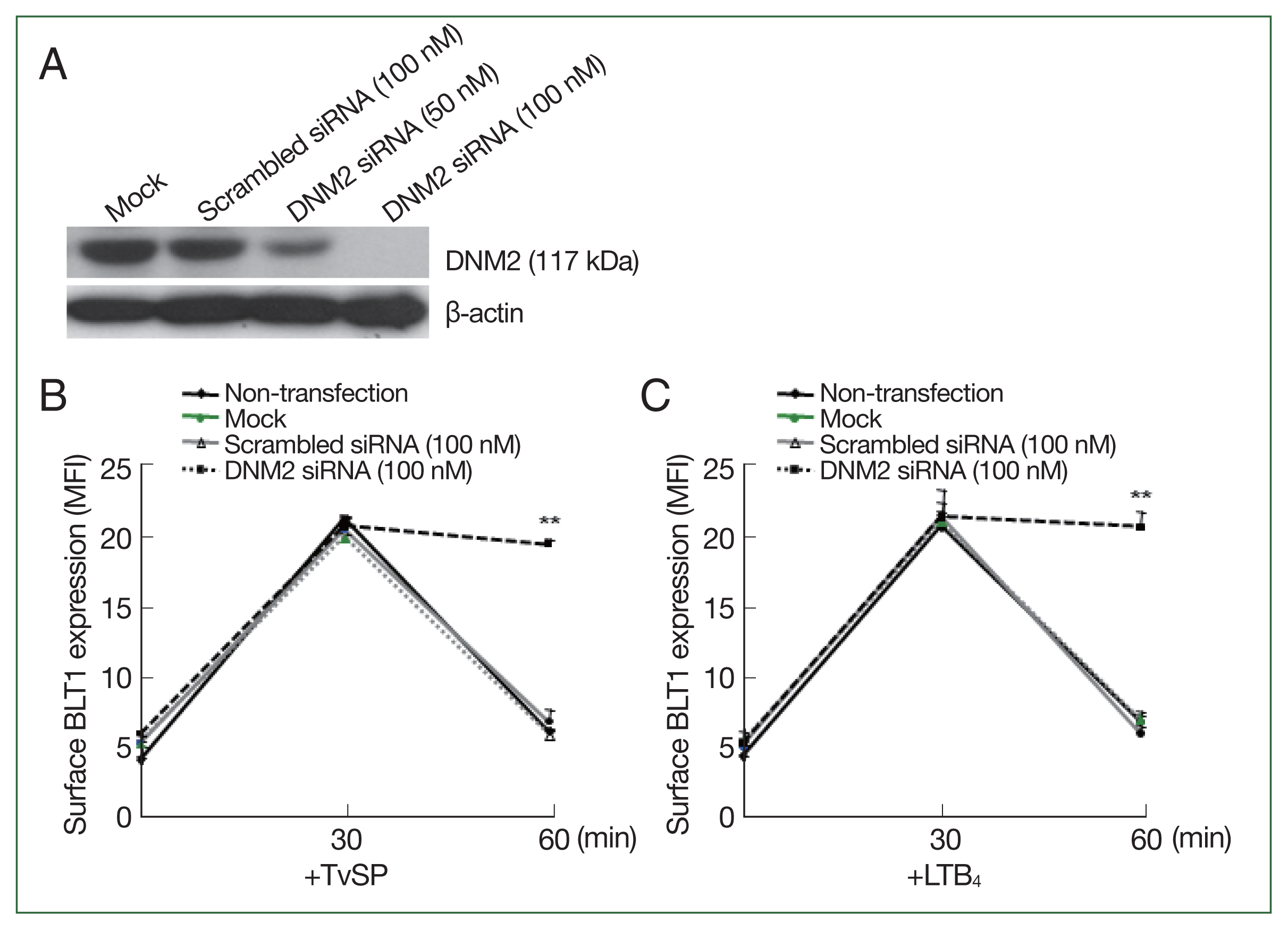
We investigated whether dynamin was directly involved in BLT1 surface trafficking induced by TvSP using dynamin-2 siRNA.
Fig. 6A shows that dynamin-2 knockdown using 100 nM siRNA was successfully. Next, we observed the surface BLT1 expression induced by TvSP in dynamin-2 knocked HMC-1 cells. In HMC-1 cells treated with scrambled siRNA, TvSP-induced BLT1 surface expression was maximal at 30 min; however, at 60 min, BLT1 surface expression rapidly decreased to the expression level before TvSP stimulation. However, TvSP-induced BLT1 surface expression in dynamin-2 knocked HMC-1 cells peaked at 30 min, but remained at the peak level even at 60 min. The results of BLT1 surface expression in HMC-1 cells treated with LTB
4, which was used as a positive control, were similar to those treated with TvSP (
Fig. 6B, C).
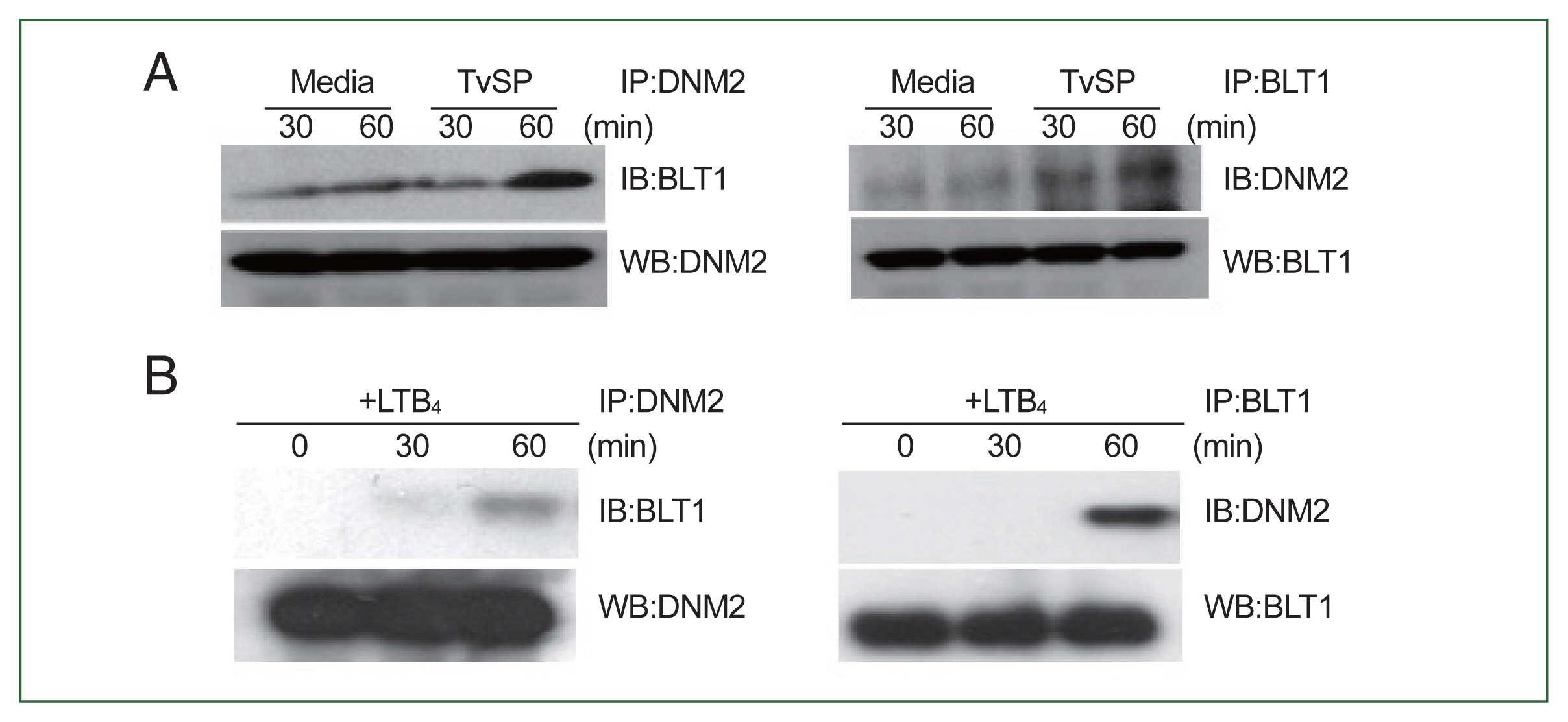
We performed co-IP to investigate the interaction between dynamin-2 and BLT1 in TvSP-stimulated HMC-1 cells. As shown in
Fig. 7A, dynamin-2 interacted weakly with BLT1 in the group not treated with TvSP. However, the interaction between dynamin-2 and BLT1 was strongly induced in HMC-1 cells stimulated with TvSP for 60 min compared to that in cells stimulated with medium alone. Similarly, LTB
4 stimulation for 60 min strongly induced an interaction between dynamin-2 and BLT1 in HMC-1 cells compared to that in cells stimulated with medium alone. However, no interaction between dynamin-2 and BLT1 was observed in the group not treated with LTB
4, or in HMC-1 cells stimulated with LTB
4 for 30 min (
Fig. 7B).
Discussion
In this study, we found that
T. vaginalis-secreted LTB
4 induces IL-8 production in HMC-1 cells through dynamin-2-mediated endocytosis of BLT1 and phosphorylation of NF-κB. We previously reported that various lipid mediators, such as LTB
4 and CysLT, are present in TvSP and that LTB
4 receptor (BLT) are involved in TvSP-induced IL-8 production in HMC-1 cells [
5,
6,
8]. In this study, we also found that inhibition of BLT receptor activity using BLT1 siRNA or BLT2 siRNA significantly reduced TvSP-induced IL-8 production. Disruption of lipid rafts-associated signaling by pretreatment with lipid raft inhibitor MβCD suppressed the IL-8 production and NF-κB activation in TvSP-stimulated HMC-1 cells. Furthermore, blocking of receptor endocytosis process by pretreatment with dynasore suppressed TvSP- or LTB
4-induced IL-8 production. Surface translocated BLT1 by stimulation with TvSP or LTB
4 was internalized at 60 min after stimulation. TvSP- or LTB
4-induced BLT1 internalization was prevented by pretreatment with dynamin-2 siRNA. A strong interaction between dynamin-2 and BLT1 was also observed at 60 min after stimulation with TvSP or LTB
4. These findings suggest that dynamin-2-mediated BLT1 endocytosis plays an important role in the signaling for NF-κB activation and IL-8 production in HMC-1 cells during trichomonad infection in human beings.
Lipid rafts play important roles in trafficking and GPCR signaling for the activation of immune cells [
20,
21]. Cholesterol-rich lipid rafts are abundant in the plasma membrane and contain many receptors that respond to external stimuli. GPCRs and associated signaling molecules, including heterotrimeric G proteins, trafficking proteins, secondary messengers, related kinases, and phosphatases, are mainly distributed in lipid rafts [
22]. Therefore, lipid rafts are essential for GPCR transport and signaling [
20–
22]. For example, lipid raft complexes are critical for LPS-mediated Toll-like receptors 4 activation and GPCR signaling [
21]. Direct binding of FcγRI to the GPCR receptor BLT1 on lipid rafts activates downstream intracellular signaling pathways in murine macrophages [
23]. Our previous study showed that pretreatment with G protein inhibitor pertussis toxin reduced IL-8 production by TvSP in mast cells [
8]. In this study, we showed that TvSP-induced IL-8 production was significantly reduced when lipid rafts in HMC-1 cells were disrupted by MβCD treatment. These results suggest that lipid rafts play an important role in GPCR BLT-mediated signaling during TvSP-induced mast cell activation.
Endosomes and NF-κB activation are intricately linked through multiple mechanisms, particularly in the context of immune signaling and inflammation. For example, many receptors involved in NF-κB activation are internalized via receptor-mediated endocytosis [
23–
25]. These receptors include those for cytokines (such as tumor necrosis factor-α and IL-1β), growth factors, and pattern recognition receptors like Toll-like receptors [
23–
25]. Upon ligand binding, these receptors are internalized by endosomes. Endocytosis and NF-κB signaling are 2 distinct biological processes that can interact in several ways within cells, particularly in the context of immune responses and cellular signaling. Some regulators of NF-κB signaling, such as cytokines and their receptors, are internalized via receptor-mediated endocytosis [
24,
25]. This internalization process can influence NF-κB activation by modulating the availability of these receptors on the cell surface.
In particular, dynamin-mediated endocytosis is critical for GPCR-mediated signaling during immune cell activation [
5,
13,
22]. For example, the inhibition of dynamin-dependent endocytosis interferes with type III IFN expression in bacteria-infected human monocyte-derived DCs [
26]. In addition, dynamin-dependent endocytosis is necessary for thrombin and tumor necrosis factor-α-mediated activation of NOX1-dependent signaling via PI3K/Akt-ATF-1 pathway [
27]. Dynamin-mediated endocytosis of TCR can continue to signal and enable sustained signaling and cell proliferation [
28]. In this study, we showed that dynamin 2 is required for BLT1 internalization in TvSP- or LTB
4-stimulated HMC-1 cells. Treatment of HMC-1 cells with dynamin 2 siRNA blocked BLT1 internalization in HMC-1 cells stimulated with TvSP or LTB
4. This demonstrates that dynamin-2 is involved in the endocytosis of BLT1, which was translocated to the cell membrane from the intracellular area when HMC-1 cells were stimulated with LTB
4 or TvSP. Our results are consistent with the report that BLT1 endocytosis is dynamin-dependent [
29]. After internalization, BLT1 can persist in signaling from endosomal compartments, thereby influencing downstream signaling pathways such as MAPK activation and NF-κB signaling.
The human cells have 2 types of LTB
4 receptor (BLT), BLT1 and BLT2 [
29]. BLT1 (a high-affinity LTB
4 receptor) is known binds only to LTB
4 even in the presence of other lipids [
29]. In contrast, BLT2 (a low-affinity LTB
4 receptor) is known to bind not only to LTB
4 but also to other lipids such as 12(S)-Hydroxyeicosatetraenoic acid (12(S)-HETE), 12(S)-hydroxyeicosatetraenoic acid (12(S)-HPETE), and 15(S)-HETE [
29]. In this study, we investigated the surface expression of BLT1 and BLT2 with or without LTB
4 (
Supplementary Fig. S1). BLT2 was expressed only on the cell surface of HMC-1 cells with or without LTB
4 stimulation (
Supplementary Fig. S1B). However, BLT1 was highly expressed in intracellular area rather than cell surface at resting state. In contrast, surface expression of BLT1 significantly increased upon stimulation with LTB
4 (
Supplementary Fig. S1A). These results suggest that BLT2, which is abundantly expressed on the cell surface during the early stage of exogenous LTB
4 stimulation, may first bind to LTB
4, transduce the signal into the cell, and then amplify the LTB
4 signal through surface trafficking of BLT1. In addition, we examined the kinetics of the surface/intracellular expression of BLT1 in TvSP- or LTB
4-stimulated HMC-1 cells for up to 60 min. In the resting state of HMC-1 cells, BLT1 expression was abundantly distributed in the intracellular areas, but not at the cell surface. In HMC-1 cells stimulated with TvSP or LTB
4 for 10 min, the surface expression of BLT1 increased and peaked 30 min after stimulation. Sixty minutes after stimulation, the surface expression of BLT1 decreased rapidly and returned to the initial level before stimulation. In contrast, the intracellular expression of BLT1 was the highest before stimulation, and the intracellular expression level of BLT1 rapidly decreased within 1 min of stimulation with TvSP or LTB
4. Finally, the intracellular expression of BLT1 gradually decreased until 30 min and then recovered to the initial expression level at 60 min after stimulation. Taken all together, these results suggest that at the initial stage of exogenous LTB
4 stimulation, BLT2 binds to LTB
4 and transduces the signal into the cells. Such signal transduction is likely to subsequently induce surface migration of BLT1 through SNAP23-dependent exocytosis in human mast cells induced by
T. vaginalis-secreted LTB
4 [
30]. After moving to the cell surface, BLT1 binds strongly to LTB
4 and transmits powerful signals inside the cell through dynamin 2-dependent endocytosis of BLT1 during
T. vaginalis infection.
In conclusion, we demonstrated that dynamin 2-dependent BLT1 endocytosis is an important process to induce NF-κB activation for IL-8 production in HMC-1 cells induced by T. vaginalis-derived LTB4. Our study opens new avenues on signal transmission between parasite and host interactions, which provides insight into the pathogenesis of trichomoniasis.
Notes
-
Author contributions
Conceptualization: Lee YA, Shin MH
Formal analysis: Lee YA
Funding acquisition: Lee YA, Shin MH
Investigation: Lee YA
Methodology: Lee YA
Project administration: Shin MH
Software: Shin MH
Supervision: Shin MH
Visualization: Lee YA, Shin MH
Writing – original draft: Lee YA, Shin MH
Writing – review & editing: Lee YA, Shin MH
-
The authors declare no conflict of interest related to this study.
Supplementary Information
Acknowledgment
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (NRF-2020R1I1A1A01064838). Prof. JS Ryu Hanyang University Colleage of Medicine in acknowledged for kind alonation of the T016 strain of T. vaginalis.
Fig. 1BLTs is required for IL-8 secretion in HMC-1 cells induced by TvSP. (A) BLT1 protein expression levels by immunoblotting following silencing of BLT1 gene by siRNA in HMC-1 cells. (B) BLT2 protein levels by immunoblotting following silencing of BLT2 gene by siRNA in HMC-1 cells. At 72 h post-transfection, whole-cell lysate from HMC-1 cells transfected with vehicle alone (Mock), control scrambled siRNA (100 nM) or BLT1 siRNA (100 nM) were subjected to immunoblotting with anti-BLT1, anti-BLT2, or anti-β-actin antibody as loading control. Blots are representative of 3 independent experiments. (C) Effect of BLT1 siRNA transfection on TvSP-induced IL-8 secretion in HMC-1 cells. (D) Effect of BLT2 siRNA on TvSP-induced IL-8 secretion in HMC-1 cells. HMC-1 cells were stimulated for 16 h with or without TvSP. The amount of IL-8 in culture supernatant was measured by human IL-8 ELISA. Data are expressed as the mean±SD from 4 independent experiments. Significant differences from the value obtained with cells incubated with medium alone are shown. LTB4, leukotriene B4; HMC-1, human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; siRNA, short interfering RNA; IL-8, interleukin-8; BLT1, high affinity LTB4 receptor; BLT2, low affinity LTB4 receptor. *P<0.05.

Fig. 2Effects of lipid raft inhibitor (MβCD) on TvSP-induced IL-8 production in HMC-1 cells. HMC-1 cells were stimulated for 16 h with or without TvSP in the presence or absence of MβCD. The amount of IL-8 in culture supernatant was measured by human IL-8 ELISA. Data are expressed as the mean±SD from 4 independent experiments. Significant differences from the value obtained with cells incubated with medium alone are shown. LTB4, leukotriene B4; HMC-1, Human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; IL-8, interleukin-8; MβCD, methyl-β-cyclodextrin. *P<0.05, **P<0.01.

Fig. 3Effects of dynamin inhibitor (Dynasore) on IL-8 production in HMC-1 cells stimulated with TvSP or LTB4. HMC-1 cells were stimulated for 6 (A) or 16 h (B) with or without TvSP in the presence or absence of Dynasore. The amount of IL-8 in culture supernatant was measured by human IL-8 ELISA. Data are expressed as the mean±SD from 4 independent experiments. Significant differences from the values obtained with cells incubated with DMSO in TvSP- or LTB4-treated groups are shown. LTB4, Leukotriene B4; HMC-1, Human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; IL-8, interleukin-8. *P<0.05, **P<0.01.

Fig. 4Effect of Dynasore on NF-κB phosphorylation in HMC-1 cells stimulated with TvSP or LTB4. (A) Effect of Dynasore on phosphorylation of NF-κB in TvSP-stimulated HMC-1 cells. HMC-1 cells were stimulated for 30 min with or without TvSP. (B) Effect of Dynasore on phosphorylation of NF-κB in LTB4-stimulated HMC-1 cells. HMC-1 cells were stimulated for 30 min with or without LTB4. After stimulation, whole cell lysates were subjected to SDS-PAGE and immunoblotted with antibody specific for phospho-NF-κB, or β-actin as a control. The images are representative of 3 independent experiments with similar results. LTB4, leukotriene B4; HMC-1, human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; NF-κB, nuclear factor kappa B.

Fig. 5Kinetics of trafficking of BLT1 in HMC-1 cells stimulated for up to 60 min with TvSP (A) or LTB4 (B). Cells were stained with anti-BLT1 antibody and then measured by Flow cytometry. LTB4 was used as a positive control. Data are presented as the means±SD from 3 independent experiments. LTB4, leukotriene B4; HMC-1, human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; BLT1, LTB4 receptor. *P<0.05; **P<0.01.

Fig. 6Effect of dynamin-2 siRNA transfection on internalization of BLT1 in HMC-1 cells induced by TvSP or LTB4. (A) Dynamin-2 protein levels by immunoblotting following silencing of dynamin-2 gene by siRNA in HMC-1 cells. At 72 h post-transfection, whole-cell lysate from HMC-1 cells transfected with vehicle alone (Mock), control scrambled siRNA (100 nM) or dynamin 2 siRNA (100 nM) were subjected to immunoblotting with anti-BLT1 or anti-β-actin antibody as loading control. Blots are representative of 3 independent experiments. Surface and intracellular expression of BLT1 in HMC-1 cells stimulated for up to 60 min with TvSP (B) or LTB4 (C) cells were stained with anti-BLT1 antibody and then measured by Flow cytometry. LTB4 was used as a positive control. Data are presented as the means±SD from 3 independent experiments. LTB4, Leukotriene B4; HMC-1, Human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; siRNA, short interfering RNA; DNM2, dynamin-2; BLT1, LTB4 receptor. **P<0.01.

Fig. 7Interaction of dynamin-2 with BLT1 in TvSP (A) or LTB4 (B)-stimulated HMC-1 cells. HMC-1 cells (1×107/sample) were stimulated with TvSP or LTB4 for 30 or 60 min. Cells were precipitated with anti-dynamin-2 or BLT1 antibodies for 18 h and then blotted with anti-BLT1 or dynamin-2 antibodies. Interaction of dynamin-2 and BLT1 was evaluated by coimmunoprecipitation. Anti-β-actin antibody was used as loading control. The images are representative of 3 independent experiments with similar results. LTB4, Leukotriene B4; HMC-1, human mast cell line; TvSP, Trichomonas vaginalis-derived secretory products; BLT1, LTB4 receptor; DNM2, dynamin-2; IB, westernblotting after IP; WB, westernblotting in cell lysate.

References
- 1. Ryu JS, Min DY.
Trichomonas vaginalis and trichomoniasis in the Republic of Korea. Korean J Parasitol 2006;44(2):101-116.
https://doi.org/10.3347/kjp.2006.44.2.101
- 2. Han IH, Kim JH, Ryu JS. Inflammatory response to Trichomonas vaginalis in the pathogenesis of prostatitis and benign prostatic hyperplasia. Parasites Hosts Dis 2023;61(1):2-14.
https://doi.org/10.3347/PHD.22160
- 3. Mercer F, Johnson PJ.
Trichomonas vaginalis: pathogenesis, symbiont interactions, and host cell immune responses. Trends Parasitol 2018;34(8):683-693.
https://doi.org/10.1016/j.pt.2018.05.006
- 4. Szempruch AJ, Dennison L, Kieft R, Harrington JM, Hajduk SL. Sending a message: extracellular vesicles of pathogenic protozoan parasites. Nat Rev Microbiol 2016;14(11):669-675.
https://doi.org/10.1038/nrmicro.2016.110
- 5. Preta G, Cronin JG, Sheldon IM. Dynasore - not just a dynamin inhibitor. Cell Commun Signal 2015;13:24.
https://doi.org/10.1186/s12964-015-0102-1
- 6. Kim HS, Kawakami Y, Kasakura K, Kawakami T. Recent advances in mast cell activation and regulation. F1000Res 2020;9:F1000 Faculty Rev-196https://doi.org/10.12688/f1000research.22037.1
- 7. Shaio MF, Lin PR, Lee CS, Hou SC, Tang P, et al. A novel neutrophil-activating factor released by Trichomonas vaginalis
. Infect Immun 1992;60(11):4475-4482.
https://doi.org/10.1128/iai.60.11.4475-4482.1992
- 8. Nam YH, Min D, Kim HP, Song KJ, Kim KA, et al. Leukotriene B4 receptor BLT-mediated phosphorylation of NF-κB and CREB is involved in IL-8 production in human mast cells induced by Trichomonas vaginalis-derived secretory products. Microbes Infect 2011;13(14–15):1211-1220.
https://doi.org/10.1016/j.micinf.2011.07.006
- 9. Min A, Lee YA, Kim KA, Shin MH. BLT1-mediated O-GlcNAcylation is required for NOX2-dependent migration, exocytotic degranulation and IL-8 release of human mast cell induced by Trichomonas vaginalis-secreted LTB(4). Microbes Infect 2018;20(6):376-384.
https://doi.org/10.1016/j.micinf.2018.05.005
- 10. Kobayashi TK, Fujimoto T, Okamoto H, Yuasa M, Sawaragi I. Association of mast cells with vaginal trichomoniasis in endocervical smears. Acta Cytol 1983;27(2):133-137.
- 11. Narantsogt G, Min A, Nam YH, Lee YA, Kim KA, et al. Activation of MAPK is required for ROS generation and exocytosis in HMC-1 cells induced by Trichomonas vaginalis-derived secretory products. Korean J Parasitol 2015;53(5):597-603.
https://doi.org/10.3347/kjp.2015.53.5.597
- 12. Cardamone C, Parente R, Feo GD, Triggiani M. Mast cells as effector cells of innate immunity and regulators of adaptive immunity. Immunol Lett 2016;178:10-14.
https://doi.org/10.1016/j.imlet.2016.07.003
- 13. Calebiro D, Godbole A. Internalization of G-protein-coupled receptors: implication in receptor function, physiology and diseases. Best Pract Res Clin Endocrinol Metab 2018;32(2):83-91.
https://doi.org/10.1016/j.beem.2018.01.004
- 14. Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol 2012;13(2):75-88.
https://doi.org/10.1038/nrm3266
- 15. Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 2004;5(2):133-147.
https://doi.org/10.1038/nrm1313
- 16. Kim YN, Bertics PJ. The endocytosis-linked protein dynamin associates with caveolin-1 and is tyrosine phosphorylated in response to the activation of a noninternalizing epidermal growth factor receptor mutant. Endocrinology 2002;143(5):1726-1731.
https://doi.org/10.1210/endo.143.5.8814
- 17. van Dam EM, Stoorvogel W. Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol Biol Cell 2002;13(1):169-182.
https://doi.org/10.1091/mbc.01-07-0380
- 18. Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem 1999;274(3):1185-1188.
https://doi.org/10.1074/jbc.274.3.1185
- 19. Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol 2010;282:135-163.
https://doi.org/10.1016/S1937-6448(10)82003-9
- 20. Silveira E, Souza AM, Mazucato VM, Jamur MC, Oliver C. Lipid rafts in mast cell biology. J Lipids 2011;2011:752906.
https://doi.org/10.1155/2011/752906
- 21. Varshney P, Yadav V, Saini N. Lipid rafts in immune signalling: current progress and future perspective. Immunology 2016;149(1):13-24.
https://doi.org/10.1111/imm.12617
- 22. Villar VA, Cuevas S, Zheng X, Jose PA. Localization and signaling of GPCRs in lipid rafts. Methods Cell Biol 2016;132:3-23.
https://doi.org/10.1016/bs.mcb.2015.11.008
- 23. Serezani CH, Aronoff DM, Sitrin RG, Peters-Golden M. FcgammaRI ligation leads to a complex with BLT1 in lipid rafts that enhances rat lung macrophage antimicrobial functions. Blood 2009;114(15):3316-3324.
https://doi.org/10.1182/blood-2009-01-199919
- 24. Hermanns HM, Wohlfahrt J, Mais C, Hergovits S, Jahn D, et al. Endocytosis of pro-inflammatory cytokine receptors and its relevance for signal transduction. Biol Chem 2016;397(8):695-708.
https://doi.org/10.1515/hsz-2015-0277
- 25. Zhao Q, Qian Y, Li R, Tan B, Han H, et al. Norcantharidin facilitates LPS-mediated immune responses by up-regulation of AKT/NF-kappaB signaling in macrophages. PLoS One 2012;7(9):e44956.
https://doi.org/10.1371/journal.pone.0044956
- 26. Pietilä TE, Latvala S, Osterlund P, Julkunen I. Inhibition of dynamin-dependent endocytosis interferes with type III IFN expression in bacteria-infected human monocyte-derived DCs. J Leukoc Biol 2010;88(4):665-674.
https://doi.org/10.1189/jlb.1009651
- 27. Miller FJ Jr, Chu X, Stanic B, Tian X, Sharma RV, et al. A differential role for endocytosis in receptor-mediated activation of Nox1. Antioxid Redox Signal 2010;12(5):583-593.
https://doi.org/10.1089/ars.2009.2857
- 28. Willinger T, Staron M, Ferguson SM, De Camilli P, Flavell RA. Dynamin 2-dependent endocytosis sustains T-cell receptor signaling and drives metabolic reprogramming in T lymphocytes. Proc Natl Acad Sci U S A 2015;112(14):4423-4428.
https://doi.org/10.1073/pnas.1504279112
- 29. Subramanian BC, Majumdar R, Parent CA. The role of the LTB(4)-BLT1 axis in chemotactic gradient sensing and directed leukocyte migration. Semin Immunol 2017;33:16-29.
https://doi.org/10.1016/j.smim.2017.07.002
- 30. Min A, Lee YA, Kim KA, El-Benna J, Shin MH. SNAP23-dependent surface translocation of leukotriene B4 (LTB4) receptor 1 is essential for NOX2-mediated exocytotic degranulation in human mast cells induced by Trichomonas vaginalis-secreted LTB4
. Infect Immun 2016;85(1):e00526-16.
https://doi.org/10.1128/IAI.00526-16