Abstract
The furcocercus cercariae of Neodiplostomum seoulense (Digenea: Neodiplostomidae) penetrate the skins of tadpoles and shed their tails. The speculated mechanism of this tail loss was physical efforts required to produce a vigorous zigzag motion during skin penetration; no other mechanism has been proposed. We examined the relationship between the host serum and cercarial tail loss. Cercariae of N. seoulense were collected from experimentally infected Segmentina hemisphaerula, and lots of 300 cercariae were cultured in medium 199 contained several types of sera. Cercarial tail degradation was induced in all media, but all the cercariae cultured except those cultured in media containing fetal bovine serum (FBS) died within 48 hr. After 72 hr cultivation in media containing FBS, cercarial tail degradation was induced in 67.0%; in continuous cultivation 13.3% of larvae survived for 7 days. Tail degradation did not occur in the absence of serum and when serum was heat inactivated at 56℃ for 30 min. The addition of 20 mM ethylenediaminetetraacetic acid (EDTA) blocked cercarial tail degradation completely. Moreover, the addition of 20 mM MgCl2 restored tail degradation blocked by EDTA. These results suggest that the alternative complement pathway is related with the N. seoulense cercarial tail degradation induced by serum.
-
Key words: Neodiplostomum seoulense, cercaria, tail degradation, complement, serum, EDTA
INTRODUCTION
The life cycle of
Neodiplostomum seoulense Seo, Rim and Lee, 1964 (Digenea: Diplostomatidae) had been clarified by several laboratory and field based studies, and
Hippeutis cantori,
Segmentina hemisphaerula and
Austropeplea ollula were identified as its first intermediate hosts (
Seo et al., 1988;
Chung et al., 1996,
2002). It was reported that the tadpoles of
Rana nigromaculata serve as the only second intermediate host (
Hong et al., 1982,
1985).
In previous studies (
Seo et al., 1988),
N. seoulense cercariae from snails were observed to attach to the skins of tadpoles using their suckers, and then to invade into the tadpole abdominal cavity. Moreover, cercarial tail loss was observed after penetration, but the only suggested mechanism of the tail loss concerned the physical effort required for the penetrating cercariae to produce a vigorous zigzag motion. No other mechanism related to this tail loss and the fate of detached tails has been proposed. In the process of cercarial invasion and metacercarial development, shedding of the cercarial tail is not merely throwing away a useless body portion, but is regarded as an important step related with various physiological signals (
Fried and Graczyk, 1997). Thus, we studied the processes and mechanisms of the cercarial tail loss in
N. seoulense.
We noted that this penetration process is similar to that of
Schistosoma species. The processes and mechanisms of their cercarial tail loss had been clarified by in vitro cercarial cultivation. In the case of schistosomes, the physical force produced by penetrating cercariae (
Stirewalt, 1974) and the host's complement system (
Eveland and Morse, 1975;
Yasuraoka et al., 1978;
Greenblatt et al., 1979) were revealed as 2 important factors for tail loss. In the present work, we cultured
N. seoulense cercariae in media containing several kinds of sera to induce tail loss, and examined whether the complement system is involved in this tail shedding process.
MATERIALS AND METHODS
Collection of cercariae
The miracidia of
N. seoulense were obtained from eggs as described by earlier studies (
Chung et al., 1996,
2002). Each laboratory-reared
Segmentina hemisphaerula snail was exposed to 10-20 miracidia in a 24-well plastic plate (SPL Lifesciences, Pocheon, Korea) containing aerated tap water. After 2 weeks, the cercarial release was examined in infected snails at 700 lux illumination. Cercariae were first detected from the 19
th to the 32
th day after miracidial challenge. Cercariae were washed 5 times with sterile distilled water containing 100 units/ml of penicillin and 100 µg/ml of streptomycin (Sigma Co., St. Louis, Missouri, USA), collected (
Fig. 1A), and promptly transferred to culture media for in vitro cultivation.
Medium 199 was used as the basic culture medium. In our preliminary studies, this medium was confirmed to be the most suitable medium for this purpose (data not shown). Penicillin (100 units/ml) and streptomycin (100 µg/ml) were added to medium to prevent contamination. All cultivation reagents were purchased from Sigma Co. A 5.4 ml volume of medium 199 was placed in each well of a 6-well culture plate (SPL Lifesciences) for in vitro cultivation, and a total of 100 cercariae of N. seoulense were added to each well, followed by 0.6 ml (10% v/v) of serum, i.e., FBS, horse serum, chicken serum, human serum, or African clawed frog (Xenopus laevis) serum. FBS, horse serum, and chicken serum were purchased from Sigma Co., human serum and African clawed frog serum from a normal healthy man and a laboratory-bred adult female frog. Cultivation was performed at 30 ± 1℃ under 5% CO2, and the media were changed daily. Cultivations were continued until all cercariae died, and all cultivations were repeated twice. Morphologic changes in cercariae were examined during culture under an inverted microscope.
Blockage of the complement pathways
Serum was preheated at 56℃ for 30 min to inactivate complement. The chelator EDTA was used to block the classical and alternative complement pathways, and another chelator ethylene glycol-bis (β-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) to block the classical complement pathway (
Nelson et al., 2000). These agents were prepared as 200 mM stock solutions and stored at 4℃ until use (
Fine et al., 1972). To block the complement pathways, stock solutions were added to medium to a final concentration of 20 mM. MgCl
2 solution was used to restore the classical and alternative pathways blocked by EDTA. To evaluate the complement pathways related to cercarial tail loss, 50
N. seoulense cercariae were cultured in medium 199 alone, or in medium 199 containing heat inactivated serum, 20 mM EDTA added serum, 20 mM EGTA added serum, or 20 mM EDTA plus 20 mM MgCl
2 added serum. All cultures were repeated twice.
RESULTS
Cercarial tail degradation induced by sera
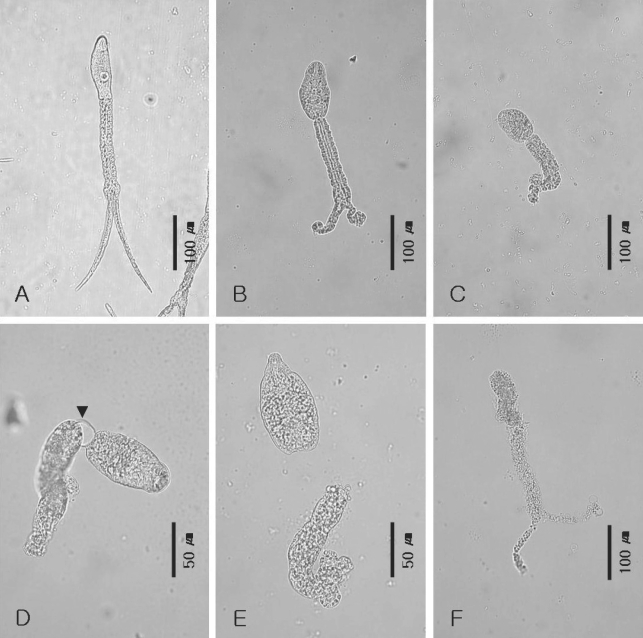
Cercariae added to culture media (
Fig. 1A) remained motionless for 30 min, and then slowly recovered from 30 min to 1 hr after being added to media. In all media added 10 % sera, rolling up of cercarial tail tips (
Fig. 1B) was observed between 1 and 2 hr after addition to media, and cercariae were observed to simultaneously show a vigorous shaking movement. From about 3 hr, tail degradation (
Fig. 1C) was evident in some cercariae, in combination with a continuous shaking movement. However, all cercariae died within 24 hr in media containing chicken serum, human serum, and African clawed frog serum, and within 48 hr in media containing horse serum. In fact, they only survived in media containing FBS, in which separation of the cercarial body and degraded tail was clearly observed from about 4 hr to 72 hr. The tail degradation and separation times tended to vary for each cercaria, but the sequence adopted was identical for all, as follows; initially, the bifurcated tail tips of cercariae rolled up and degraded from their terminal portions (
Fig. 1B), followed by tail stem degradation (
Fig. 1C), the formation of a bubble between the cercarial body and tail stem (
Fig. 1D), and finally cercarial body and degraded tail separation (
Fig. 1E). The survival rate of the worms in media containing FBS was 86.3% at 72 hr, when the separation of body and tail occurred in 19.3% of the cultured 300 cercariae and 67.0 % were alive with a degraded tail. Some cercariae died during tail degradation (
Fig. 1F). After 3 days, further separation of cecarial bodies and tails ceased, and worm death accelerated. In total 13.3% of 300 cercariae survived until day 7, but all were dead at day 12.
Cercariae cultured in medium 199 showed no changes, all died within 5 days. For cercariae in medium 199 containing 10% heat inactivated FBS and the medium 199 containing 10% FBS plus 20 mM EDTA, rolling up changes of bifurcated tail terminals were observed in some cercariae on day 3, but no tail degradation was observed. The addition of 20 mM EGTA caused 14.2% of the cultured 155 cercariae to lose their tails on day 3, when 45.8% remained alive with a degraded tail. In 10% FBS contained 20 mM EDTA plus 20 mM MgCl2, 12.8% of the cultured 149 cercariae had lost their tails on day 3 and 36.2% remained alive with a degraded tail.
DISCUSSION
The metacercarial stage of
N. seoulense has no cystic wall, and thus this type of metacercarial larva is called a mesocercaria. For the mesocercarial development, the cercariae of
N. seoulense use the tadpole of
R. nigromaculata as a second intermediate host in their natural life cycle. In earlier experimental infection studies, cercarial tail loss was observed in the tadpole (
Seo et al., 1988), but the mechanism of this tail loss remains unknown. In our experiment using
N. seoulense cercariae, cercarial tail degradation was found to be induced in media containing sera, and this occurred in combination with shaking movements by the cercariae,and these phenomena continued until cercarial tail loss. Thus, the active movements of cercariae and tadpole serum are considered as important aspects of the mechanism that leads to
N. seoulense tail loss.
Our complement blocking study revealed that the alternative complement pathway is related to serum-induced
N. seoulense cercarial tail loss; similar results have been reported in human
Schistosoma species (
Eveland and Morse, 1975;
Yasuraoka et al., 1978;
Greenblatt et al., 1979). In schistosomes, C3-depleted sera or one of the terminal components C5 to C8 was used to confirm the above fact, but we adopted a simplified method using chelators (
Nelson et al., 2000). In
S. mansoni, the glycocalyx on the cercarial surface is known to be a strong activator of the alternative complement pathway (
Samuelson and Caulfield, 1986), and in
N. seoulense, similar mechanism seems to be related to complement-mediated cercarial tail degradation.
Several types of sera were tested in the present study, but only FBS induced complete cercarial tail loss and its prolonged survival. Serum is a mixture of various nutrients, minerals, hormones, and complements, and its content is different in various animals (
Baker et al., 1988;
Freshney, 1993). So its effect in parasite's culture could be different according to its content. In our preliminary studies, we tested various combinations of media, sera, and other supplements, and medium 199 plus 10% FBS was found to be best (data not shown). Biologically, tadpole serum is regarded as the most favorable agent for inducing cercarial tail loss, but its collection is practically almost impossible. Thus, we regard our cultivation system as a useful research tool for developmental studies on
N. seoulense mesocercariae.
According to an earlier report, mesocercariae older than 14 days can infect the final host (
Lee et al., 1986). Unfortunately, our cultivations were terminated on day 12, and thus, we could not confirm the infectivity of cultured mesocercariae. However, the mesocercarial sizes and shapes of 7 days of cultured were similar to those of mesocercariae used to infected tadpoles. It is hoped that future attempts to prolong their survival in culture will allow them to complete their life cycle in vitro.
References
Fig. 1Process of N. seoulense cercarial tail loss in medium 199 containing 10% fetal bovine serum. A. A cercaria released from an experimentally infected Segmentina hemishaerula (× 200). B. Rolling up change and degradation of bifurcated tail terminals (× 200). C. Degradation of tail stem and furcae (× 200), D. Bubble (arrow head) appearance between cercarial body and tail stem (× 400). E. Complete separation of body and degraded tail (× 400). F. Dead cercaria during cultivation (× 200).