Abstract
As a preliminary study for the explanation of pathobiology of Neodiplostomum seoulense infection, a 54 kDa protease was purified from the crude extract of adult worms by sequential chromatographic methods. The crude extract was subjected to DEAE-Sepharose Fast Flow column, and protein was eluted using 25 mM Tris-HCl (pH 7.4) containing 0.05, 0.1, 0.2 and 0.4 M NaCl in stepwise elution. The 0.2 M NaCl fraction was further purified by Q-Sepharose chromatography and protein was eluted using 20 mM sodium acetate (pH 6.4) containing 0.05, 0.1, 0.2 and 0.3 M NaCl, respectively. The 0.1M NaCl fraction showed a single protein band on SDS-PAGE carried out on a 7.5-15% gradient gel. The proteolytic activities of the purified enzyme were specifically inhibited by L-trans-epoxy-succinylleucylamide (4-guanidino) butane (E-64) and iodoacetic acid. The enzyme, cysteine protease, showed the maximum proteolytic activity at pH 6.0 in 0.1 M buffer, and degraded extracellular matrix proteins such as collagen and fibronectin with different activities. It is suggested that the cysteine protease may play a role in the nutrient uptake of N. seoulense from the host intestine.
-
Key words: Neodiplostomum seoulense, cysteine protease, pathobiology, nutrition
INTRODUCTION
Neodiplostomum seoulense is an intestinal trematode which can infect humans by the ingestion of raw or improperly cooked snakes or frogs (
Seo, 1990). Infected persons may experience gastrointestinal symptoms such as epigastric discomfort or pain, and diarrhea (
Seo et al., 1982). In mice heavily infected with 1,000 metacercariae of
N. seoulense, severe clinical course was produced including severe diarrhea, malnutrition, marked weight loss, intestinal bleeding, and finally a high mortality of mice (
Huh et al., 1988). Various strains of mice have been shown to be killed by
N. seoulense infection due to irreversible damage to their intestine; even a small number, 25 metacercariae, induced a fatal outcome in C3H/HeJ mice (
Kook et al., 1998). Worms were found to entrap the villi with the tribocytic organ and ventral curvature of the forebody, producing characteristic pathological changes with villous atrophy and crypt hyperplasia, especially in the duodenum (
Huh et al., 1988). The destruction of the villi has been suggested as the outcome of mechanical or biochemical actions of
N. seoulense (
Huh and Song, 1993).
The tribocytic organ of
N. seoulense has been considered to have lytic activities on the host tissues (
Huh et al., 1988,
1990), the secretions of which may include several kinds of proteases. Parasitic proteases are known to play important roles in parasite survival in the host by facilitation of invasion and migration through the host tissue, nutritional uptake, and evasion from host immune responses (
McKerrow, 1989). As a preliminary study for the elucidation of possible roles of proteases in the pathogenesis of
N. seoulense infection, the present study was performed to purify a protease from the crude extract of adult worms and to observe its proteolytic activities against macromolecules.
MATERIALS AND METHODS
Preparation of the crude extract
Metacercariae of
N. seoulense were collected from peptic digestion of the viscera of the snake,
Rhabdophis tigrinus tigrinus. Forty Sprague-Dawley rats, 150 g, were fed orally with 500 metacercariae using a gavage needle, and killed 2 weeks after infection. In order to harvest as many adult worms as possible, rats were immunosuppressed by the intramuscular injection of methylprednisolone, a single dose of 25 mg/kg, once a week. Adult flukes were recovered from the small intestine of rats, and homogenized in 0.1 M phosphate buffered saline (pH 7.4) after washing three times. The supernatant was regarded as the crude extract after centrifugation at 20,000
g for 1 hr. All procedures for enzyme purification were carried out at 4℃, unless otherwise specified. The protein content was measured by the method of Lowry et al. (
1951).
The most susceptible substrate of the crude extract was screened using five fluorogenic substrates; carboxybenzoyl-phenylalanyl-arginyl-7-amino-4-methylcoumarin (CBZ-phe-arg-AMC), carboxybenzoyl-arginyl-arginyl-7-amino-4-methylcoumarin (CBZ-arg-arg-AMC), carboxybenzoyl-alanyl-alanyl-prolyl-phenylalanyl-7-amino-4-methylcoumarin (CBZ-ala-ala-pro-phe-AMC), N-succinyl-alanyl-alanyl-alanyl-7-amino-4-methylcoumarin (Suc-ala-ala-ala-AMC), and glycyl-prolyl-leucyl-glycyl-prolyl-7-amino-4-methylcoumarin (Gly-pro-leu-gly-pro-AMC) (Sigma, St. Louis, USA).
The reaction mixtures comprised 0.44 ml of buffer, 20 µl enzyme solution, 20 µl dithiothreitol (DTT), and 20 µl substrate. After incubation for 1 hr at 37℃, the reaction was stopped by the addition of 10 µl iodoacetic acid (IAA) and 0.4 ml 7.2% ZnSO4. The enzyme activity was assayed by measuring the released AMC using a DNA fluorometer TKO 100 (excitation wavelength=380 nm, emission wavelength=460 nm) (Hoefer, San Francisco, USA). One unit of the enzyme activity was expressed as the amount that released 1 µM AMC for 1 hr.
Purification of the enzyme
The crude extract of N. seoulense adults was applied to a DEAE-Sepharose Fast Flow column (Pharmacia, Piscataway, USA), 1.6×4 cm long, pre-equilibrated with 25 mM Tris-HCl (pH 7.4). Elution of proteins was carried out with the same buffer containing 0, 0.05, 0.1, 0.2 or 0.4 M NaCl at a flow rate of 50 ml/hr. Fractions with high enzyme activities were pooled, dialysed, lyophilized, and reconstituted with 0.5 ml sodium acetate buffer (20 mM, pH 6.4) for further purification.
A Q-Sepharose column (Pharmacia), 1.6×4 cm long, was pre-equilibrated with 20 mM sodium acetate (pH 6.4) and used for final purification of the enzyme. A total of 0.45 ml of enzyme solution was eluted with starting buffer containing 0, 0.05, 0.1, 0.2 or 0.3 M NaCl in a stepwise fashion. Active fractions showing a single protein band, revealed by SDS-PAGE using a 7.5-15% separating gel, were lyophilized, and reconstituted with 0.2 ml sodium acetate (20 mM, pH 6.4). The standard molecular weight markers used were phosphorylase B (94 kDa), albumin (67), ovalbumin (43), carbonic anhydrase (30), trypsin inhibitor (20.1), and α- lactalbumin (14.4) (Pharmacia).
Biochemical characteristics
Twenty microliters of the purified enzyme was incubated in 0.05, 0.1, 0.2, 0.3, 0.4, and 0.5 M sodium acetate (420 µl each, pH 5.0) for 3 hr at 37℃. The optimal pH was also determined in sodium acetate (0.1 M) at pH 4, 4.5, 5, and 5.5, in sodium phosphate (0.1 M) at pH 6, 6.5, 7, and 7.5, and in Tris-HCl (0.1 M) at pH 8.0, respectively.
To observe the modulation effect on enzyme activity, 10 µl of enzyme was reacted with various effectors for 20 min at room temperature, and then 20 µl DTT and 10 µl substrate were added to the mixture. Tested effectors were DTT (5 mM), L-trans-epoxysuccinylleucylamido (4-guanidino) butane (E-64, 0.01 mM), IAA (1 mM), leupeptin (0.1 mM), 4-(amidinophenyl) methanesulfonyl fluoride (APMSF, 0.1 mM), aprotinin (10 µg/ml), ethylene diamine tetraacetate (EDTA, 2 mM), and 1,10-phenanthroline (0.1 mM) (Sigma).
Cleaving activities of the enzyme
Degradation of the enzyme was determined using three kinds of macromolecular substrates; acid soluble calf skin collagen (type I, Boehringer Mannheim Biochemicals, Germany), hemoglobin (Sigma), and fibronectin isolated from human plasma. Five duplicate reaction mixtures were composed of 20 µl of diluted enzyme (8 µg of protein), and 5 µl of 20 mM sodium acetate (pH 6.4), making a total volume of 100 µl, which included macromolecules for proteolysis. The amounts of collagen, fibronectin, and hemoglobin added to the mixtures were 20 µl (80 µg), 35 µl (70 µg), and 20 µl (100 µg), respectively. After incubation of the mixtures for 1, 3, 5, and 12 hr at 37℃, digestive products were visualized by SDS-PAGE.
RESULTS
Although a similar pattern of activity was observed against other two substrates, Suc-ala-ala-ala-AMC and Gly-pro-leu-gly-pro-AMC, the crude extract of
N. seoulense adult worms showed the highest enzyme activity when CBZ-phe-arg-AMC was used as a substrate, (
Table 1). In order to facilitate and make the enzyme purification more simple, the present study was carried out using CBZ-phe-arg-AMC as a substrate which is the most susceptible to the proteolytic activity of the crude extract.
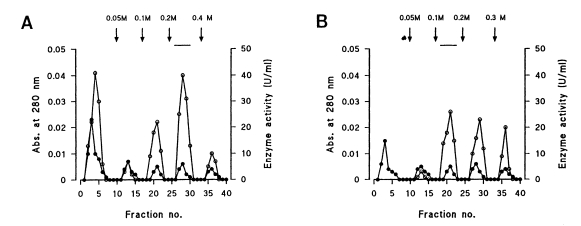
Five protein peaks were obtained by applying the crude extract to a DEAE-Sepharose Fast Flow column, and each protein peak revealed varying degrees of enzyme activities (
Fig. 1A). Among active peaks eluted by application of salt gradient, the 0.2 M NaCl fraction showed the highest proteolytic activity. The protein of interest was further purified by Q-Sepharose anion exchange chromatography, and four protein peaks were eluted after challenging the NaCl solution in stepped increments (
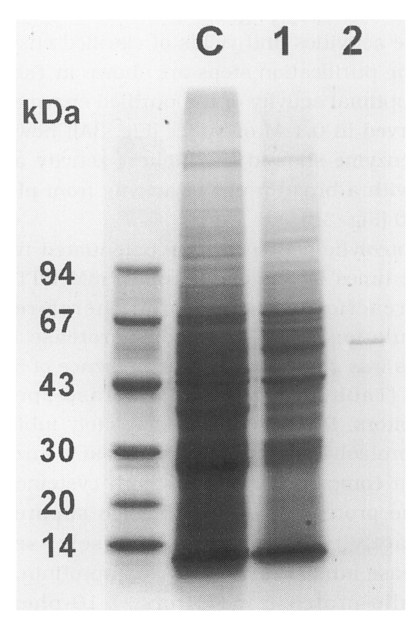
Fig. 1B). Among them, the 0.1 M NaCl fraction exhibited the highest enzyme activity. SDS-PAGE analysis for proteins in each purification step revealed a single band with a molecular weight of 54 kDa in the 0.1 M NaCl fraction in Q-Sepharose chromatography (
Fig. 2).
The activities and yields of clarified enzymes during purification steps are shown in
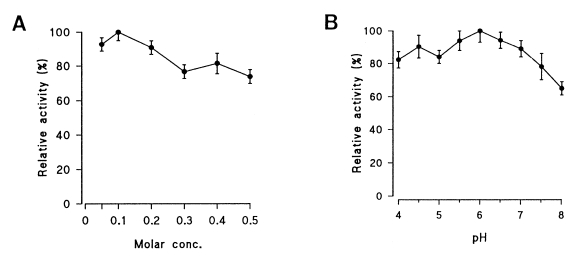
Table 2. The optimal activity of the purified enzyme was observed in 0.1 M of buffer (
Fig. 3A); however, the enzyme showed the highest activity at pH 6.0 with a broad range of activity from pH 4.0 to 7.0 (
Fig. 3B).
Proteolytic activities were potentiated two to three times by the addition of 5 mM DTT into the reaction mixtures, and therefore the modulation effect of various protease inhibitors was determined in the presence of 5 mM DTT (
Table 3). Cysteine protease specific inhibitors, E-64 and IAA, completely inhibited the proteolytic activity of the 54 kDa enzyme, and a common inhibitor of both cysteine and serine proteases, leupeptin, also suppressed the activity (
Table 3). Conversely, serine protease inhibitors, APMSF and aprotinin, and metallo-protease inhibitors, 1,10-phenanthroline and EDTA, showed no effect on the proteolytic activity at all.
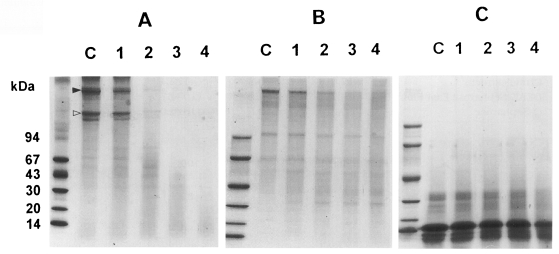
Degradation of macromolecules by the 54 kDa enzyme was observed by incubation of reaction mixtures for 1, 3, 5, and 12 hr. The enzyme began to digest collagen after 1 hr of incubation, producing degradation products. As the incubation time increased, the enzyme cleaved collagen more vigorously, and the band density gradually decreased. When incubated for 12 hr, α- and β-chains of collagen were completely hydrolysed (
Fig. 4A). The protease also digested fibronectin with less proteolytic activity compared to that against collagen, and digestive products were still observed even after 12 hr of incubation (
Fig. 4B). In the case of hemoglobin, the cleaving activities of the enzyme were negligible (
Fig. 4C).
DISCUSSION
In this study, a 54 kDa protease was finally purified from the crude extract of N. seoulense adults by sequential separation methods; DEAE-Sepharose Fast Flow and Q-Sepharose anion exchange chromatographies. The purified enzyme was most active at pH 6.0 with 0.1 M sodium acetate and its activity was greatly enhanced by the addition of 5 mM DTT. The enzyme was determined to be an acidic cysteine protease because of its cathepsin L-like activity of degrading CBZ-phe-arg-AMC, an acid pH optimum, and specific inhibition by cysteine protease inhibitors, E-64 and IAA.
Cysteine proteases of parasites have been suggested to have an extracorporeal function in the digestion of host tissues (
Rhoads and Fetterer, 1997). Maturing schistosomula and adult schistosomes degrade hemoglobin using cysteine proteases for maintenance of their viability and egg production in the host (
McKerrow and Doenhoff, 1988). A malarial cysteine protease of 28 kDa has a critical role in hemoglobin degradation within the food vacuole of
Plasmodium falciparum (
Rosenthal et al., 1988). In the present study, cysteine protease of
N. seoulense degraded extracellular matrix proteins such as type I collagen and fibronectin with different cleaving activities. The results were in agreement with proteolysis observed in cysteine proteases of
Gymnophalloides seoi, a new human intestinal trematode transmitted by raw oysters (
Choi et al., 1998a,
1998b). Therefore, the 54 kDa cysteine protease of
N. seoulense may play a significant role in the nutrient uptake by degrading host tissue proteins.
Cysteine proteases have also been known to be involved in the pathogenesis or virulence of parasites. Amoeba cysteine proteases are responsible for host tissue destruction of
Entamoeba histolytica (
Tannich, 1998). The two cysteine protease genes of
E. histolytica, EhCP1 and EhCP5, are lacking in
E. dispar, a nonpathogenic amoeba which is morphologically indistinguishable with
E. histolytica (
Bruchhaus et al., 1996). Tissue damage associated with
Naegleria fowleri has been shown to be closely related to released cysteine proteases (
Aldape et al., 1994). The role of a 54 kDa cysteine protease of
N. seoulense in the pathogenicity was not confirmed in the present study; thus, it should be evaluated further.
Excretory-secretory antigen (ESA) of N. seoulense showed proteolytic activity against CBZ-phe-arg-AMC, and the protein band of 54 kDa was identified in ESA by SDS-PAGE analysis (data not shown). We tried to purifiy the 54 kDa protein from ESA, however, unfortunately failed due to the difficulty in the preparation of large quantities of ESA. However, it suggests that the 54 kDa cysteine protease of N. seoulense may be secreted into the environment of the worm, and may play important roles in parasite survival and pathogenesis. Therefore, it is highly needed to purifiy the 54 kDa protein from ESA and compare its activity to that of the cysteine protease purified in the present study in order to explain the function of the protease accurately.
At present, the localization of the cysteine protease secretion in
N. seoulense is not clear, but the most possible organs for its secretion are the intestine or the tribocytic organ. The tribocytic organ of
N. seoulense has been regarded as the organ for absorptive and secretory functions, and is thought to be important in evoking symptoms and pathological lesions in the final host (
Huh et al., 1990). Activities of alkaline phosphatase, acid phosphatase, and non-specific esterase have been observed on the surface and excretes of the tribocytic organ by enzyme histochemistry (
Huh et al., 1990;
Huh, 1993). Transmission electron microscopic observations revealed type III cells with many electron dense granules, which were regarded as glandular cells specific to the tribocytic organ (
Huh and Song, 1993). Immunohistochemical localization of the protease secretion in
N. seoulense needs to be further elucidated.
A number of enzymes of different families or having different molecular weights has been described from various parasites.
Entamoeba histolytica secretes several cysteine proteases with relative molecular weights ranging from 96 to 16 kDa (
Montfort et al., 1994).
Paragonimus westermani secretes a number of cysteine proteases, the activities of which change according to the maturation of the worm (
Chung et al., 1997). In the present study, protein peaks obtained during the purification of the adult cysteine protease showed variable degrees of enzyme activities against CBZ-phe-arg-MNA. Therefore,
N. seoulense adults may have several cysteine proteases, probably having different molecular weights, the purification and characterization of which will provide more information for the understanding of host-parasite relationships.
The crude extract of N. seoulense adult worms in the present study showed proteolytic activities against fluorogenic substrates such as Suc-ala-ala-ala-AMC and Gly-pro-leu-gly-pro-AMC as well as CBZ-phe-arg-AMC. This result indicates that there are several kinds of proteases other than cysteine proteases in the crude extract of N. seoulense. More extensive enzymatic study is necessary for the understanding and explanation of the pathogenesis in N. seoulense infection in terms of biochemical aspects.
ACKNOWLEDGEMENTS
We express our sincere gratitude to Dr. Young-Bae Chung, Department of Parasitology, Seoul National University College of Medicine, for his helpful advice in the enzyme characterization. We would also recognize Miss Yeon Hee Lim, Department of Parasitology, Seoul National University College of Medicine, for her technical assistance in the enzyme purification.
References
- 1. Aldape K, Huizinga H, Bouvier J, McKerrow J. Naegleria fowleri: Characterization of a secreted histolytic cysteine proteinase. Exp Parasitol 1994;78:230-241.
- 2. Bruchhaus I, Jacobs T, Leippe M, Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol Microbiol 1996;22:255-263.
- 3. Choi MH, Chai JY, Lee SH. Purification and characterization of a 16-kDa cysteine proteinase of Gymnophalloides seoi (Gymnophallidae) metacercariae. J Parasitol 1998a;84:350-355.
- 4. Choi MH, Park WJ, Park YK, Chai JY, Lee SH. Isolation and characterization of a 40 kDa cysteine protease from Gymnophalloides seoi adult worms. Korean J Parasitol 1998b;36:133-141.
- 5. Chung YB, Kong Y, Yang HJ, Kang SY, Cho SY. Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol 1997;83:902-907.
- 6. Huh S. Activities of acid phosphatase and non-specific esterase are present in the tribocytic organ and the caecum of Fibricola seoulensis. Korean J Parasitol 1993;31:165-167.
- 7. Huh S, Chai JY, Hong ST, Lee SH. Clinical and histopathological findings in mice heavily infected with Fibricola seoulensis. Korean J Parasitol 1988;26:45-53.
- 8. Huh S, Lee SH, Seo BS. Histochemical findings of the tribocytic organ and tegument of Fibricola seoulensis. Korean J Parasitol 1990;28:155-160.
- 9. Huh S, Song HB. Transmission electron microscopic findings of the tribocytic organ of Fibricola seoulensis. Korean J Parasitol 1993;31:315-320.
- 10. Kook J, Nawa Y, Lee SH, Chai JY. Pathogenicity and lethality of a minute intestinal fluke, Neodiplostomum seoulense, to various strains of mice. J Parasitol 1998;84:1178-1183.
- 11. Lowry OH, Rosebrough N, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-275.
- 12. McKerrow JH. Minireview: Parasite proteases. Exp Parasitol 1989;68:111-115.
- 13. McKerrow JH, Doenhoff MJ. Schistosome proteases. Parasitol Today 1988;4:334-340.
- 14. Montfort I, Pérez-Tamayo R, Pérez-Montfort R, Canto AG, Olivos A. Purification and immunologic characterization of a 30-kDa cysteine proteinase of Entamoeba histolytica. Parasitol Res 1994;80:607-613.
- 15. Rhoads ML, Fetterer RH. Extracellular matrix: A tool for defining the extracorporeal function of parasite proteases. Parasitol Today 1997;13:119-122.
- 16. Rosenthal PJ, McKerrow JH, Aikawa M, Nagasawa H, Leech JH. A malarial cysteine protease is necessary for hemoglobin degradation by Plasmodium falciparum. J Clin Invest 1988;82:1560-1565.
- 17. Seo BS. Fibricola seoulensis Seo, Rim and Lee, 1964 (Trematoda) and fibricoliasis in man. Seoul J Med 1990;31:61-96.
- 18. Seo BS, Lee SH, Hong ST, Hong SJ, Kim CY, Lee HY. Studies on intestinal trematodes in Korea V. A human case infected by Fibricola seoulensis (Trematoda: Diplostomatidae). Korean J Parasitol 1982;20:93-99.
- 19. Tannich E. Entamoeba histolytica and E. dispar: comparison of molecules considered important for host tissue destruction. Trans R Soc Trop Med Hyg 1998;92:593-596.
Fig. 1Elution profile of cysteine protease of Neodiplostomum seoulense adult worms on DEAE-Sepharose Fast Flow (A) and Q-Sepharose column chromatographies (B). Fractions were assayed for activity on CBZ-phe-arg-AMC (blank circle) and monitored for protein content (filled circle) at 280 nm. Fractions with high enzyme activities were pooled, as shown by the bar (-). Vertical arrows indicate stepped salt gradients.

Fig. 2SDS-PAGE analysis of proteins purified from the adult crude extract of Neodiplostomum seoulense according to sequential chromatographic steps. C, crude extract; 1, binding peak from DEAE-Sepharose Fast Flow; 2, binding peak from Q-Sepharose.

Fig. 3Effects of molar concentration (A) and pH (B) on proteolytic activity of the cysteine protease. Mean ± SD, n=3.

Fig. 4Degradation of cysteine protease against collagen (A), fibronectin (B), and hemoglobin (C). C, macromolecules only; Lane 1-4, macromolecules incubated at 37℃ with the enzyme for 1, 3, 5 and 12 hr, respectively. Markings indicate α- (△) and β-chains (▲) of collagen.

Table 1.Substrate specificity of the crude extract of Neodiplostomum seoulense adults
Table 1.
|
Substrates |
Enzyme activity (U/ml) |
Specific activity (U/mg) |
|
CBZ-phe-arg-AMC |
237.6 |
23.1 |
|
CBZ-arg-arg-AMC |
2.9 |
0.3 |
|
CBZ-ala-ala-pro-phe-AMC |
96.9 |
9.4 |
|
Suc-ala-ala-ala-AMC |
220.0 |
21.4 |
|
Gly-pro-leu-gly-pro-AMC |
228.5 |
22.2 |
Table 2.Summary of purification from the crude extract to the cysteine protease of Neodiplostomum seoulense adults
Table 2.
|
Step |
Total protein (mg) |
Total activity (units) |
Specific activity (units/mg) |
Purification (fold) |
Recovery (%) |
|
Crude extract |
17.0 |
2930.8 |
172.4 |
1.0 |
100 |
|
DEAE-Sepharose Fast Flow |
0.7 |
721.5 |
1030.7 |
6.0 |
24.6 |
|
Q-Sepharose |
0.3 |
502.5 |
1675.0 |
9.7 |
17.1 |
Table 3.Modulatory effects of inhibitors on the enzyme activity
Table 3.
|
Inhibitors |
Final concentration |
Relative activitya)
|
|
Control (DTT-activated) |
5 mM |
100.0 ± 7.0 |
|
without DTT |
|
37.4 ± 4.6 |
|
E-64 |
0.01 mM |
0.4 ± 0.1 |
|
IAA |
1 mM |
0.1 ± 0.04 |
|
Leupeptin |
0.1 mM |
11.6 ± 2.5 |
|
APMSF |
0.1 mM |
119.5 ± 9.6 |
|
Aprotinin |
10 μg/ml |
121.7 ± 6.6 |
|
1,10-phenanthroline |
0.1 mM |
120.4 ± 8.7 |
|
EDTA |
2 mM |
134.4 ± 10.2 |