Abstract
We developed a new concentration kit, called the ParaEgg (PE), for easy detection trematode eggs from fecal samples in endemic areas of clonorchiasis and metagonimiasis in Korea. To create a standard of detection efficiency, 120 fecal samples were examined using the water–ether concentration method (WECM). The PE kit and Mini ParaSep (PS) kit were used to compare the detection sensitivity of 100 egg-positive and 20 egg-negative samples in WECM. Additionally, stool samples, which were intentionally spiked with 10, 20, and 30 Clonorchis sinensis eggs, were evaluated to assess the sensitivity in low-infection cases. The PE and PS kits showed detection rates of 100% and 92%, respectively, from 100 egg-positive samples in WECM. Meanwhile, eggs were detected in 3 (PE) and 2 (PS) out of 20 egg-negative samples in WECM. The PE kit detected the highest number of eggs per gram of feces (727 on average), followed by the WECM (524) and PS kit (432). In fecal samples that were intentionally spiked with 10, 20, and 30 C. sinensis eggs, PE only detected eggs 2 out of 5 samples in 10 eggs spiked (40%), and the detection rates were 80% and 100%, respectively. The PE kit enabled a more accurate identification of trematode eggs because of the clearance of small fecal debris in the microscopic field. In conclusion, the PE kit is obviously helpful to detect and identify trematode eggs in stool examinations especially in endemic areas of clonorchiasis and metagonimiasis.
-
Key words: Helminth eggs, fecal examination, microscopic detection
Introduction
The rate of infection by intestinal parasites has steadily declined in Korea. In particular, soil-transmitted helminths, including
Ascaris lumbricoides and
Trichuris trichiura, were declared eliminated from Korea in 2001 [
1,
2]. However, in some regions of Korea, the incidence of foodborne helminth infections caused by
Clonorchis sinensis and
Metagonimus spp. remain high because of the consumption of raw or improperly cooked freshwater fish [
3,
4]. Based on the results of the Domestic Indigenous Parasite Disease Investigation Project from 2011 to 2020, clonorchiasis and metagonimiasis accounted for over 95% of intestinal parasitic infections in Korea [
5]. Infections with foodborne helminths, including
C. sinensis and
Metagonimus spp., are the main public health problems with clinical symptoms in endemic Korean riverside areas. In particular, clonorchiasis, which is caused by
C. sinensis, causes liver cirrhosis and even induces cholangiocarcinoma [
6,
7].
C. sinensis is classified as a group 1 carcinogen by the International Agency for Research on Cancer [
8]. Therefore, clonorchiasis continues to pose a serious threat to public health in Korea, and an accurate diagnosis is important to eliminate this endemic disease [
7].
Fecal examination is the gold standard for diagnosing intestinal parasitic diseases, including clonorchiasis and metagonimiasis. According to previous studies, the preparation of stool samples is an important factor that affects the diagnosis results [
9,
10]. Based on the World Health Organization and the US Centers for Disease Control and Prevention, the Kato–Katz egg counting technique (KKECT) and formalin–ether sedimentation technique (FEST) are the gold standard for fecal examinations (quantitative and qualitative method) [
11,
12]. The KKECT is used in field surveys for many populations because it is simple, fast, and inexpensive [
13]. However, this method cannot differentiate small trematode eggs from opisthorchiid and heterophyid flukes. By contrast, FEST is more efficient than the KKECT in detecting small trematode eggs, including
C. sinensis and
Metagonimus spp. [
14,
15]. The Korea Disease Control and Prevention Agency (KDCA) endorses the water–ether concentration method (WECM), which is derived from FEST and uses water instead of formalin. However, the WECM is a labor-intensive and time-consuming method [
16]. Therefore, a more sensitive and convenient method for detecting helminth eggs in fecal samples needs to be developed. In the present study, we developed a new concentration kit (ParaEgg [PE], KR 10-1057975) for easily detecting small trematode eggs in fecal samples from endemic areas of clonorchiasis and metagonimiasis.
Materials and Methods
Ethics statement
Ethical approval was waived because this study was conducted to evaluate public welfare through a fact-finding survey (Infectious Disease Control and Prevention Act, Article 17(1)).
Standard stool samples to be examined
Standard stool samples were made based on the WECM. Thus, the number of positive (n=100) and negative (n=20) samples were identical based on the findings of the WECM. Twenty negative samples were selected from regions with a high positivity rate for disease occurrence. Moreover, the number of eggs per gram of feces (EPG) was categorized from the findings of the WECM: low (EPG 0–49), medium (EPG 50–99), and high (EPG≥100) infection. To determine the recovery rate, negative stool samples were spiked with 10, 20, and 30 C. sinensis eggs.
Fecal sample preparation using the WECM
Fecal samples (1 g) were homogenized in 13 ml of water and passed through 2 layers of gauze to remove large debris [
17]. The suspension was centrifuged at 3,000 rpm (1,977 g) for 3 min. After discarding the supernatant, the sediment was resuspended in 10 ml of water and vigorously mixed using a loop for at least 1 min. Afterward, the suspension was added with 3 ml of ethyl ether and mixed thoroughly to separate fat from the feces. The mixed solution was centrifuged at 3,000 rpm (1,977 g) for 3 min, and the supernatant was discarded. The obtained sediment was examined under a light microscope.
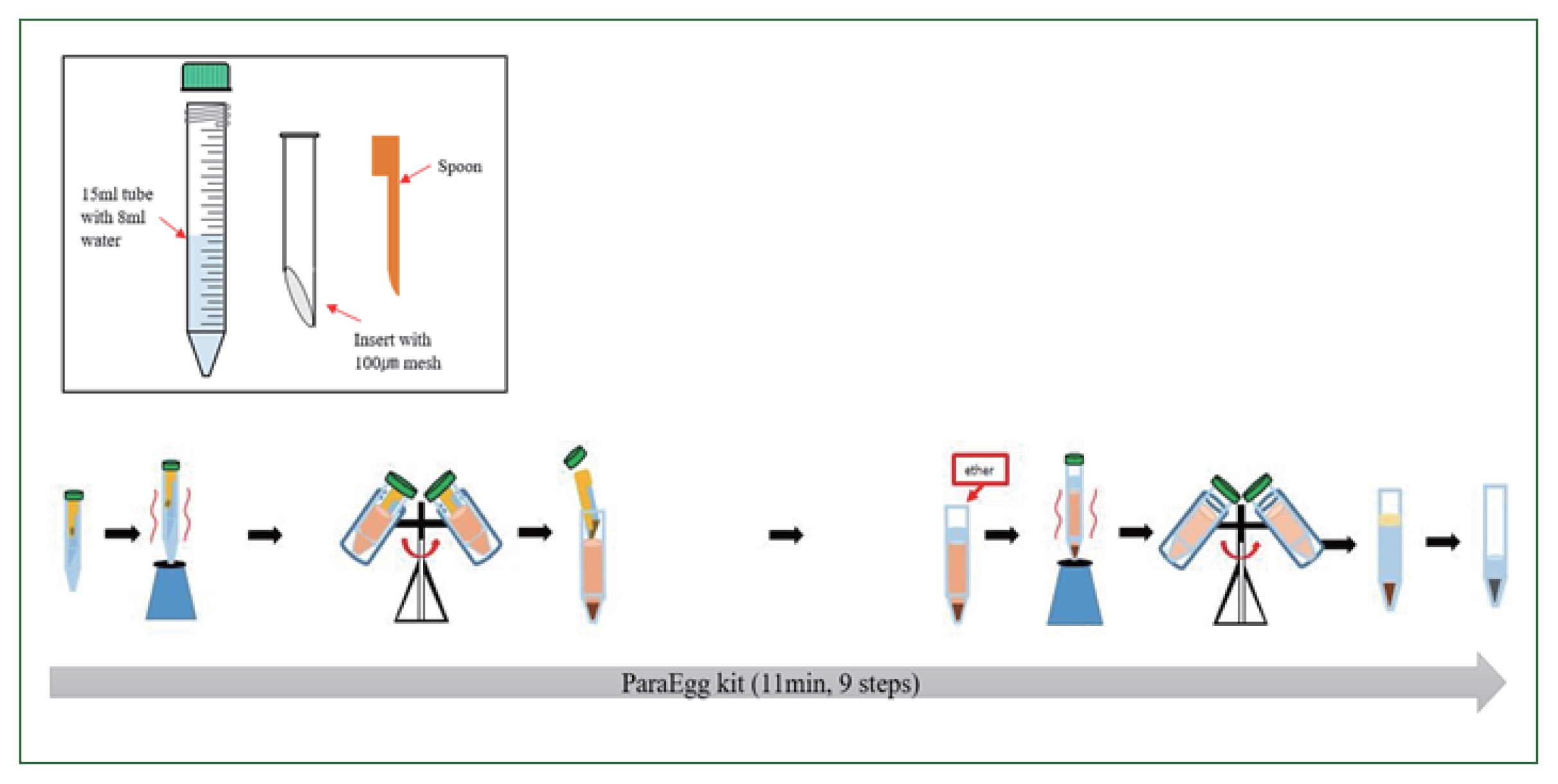
The PE kit (KR 10-1057975) comprises an integral configuration with a body, an insert, and a spoon (
Fig. 1). The insert uses a 100-μm mesh that is placed diagonally to achieve effective filtration of debris and collection of eggs. To prepare the PE for use, the “insert” was placed into the “body” (15 ml conical tube) containing 8 ml of buffer. The fecal sample (0.5 g) was added using the spoon, and the sample was vortexed to emulsify the mixture for suspension. The tube was centrifuged at 2,000 rpm (879 g) for 3 min. Thereafter, the “insert” was discarded. Next, 3 ml of ethyl ether was added to the tube to concentrate and separate parasitic eggs from fecal matter comprising vegetable and/or meat fibers. The mixture was then vortexed and centrifuged at 3,000 rpm (1,977 g) for 3 min, and the supernatant was discarded. Finally, the parasitic eggs contained in the formed pellet were examined under a light microscope.
The Mini ParaSep Faecal Parasite Concentrator (PS; Diasys Europe, Berkshire, UK) method was performed in accordance with the manufacturer’s instructions. Fecal samples (0.5 g) were placed in a tube containing 2.4 ml of buffer (10% formalin fixative and Triton X) and then added with 1 ml of ethyl acetate. The mixture was then vortexed to allow emulsification. The tube was inverted and centrifuged at 1,200 g for 3 min. Thereafter, the top layers of the supernatant were discarded, and the sediment was examined under a light microscope.
Microscopy to detect helminth eggs
To detect parasite eggs using a microscope, the pellet was resuspended in 1 ml of water, and a drop (30 μl) of each sample was examined at magnifications of 100× and 400×. Once the total egg count was obtained, the EPG was calculated. To compare the clearance of samples for microscopic observation, images were captured using the Rebel Hybrid Microscope (Discover Echo, San Diego, CA, USA) at magnifications of 100× and 400×.
Statistical analysis
Statistical analyses were performed using Microsoft Excel 2016 for Windows (Microsoft, Redmond, WA, USA) and SPSS 25 (SPSS, Chicago, IL, USA). The diagnostic performance was evaluated based on WECM as the reference standard. Statistical significance was considered at P<0.01.
Results
Detection rate of eggs and EPG using the 3 methods tested
The results of sensitivity tests with positive and negative samples using WECM are shown in
Table 1. Of the 100 positive samples, PE had a sensitivity of 100%, whereas PS only had a sensitivity of 92%. Furthermore, PS only detected eggs from low-EPG samples with a sensitivity of 74%. From the 20 samples deemed negative for infectious parasites by WECM, PE detected eggs in 3 samples, whereas PS detected eggs in 2 samples. These results indicate that PE had the highest sensitivity. The PE concentration method identified 103 positive samples, with egg counts ranging from 12 EPG to 8,232 EPG. WECM identified 100 positive samples with significantly lower egg counts, ranging from 5 EPG to 5,060 EPG. Meanwhile, PS identified 102 positive samples, with the lowest EPG values with an average of 432 eggs.
To assess the sensitivity in low-infection samples, negative stool samples were spiked with 10, 20, and 30
C. sinensis eggs, and the egg detection sensitivity was compared among the 3 methods. The results of this experiment are shown in
Table 2. Although the WECM and PS methods could not detect eggs in samples spiked with 10 eggs, the PE method detected eggs in 2 out of 5 samples. The number of egg-positive samples and recovered eggs did not differ between the PE and WECM samples spiked with 20 and 30 eggs. However, the number of egg-positive samples using the PS method was only 3 (60%). Moreover, the PS method showed a low recovery of samples spiked with 20 and 30 eggs compared with the other methods in
Table 3.
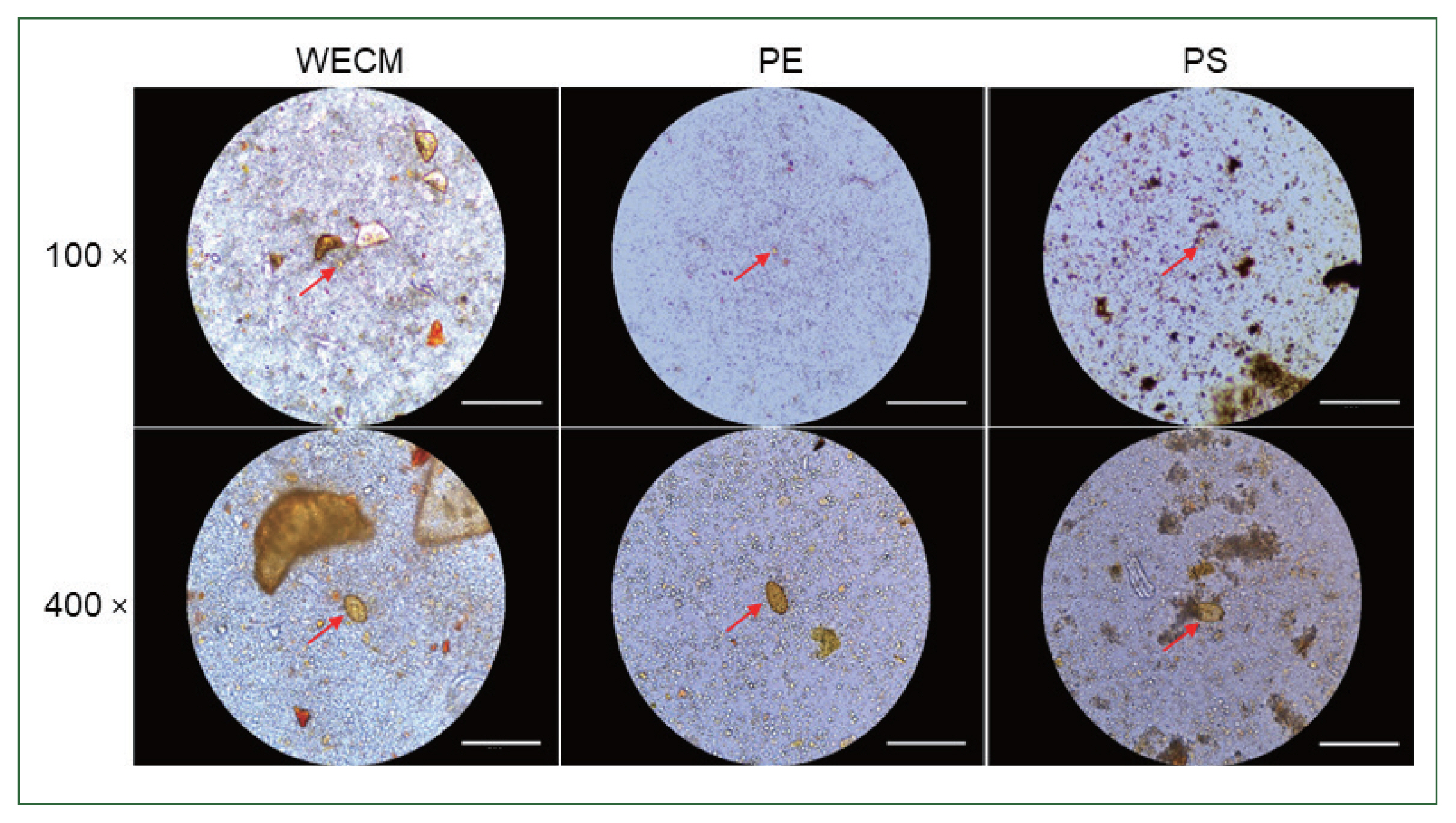
The amounts of debris observed in the 3 preparation methods were significantly different. The debris observed in the WECM was larger than those observed in the PE and PS methods. Although large-sized particles, such as those observed in the PE method, were not visible in the PS method, more debris was observed in the PS method than in the PE method (
Fig. 2).
Discussion
Traditionally, the examination of parasites in feces has been conducted using manual concentration techniques with toxic organic solvents including formalin, which consequently pose a risk to the personnel processing the samples. Therefore, for several years, the KDCA has used a modified concentration technique with water instead of formalin for fecal preparation, termed the WECM. Despite the improvement, the WECM is still a laborious and time-consuming process to perform. The PE kit (Patent no. 10-2561898 invented by the KDCA) is an easy-to-handle, closed plastic system requiring minimal manipulation, providing a clear safety advantage. It comprises a buffer and an adapted filter. Moreover, the personnel using the kit can place a fixed amount of feces directly into the device with the spoon provided, thereby maintaining consistency in testing of multiple samples.
The present study aimed to evaluate the performance of the PE kit, WECM, and PS kit for diagnosing intestinal parasitic infections with
C. sinensis and
Metagonimus spp. The results of qualitative and quantitative evaluations showed that the PE method exhibited superior detection rate compared with the WECM and PS methods. Furthermore, in the present study, eggs were detected using PE in negative samples that went undetected by WECM. The PS kit was developed and commercialized to simplify the formalin–ether concentration method and minimize specimen handling within a disposable enclosed system [
18,
19]. However, in the present study, PS was not suitable for low-EPG samples infected with
C. sinensis [
20]. The larger size of filters in the PS kit is intended for detecting large eggs, including those of
Schistosoma mansoni and
Hymenolepis nana [
21]. However, for detecting small eggs, such as those of
C. sinensis or
Metagonimus spp., which are more prevalent in Korea, the fine filter of the PE kit is considered more suitable. Although we only showed the detection results of
C. sinensis and
Metagonimus spp. in the present study, we also detected
Ascaris lumbricoides and
Paragonimus westermani in the survey (data not shown). The PE kit also has another big advantage: the mesh size can be changed depending on the purpose of the experiment, which can be applied to smaller or larger eggs. Moreover, compared with the WECM, closed concentration systems, including the PE and PS kits, can protect against toxic hazards and are less laborious and time-consuming as they use a vortex for mixing and homogenizing operations. When comparing the appearance of residual fragments of foreign substances in fecal samples under a microscope after preparation, the PE kit provided a relatively clean state, allowing for clear observation of eggs. We believe that the amounts of debris seen in the deposit increased with pore size above the recommended value, making it more difficult to observe. According to previous studies, background clearing is better in PS than in the formalin–ethyl acetate sedimentation technique [
22,
23]. However, our results showed that PE exhibited better cleaning effects (or debris removal) compared with PS. The reason for this is because PE uses a finer mesh size than the gauze in WECM or plastic mesh in PS.
Although the PE kit may be more expensive than the conventional WECM, it is faster, more convenient, and easier to use. Additionally, it allows diagnosis with a smaller sample size. The PE kit demonstrated higher sensitivity and provided better background clearance than the PS kit. It also exhibited better performance in qualitative and quantitative diagnoses. In conclusion, the new PE kit is convenient and has excellent sensitivity, making it a promising tool for accurately diagnosing intestinal parasite infections.
Notes
-
Author contributions
Conceptualization: Ju JW, Lee HI, Lee MR
Formal analysis: Lee E
Investigation: Back SO
Project administration: Lee HI
-
The authors declare no conflict of interest related to this study.
Acknowledgment
This work was supported by a grant (6331-311-210-13, 2023) from the Korea Disease Control and Prevention Agency (KDCA), Korea.
Fig. 1Workflows of stool sample preparation with the ParaEgg kit.
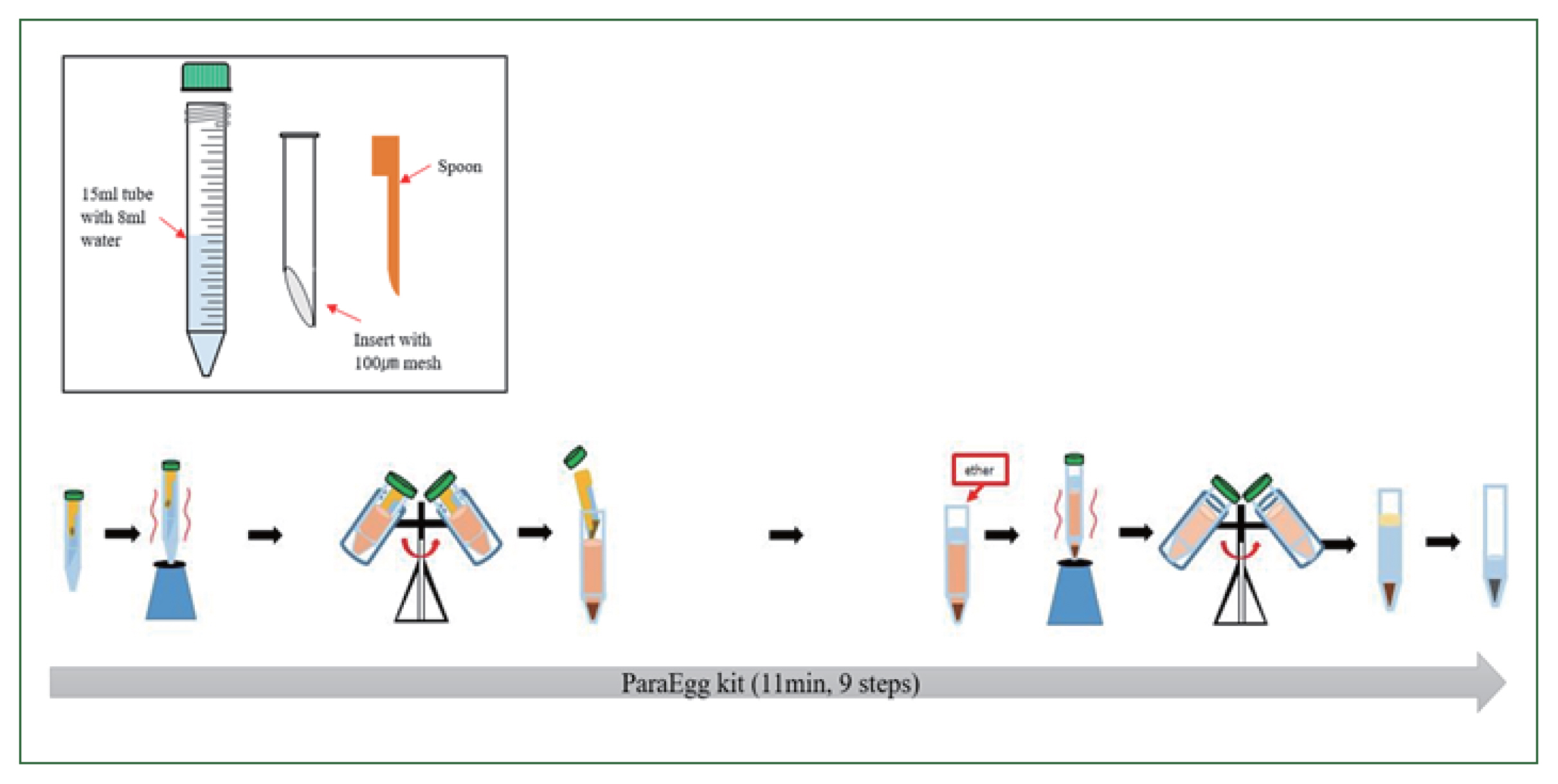
Fig. 2Comparison of eggs and foreign particles under a microscope after preparation a single sample. Images were captured at 100× and 400× magnification by WECM, PE, and PS. Bar=270 μm.
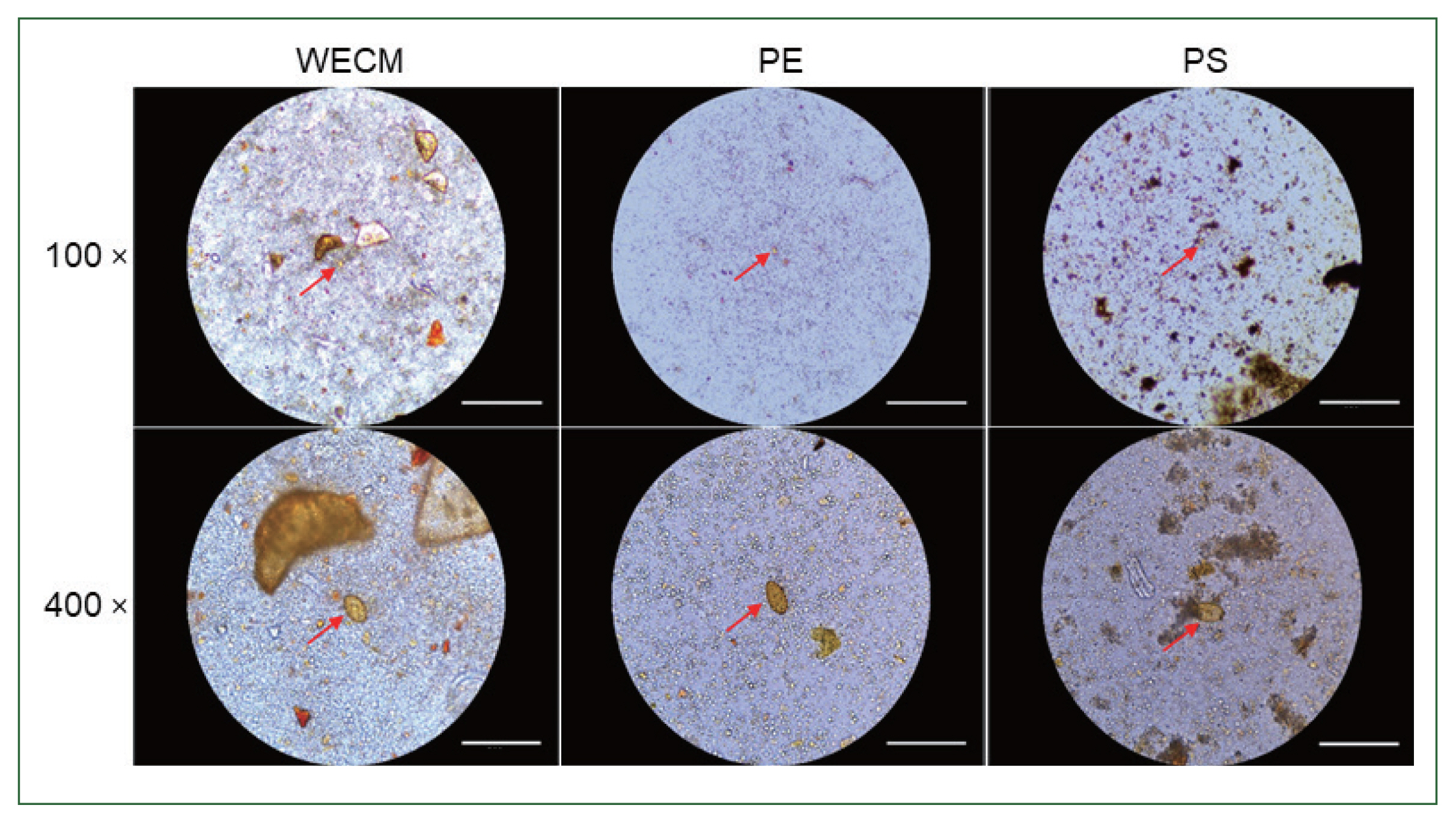
Table 1Detection rate of eggs in the standard stool samples (100 positive and 20 negative determined by WECM) by the PE and PS methods
Table 1
|
Samples |
No. of egg positive (%) |
No. of egg negative (%) |
P-value |
|
|
|
WECM |
PE |
PS |
WECM |
PE |
PS |
|
No. of positive |
Higha
|
64 |
64 (100) |
64 (100) |
63 (98) |
0 |
0 |
1 (1.6) |
|
|
Mediumb
|
17 |
17 (100) |
17 (100) |
15 (88) |
0 |
0 |
2 (11.8) |
|
|
Lowc
|
19 |
19 (100) |
19 (100) |
14 (74) |
0 |
0 |
5 (26.3) |
|
|
Total |
100 |
100 (100) |
100 (100) |
92 (92) |
0 |
0 |
8 (8.0) |
0.01 |
|
|
No. of negative |
|
20 |
0 |
3 (15) |
2 (10) |
20 (100) |
17 (85) |
18 (90) |
0.01 |
Table 2Comparison of quantitative egg detection by 3 methods
Table 2
|
Concentration method |
No. of positive samples |
EPG |
|
Mean±SD |
Median |
Min |
Max |
|
WECM |
100 |
524±849 |
189 |
5 |
5,060 |
|
PE |
103 |
727±1,280 |
246 |
12 |
8,232 |
|
PS |
94 |
432±753 |
132 |
12 |
5,026 |
Table 3Detection rate of eggs in the spiked stool samples by 3 methods tested
Table 3
|
No. of eggs spiked |
No. of samples |
No. (%) of sample positive (%) |
No. of recovered eggs |
|
|
|
WECM |
PE |
PS |
WECM |
PE |
PS |
|
10 eggs |
5 |
0 |
2 (40) |
0 |
0 |
2.8±3.9 |
0 |
|
|
20 eggs |
5 |
4 (80) |
4 (80) |
1 (20) |
8.2±4.7 |
4.6±3.0 |
2.0±4.5 |
|
|
30 eggs |
5 |
5 (100) |
5 (100) |
3 (60) |
12.4±4.5 |
16.4±14.8 |
10.8±14.8 |
References
- 1. Hong ST, Yong TS. Review of successful control of parasitic infections in Korea. Infect Chemother 2020;52(3):427-440.
https://doi.org/10.3947/ic.2020.52.3.427
- 2. World Health Organization. Communicable diseses: control of schistosomiasis and soil-transmitted helminth infections. Fifty-fourth World Health Assembly 2001;WHA54(3):A54/10.
- 3. Jeong YI, Shin HE, Lee SE, Cheun HI, Ju JW, et al. Prevalence of Clonorchis sinensis infection among residents along 5 major rivers in the Republic of Korea. Korean J Parasitol 2016;54(2):215-219.
https://doi.org/10.3347/kjp.2016.54.2.215
- 4. Yoo WG, Sohn WM, Na BK. Current status of Clonorchis sinensis and clonorchiasis in Korea: epidemiological perspectives integrating the data from human and intermediate hosts. Parasitology 2022;149(10):1296-1305.
https://doi.org/10.1017/S0031182022000798
- 5. Lee MR, Shin HE, Back SO, Lee YJ, Lee HI, et al. Status of helminthic infections in residents around River Basins in the Republic of Korea for 10 years (2011–2020). Korean J Parasitol 2022;60(3):187-194.
https://doi.org/10.3347/kjp.2022.60.3.187
- 6. Chai JY, Hong ST, Choi MH, Shin EH, Bae YM, et al. Clinical Parasitology. Seoul national University Press; Seoul, Korea. 2011, pp 358-368. pp 372-384.
- 7. Kim HS, Nam HW, Ahn HJ, Kim D, Kim YH. Relationship between Clonorchis sinensis infection and cholangiocarcinoma in Korea. Korean J Parasitol 2022;60(4):261-271.
https://doi.org/10.3347/kjp.2022.60.4.261
- 8. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, et al. A review of human carcinogens-part B: biological agents. Lancet Oncol 2009;10:321-322.
https://doi.org/10.1016/s1470-2045(09)70096-8
- 9. Perry JL, Matthews JS, Miller GR. Parasite detection efficiencies of five stool concentration systems. J Clin Microbiol 1990;28(6):1094-1097.
https://doi.org/10.1128/jcm.28.6.1094-1097.1990
- 10. Barda BD, Rinaldi L, Ianniello D, Zepherine H, Salvo F, et al. Mini-FLOTAC, an innovative direct diagnostic technique for intestinal parasitic infections: experience from the field. PLoS Negl Trop Dis 2013;7(8):e2344.
https://doi.org/10.1371/journal.pntd.0002344
- 11. Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioll L. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level Guide for Managers of Control Programmes. World Health Organization; Geneva, Switzerland. 1998.
- 12. Centers for Disease Control and Prevention. Stool Specimens – Specimen Processing [Internet]; Available from: https://www.cdc.gov/dpdx/diagnosticprocedures/stool/specimenproc.html
- 13. Hong ST, Choi MH, Kim CH, Chung BS, Ji Z. The Kato-Katz method is reliable for diagnosis of Clonorchis sinensis infection. Parasitology 2003;47(1):345-347.
https://doi.org/10.1016/s0732-8893(03)00113-5
- 14. Kaewpitoon SJ, Wakkhuwatapong P, Loyd RA, Sangwalee W, Kujapun J, et al. Detection of a carcinogenic liver fluke among migrant workers by three coprological concentration methods. Trop Biomed 2017;34(4):877-885.
- 15. Hussein AH, Rashed SM, El-Hayawan IA, Aly NSM, Abou Ouf EA, et al. Intestinal parasite infections and accuracy of direct thin and thick smear, formol-ether sedimentation, centrifugal flotation, and mini-flotac techniques among patients with gastrointestinal tract disorders from the Greater Cairo region, Egypt. Am J Trop Med Hyg 2017;96(3):589-594.
https://doi.org/10.4269/ajtmh.16-0436
- 16. Won EJ, Kim J, Ryang DW. Evaluation of modified formalin-ether concentration method using Para Tube in clinical settings. Ann Lab Med 2015;35:445-448.
https://doi.org/10.3343/alm.2015.35.4.445
- 17. Smith JW, Bartlett MS. Diagnostic parasitology introduction and methods. In Lenette EH, Balows A, Hausler WJ Jr, Shadomy HJ eds, Manual of Clinical Microbiology. 4th ed. American Society for Microbiology; Washington D.C., USA. 1985, pp 595-611.
- 18. Saez AC, Manser MM, Andrews N, Chiodini PL. Comparison between the midi parasep and midi parasep solvent free (SF) faecal parasite concentrators. J Clin Pathol 2011;64(10):901-904.
https://doi.org/10.1136/jcp.2011.090639
- 19. Couturier BA, Jensen R, Arias N, Heffron M, Gubler E, et al. Clinical and analytical evaluation of a single-bial stool collection device with formalin-free fixative for improved processing and comprehensive detection of gastrointestinal parasites. J Clin Microbiol 2015;53(8):2539-2548.
https://doi.org/10.1128/JCM.00838-15
- 20. Charoensuk L, Subrungruang I, Mungthin M, Pinlaor S, Suwannabitatorn P. Comparison of stool examination techniques to detect Opisthorchis viverrini in low intensity infection. Acta Trop 2019;191:13-16.
https://doi.org/10.1016/j.actatropica.2018.12.018
- 21. Adugna S, Kebede T, Mekonnen Z, Degarege A, Liang S, et al. Diagnostic performance of Mini Parasep® solvent-free faecal parasite concentrator relative to Kato-Katz and McMaster for the diagnosis of intestinal parasitic infections. Trans R Soc Trop Med Hyg 2017;111(12):572-578.
https://doi.org/10.1093/trstmh/try010
- 22. Khanna V, Sagar S, Khanna R, Chawla K. A comparative study of formalin-ethyl acetate sedimentation technique and Mini Parasep® solvent-free method in the rapid diagnosis of intestinal parasites. Trop Parasitol 2018;8(1):29-32.
https://doi.org/10.4103/tp.TP_44_17
- 23. Mewara A, Khurana S, Gupta S, Munda VS, Singh S, et al. Diagnostic performance of Mini Parasep® solvent-free foecal parasite concentrator for the diagnosis of intestinal parasitic infections. Indian J Med Microbiol 2019;37(3):381-386.
https://doi.org/10.4103/ijmm.IJMM_19_44