Abstract
Naegleria fowleri, a brain-eating amoeba, thrives in lakes and rivers with aquatic vegetation and causes primary amoebic meningoencephalitis (PAM) in humans. Most recently, it has become such a serious problem that N. fowleri was detected in tap water in Houston, USA. Several pathogenic factors are considered very important to destroy target cells in the brain. In particular, the food-cup where N. fowleri antigen-1 (Nfa1) is located, is strongly expressed in pseudopodia involved in the movement of N. fowleri, and is involved in phagocytosis by attaching to target cells. In this article, we reviewed the role of the Nfa1 protein and its associated pathogenicity. The nfa1 gene was cloned by cDNA library immunoscreening using infection serum and immune serum. Nfa1 protein is mainly distributed in pseudopodia important to movement and vacuoles. Moreover, heat shock protein 70, cathepsin-like proteare and Nf-actin are also associated with pseudopodia in which Nfa1 is localized. Interestingly, the amount of the nfa1 gene changed as N. fowleri trophozoites transformed into cysts. Polyclonal antiserum against Nfa1 showed a protective effect against cytotoxicity of approximately 19.7%. Nfa1-specific IgA antibodies prevent N. fowleri trophozoites from adhering to the nasal mucosa, delaying invasion. The nfa1-vaccinated mice showed significantly higher levels of Nfa1-specific antibody. The duration of anti-Nfa1 IgG in the vaccinated mice lasted 12 weeks, strongly suggesting that nfa1 is a significant pathogenic gene and that Nfa1 is a pathogenic protein. Several factors related to pseudopodia and locomotion have been linked to Nfa1. A clearer function of N. fowleri targeting nfa1 with other genes might enable target-based inhibition of N. fowleri pathogenicity.
-
Key words: Naegleria fowleri, nfa1, pathogenicity, pseudopodia, tissue invasion
Introduction
Naegleria fowleri lives in lakes and rivers where aquatic plants exist and causes primary amoebic meningoencephalitis (PAM) in humans and experimental animals via the nasal passages [
1–
3]. Recently, a person in USA has been infected with
N. fowleri from tap water; Texas authorities are warning residents in some communities near Houston to stop using tap water because it could be contaminated with this deadly brain-eating microbe [
4]. If the concentration of chlorine used for water purification is very low or the purification filter is damaged, it may still be found in tap water in the future.
Naegleria fowleri produces severe disease very quickly, causing PAM, and is usually fatal within 2 weeks [
5,
6]. Non-pathogenic
N. gruberi also exists but does not cause any disease in humans or experimental animals [
7]. Therefore,
N. fowleri is the only pathogenic species among
Naegleria spp. and is known to have stronger phagocytosis, movement, and cytotoxicity in terms of pathophysiology than non-pathogenic
N. gruberi [
8,
9].
Fewer than 100 review papers have been published on the
N. fowleri pathogenicity, but recently, analysis of the full genome has identified pathogenicity and several other important genes [
10]. Several pathogenic factors that are considered very important to destroy target cells in the brain are listed in
Table 1 [
10,
11]. In particular, food-cup where
N. fowleri antigen-1 (Nfa1) is located, is strongly expressed in pseudopodia involved in the movement of
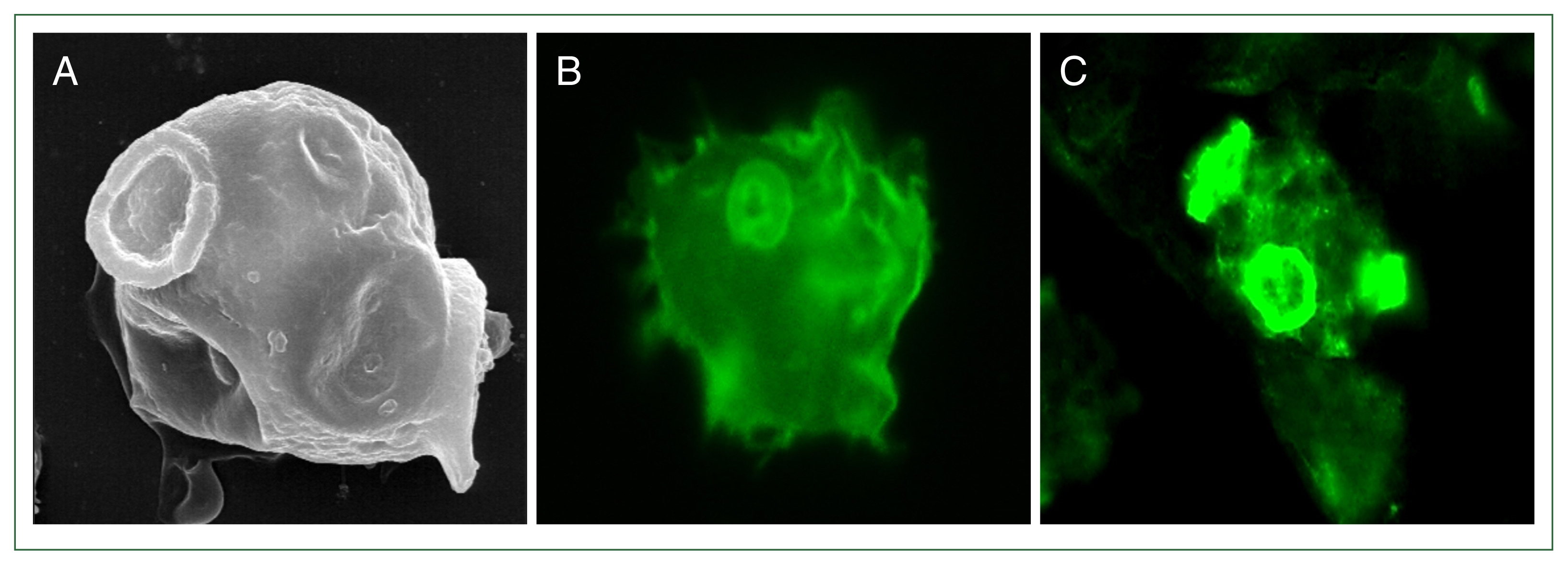
N. fowleri (
Fig. 1), and is involved in phagocytosis by attaching to target cells. After
N. fowleri attaches to a target cell, many proteolytic enzymes are secreted to aid in phagocytosis. Therefore, pseudopodia are believed to play a very important role in the contact-dependent pathogenic pathway of
N. fowleri [
12,
13]. In this study, we demonstrated that Nfa1 as pathogenic factor, immunization with recombinant Nfa1 protein, DNA vaccine expressing Nfa1, host cell invasion by various proteins,
nf-actin gene as contact dependent mechanism,
nfa1 gene in
N. fowleri encystation and transcriptome profiling, and suggested directions for future research related to
N. fowleri pathogenicity.
Identification of nfa1 gene/Nfa1 protein using infection and immune sera
Generally, when separating serum by electrophoresis, serum can be separated into albumin, alpha-1 globulin, alpha-2 globulin, beta-globulin, and gamma-globulin [
14]. The terms “infection” or “immunity” are generally not used to preface the term “serum.” However, our research team has used
N. fowleri “infection” and “immune” sera separately. Infection serum is the serum obtained from the orbital artery or heart when
N. fowleri is used in experimental animals (e.g., BALB/c mice) before the mice die due to infection [
15]. Immune serum is serum obtained from the orbital artery or heart about 4 to 6 weeks after injecting lysates obtained by lysing
N. fowleri (lysate pellets removed by centrifugation) into the peritoneal cavity of an experimental animal.
N. fowleri can infect the cerebrum via the olfactory nerve, and infection serum can be obtained through an immune response caused by factors related to movement and secretion factors such as proteases [
15]. However, immune serum can be generated through an immune reaction caused by water-soluble proteins dissolved in lysate buffer. Immunoscreening of the
N. fowleri cDNA library using infection serum and immune serum showed different results, but a common cDNA expression spot was obtained for the 2 types of sera, and this gene and protein were named
nfa1 (GenBank accession no. AF230370) and Nfa1, respectively [
15].
Analysis of basic components and functions of Nfa1 protein
The 13.1 kDa Nfa1 protein showed 43.0% identity with the myohemerythrin (myoHr) protein from
Nereis divesicolor [
15,
16], including the region that binds 2 iron atoms and a P-turn-rich N-terminal segment, especially the conserved region; and 100.0% identity with the iron binding residues [
15]. Hemerythrin is an oxygen-binding protein isolated from 4 invertebrate phylae, e.g., sipunculans, brachiopods, priapulids and annelids [
17]. It has been found in the vasculature, coelomic fluid, and muscle [
18]. One iron atom is bound to 2 histidine residues, and the other is bound to 3 histidine residues. The 2 iron atoms are connected by a glutamate residue, an aspartic acid residue, and an oxide ion [
19]. Meanwhile, when studying the structure of the Nfa1 protein, 2 potential glycosylation sites and 3 phosphorylation sites were identified [
15]. Although biochemical structural analysis of these proteins may be important, our team focused on the function analysis of the Nfa1 protein to understand and analyze the pathogenicity of
N. fowleri. Nfa1 protein is mainly distributed in pseudopodia and vacuoles [
20,
21]. In other words, it may be implied that the Nfa1 protein and proteins related to pseudopods or vacuoles may be related to the motility or cytotoxicity of
N. fowleri.
Naegleria fowleri trophozoites can cause approximately 55.3% or more cytotoxicity against target cells in 24 h [
21]. The extent of the protective effect against the cytotoxicity of
N. fowleri was confirmed using serum obtained by injecting purified recombinant Nfa1 into mice [
20,
22]. The serum (diluted 1:50 to 1:100) was found to have a protective effect against cytotoxicity of approximately 11.3% to 19.7% by the lactate dehydrogenase (LDH) release assay [
22]. Through this experiment, it was confirmed that Nfa1 may be involved in the cytotoxicity of
N. fowleri. When the monoclonal antibody (IgG2b) against Nfa1 produced in mice was extracted directly from the cerebrum of rat offspring and treated with cultured microglial cells, the cytotoxicity of
N. fowleri was reduced by about 66.3% [
23]. Cytotoxicity was further reduced by the specific monoclonal antibody compared to the polyclonal serum obtained from mice. If the ratio of serum or monoclonal antibody and the amount of
N. fowleri were optimally adjusted, a higher cytotoxicity inhibition or protective effect could be observed. This result suggests that Nfa1 is a critical factor to determine the cytotoxicity of
N. fowleri.
RNAi showed that Nfa1 is a pathogenic factor
Double-stranded RNA-mediated interference (RNAi) silences gene expression in various cells, resulting from the breakdown of RNA into short RNAs that activate ribonucleases [
24]. RNAi has been applied to
Trypanosoma [
25] and nematodes [
26]. Our research team was the first to attempt it in
Naegleria [
27,
28]. The initial hypothesis was that
Naegleria is similar in shape to early-divergent amoeba, but its internal cellular structures, such as mitochondria and Golgi apparatus, were very distinct, so RNAi could sufficiently act and there would be no problems with Nfa1 protein expression. As mentioned above, the antigenicity of Nfa1 and antibodies against Nfa1 were confirmed to have a protective effect against
N. fowleri cytotoxicity. RNAi was applied to
N. fowleri in an attempt to suppress the expression of Nfa1 protein at the gene level. Briefly, by selecting a specific site and synthesizing small interfering RNA (siRNA),
nfa1 mRNA synthesis was suppressed by about 70.0% and Nfa1 protein was suppressed by about 43.0% [
28]. This fact proves that the RNAi mechanism exists within
N. fowleri trophozoites. Additionally, with cytotoxicity being reduced by about 30.0% by siRNA, proves that the
nfa1 gene contributes to the pathogenicity of
N. fowleri. The application of RNAi to transcribe the RNA of the complementary sequence of mRNA showed consequences similar to those of the gene, Nfa1 protein, and cytotoxicity induced by siRNA. These experiments confirm that the
nfa1 gene is a pathogenic gene. Another question related to pathogenicity experiments is whether non-pathogenic
Naegleria can become pathogenic when the
nfa1 gene is transfected with non-pathogenic
Naegleria [
29]. The cytotoxicity of non-pathogenic
N. gruberi injected with the
nfa1 gene was very high, approximately 55.8% (
P<0.01). Compared with cytotoxicity caused by
N. fowleri, a difference of about 26.2% (
P<0.01) was shown [
29]. This study provided further evidence that
nfa1 gene is pathogenetic factor.
siRNA is a short sequence and can complementarily bind to the target gene more specifically. The
nfa1 gene is 360 bp in length, therefore, there was a question as to whether long RNA that complementarily binds to mRNA corresponding to open reading frame (ORF) could act like siRNA [
27]. Using synthetic double-stranded RNA of the
nfa1 gene, the
nfa1 gene and Nfa1 protein were knocked down by approximately 50.4% and 52.0%, respectively. These results suggested that the RNAi mechanism for the
nfa1 gene could be applied regardless of siRNA or 360 bp RNA [
28].
Effects of immunization with the recombinant Nfa1 (rNfa1) protein
Human sera from both healthy and PAM patients contained antibodies against the
N. fowleri antigen, showing that exposure to the amoeba is widespread [
30,
31]. A variety of studies have been conducted to assess the involvement of certain antibody isotypes, such as IgA, IgG, and IgM, in host defense against
N. fowleri infection [
32,
33]. In mice infected with
N. fowleri trophozoites, circulating IgG was detected 1 week after infection [
33]. Additional studies of experimental infections in mice and PAM patients have focused on serum antibody responses [
34,
35]. The mouse model of PAM was used to study host protective immunity to
Naegleria infection [
35,
36]. We conducted the experiment to induce protective immunity in PAM mice by immunization with the recombinant Nfa1 (rNfa1) protein [
37]. To assess the host immune response, Nfa1-specific serum IgG, IgA, and IgE levels were examined, as well as the amounts of cytokines produced by splenocytes following intraperitoneal or intranasal immunization with the rNfa1 protein. Moreover, the survival rate and mean time to death of immunized mice following a fatal challenge with
N. fowleri trophozoites were calculated. Previous research on immunity in experimental infections and PAM patients has mostly focused on serum antibody responses [
34,
35]. We attempted to explain the serological and cellular immune responses that give partial protection against
N. fowleri infection in Nfa1-immunized mice and found that the intranasal route of immunization appeared to be appropriate for in vivo models. It is generally known that mucosal IgA inhibits bacterial adherence, which is one of the most important defense mechanisms against mucosal invasion and bacterial attachment to epithelial cells [
38].
N. fowleri adhesion to host cells is an essential first stage in the infection process, and the Nfa1 protein is important in the production of pseudopodia and food-cup structure. Based on this work, we proposed that Nfa1-specific IgA antibodies can prevent
N. fowleri trophozoites from adhering to the nasal mucosa, delaying invasion. Additionally, rNfa1 protein may activate a mixed Th1/Th2/Treg immunological response [
37]. Finally, all inoculated mice survived longer when challenged with a lethal dose of
N. fowleri [
37]. These findings suggest that immunization with the rNfa1 protein can elicit partially protective immune responses, resulting in longer host survival periods in
N. fowleri infection. However, the vaccination did not improve the mice’s overall survival, indicating that further improvement of the vaccine strategy using Nfa1 protein is required.
DNA vaccine expressing nfa1 gene and application of lentiviral vector system
DNA vaccination was presented in 1990, as proven by the stimulation of protein expression following direct intramuscular injection of plasmid DNA into myocytes [
39]. This discovery could lead to endogenous protein manufacturing and a targeted immune response against those, opening up new avenues for vaccine development. It was recently revealed that DNA vaccines can protect against parasitic diseases such as amebiasis, leishmaniasis, and toxoplasmosis [
40–
42]. Viral vectors are commonly employed in the production of DNA vaccines to successfully deliver genes. Over the last few decades, retroviral vectors have been utilized in gene therapy clinical trials to treat a variety of hereditary illnesses and cancers. Retroviral vectors may transduce a variety of cell types from several animal species, correctly integrate the genetic information the vector carries into recipient cells, and produce high levels of transduced gene expression [
43]. Furthermore, lentiviral vectors have the potential to be effective instruments for in vivo gene delivery. Lentiviral vectors are simple to construct to infect a wide range of cell types, dependent on the envelope to manufacture the recombinant virus and to transduce cells regardless of replication state, both in vitro and in vivo, eventually culminating in integration into the host cell genome [
44]. Based on previous research, our research team determined that the
nfa1 gene is a suitable option for DNA vaccination since the Nfa1 protein is the essential molecule that contacts and kills the host cell in the contact-dependent pathogenesis of
N. fowleri [
45]. To establish viral vector systems and evaluate immune responses for DNA vaccination, we constructed retroviral and lentiviral vectors encoding the
nfa1 gene, and then investigated the effect of intranasal vaccination with viral particles using a mouse model (number of Animal Care Committee of Ajou University School of Medicine: AMC66) to induce immune responses in DNA vaccine strategy [
45]. In experimental animal models, the intranasal approach is particularly effective in inducing mucosal immune responses, as it needs smaller concentrations of antigens and elicits higher systemic immune responses than the oral route [
44]. The intranasal approach appears to be more appropriate than other methods for the
N. fowleri DNA vaccine model, since it efficiently inhibits amoeba adherence via a contact-dependent mechanism. It is necessary to establish factors such as viral vector selection, antigen presentation pathways, adjuvant combination, and mouse immunological state in order to optimize the vaccine against
N. fowleri infection. As mentioned above, we developed lentiviral vector systems that expressed the
nfa1 gene and successfully delivered it to the target cells [
45]. Continuously, to develop a potentially successful DNA vaccine for
N. fowleri infection, the in vivo efficacies of lentiviral vector systems expressing the
nfa1 gene should be investigated [
46]. In terms of the humoral response,
nfa1-vaccinated mice showed significantly higher levels of Nfa1-specific IgG2a and IgG1, which are associated with Th1 and Th2 responses, respectively. In addition, compared to the control groups, the duration of anti-Nfa1 IgG in the vaccinated mice was prolonged during the 12 weeks. After
N. fowleri infection, mice vaccinated with nfa1 exhibited longer mean times to death and higher survival rates (90.0%) [
46]. DNA vaccines are attractive due to their simplicity of manufacture, low cost, and promise for long-term protection [
47]. Lentiviral vectors are thought to be excellent options for vaccine vectors since they have been tested in a variety of preclinical models for gene therapy and immunization. Both a vector system and an effective adjuvant system are necessary for a vaccine to be successful. An adjuvant that enhances the effectiveness of the immune response is appropriate. Cholera toxin B subunit (CTB) and heat-labile enterotoxin B subunit (LTB) have been used as mucosal adjuvants to demonstrate the possibility of intranasal vaccination [
48,
49]. When combined with antigens, these adjuvants significantly enhance immunological responses, such as IgA production during intranasal vaccination. For these reasons, we investigated the effectiveness of mucosal adjuvants against
N. fowleri infection, such as CTB or LTB [
50]. Mice inoculated with the Nfa1 protein using LTB or CTB adjuvant had a 5 to 8 day longer mean time to death than control mice. Based on these findings, it appears that Nfa1 protein combined with CTB or LTB adjuvants strongly protects mice against
N. fowleri infection. Taken together, Nfa1 vaccination can significantly increase specific immunoglobulin and cytokine production in
N. fowleri infected mice. These studies are meaningful because they are the first to assess in vivo immune responses of DNA vaccine against
N. fowleri infection.
Several proteins including heat shock protein, cathepsin B, and other excretory proteins are involved in host cell invasion by pseudopodia
The pathogenic agents of
N. fowleri rely on both contact-dependent and contact-independent mechanisms with the host cell [
10,
34]. In contact-dependent mechanisms, Nfa1 was cloned and characterized [
15,
22]. In addition, we cloned and characterized Nf-cHSP70 (HSP, heat-shock protein) and Nf-actin as contact-dependant factors related to pathogenicity of
N. fowleri [
51–
53]. We previously revealed that the Nf-cHSP70 gene (GenBank accession no. AY684788) produces a protein of 660 amino acid residues and a molecular mass of 72 kDa [
51,
52]. With the findings of its pseudopodia-localization [
52], the recombinant Nf-cHSP70 protein exhibited significant antigenicity in mice infected with
N. fowleri, indicating that Nf-cHSP70 is involved in
N. fowleri proliferation and cytotoxicity. The pathogenicity of parasites may be associated with the characteristics of their excretory-secretory proteins (ESPs), which play a role in the contact-independent process. Antigenic proteases in
N. fowleri ESPs play a role in
N. fowleri host cell invasion as well as the cleaving capacity of host immunoglobulins, which may contribute to parasite persistence in the host through immune evasion. For these reasons, we analyzed the pathogenicity related proteins from
N. fowleri ESPs using proteomics-based approaches, and sought to find a possible role of ESPs in
N. fowleri’s ability to penetrate host cells [
54]. The findings showed that
N. fowleri ESPs contained a variety of dominant antigenic proteins, including cathepsin B protease, cysteine protease, peroxiredoxins, and thrombin receptor, as well as a variety of pathogenic proteins that are involved in the organism’s entry into host cells [
54]. Among them, our research team has concentrated on cathepsin B protease of
N. fowleri [
54]. Cathepsin B protease is a cysteine protease that is secreted into hosts and is thought to help break down ingested host proteins, such as hemoglobin [
55,
56]. We initially cloned cysteine proteases, cathepsin B (NfCPB) and cathepsin B-like protein (NfCPB-L) from cDNA library of pathogenic
N. fowleri, and then characterized their biochemical features. According to sodium dodecyl sulfate-polyacrylamide gel analysis (SDS-PAGE), the molecular weights of NfCPB and NfCPB-L were 38.4 and 34 kDa, and their open reading frames were 1,038 and 939 bp, which encode 345 and 313 amino acids, respectively [
57]. The rNfCPB and rNfCPB-L are crucial in the proteolytic effects on immunoglobulin, collagen, fibronectin, hemoglobin and albumin [
57]. Additionally, the attachment to the host tissue (proteolysis of collagen and fibronectin), immune system evasion (proteolysis of immunoglobulins), and nutrient uptake (proteolysis of albumin and hemoglobin) may be facilitated by rNfCPB and rNfCPB-L. Ultimately, the pathogenicity of
N. fowleri is complex in its ability to destroy target cells, which includes both contact-dependent mechanisms such as Nfa1 and contact-independent mechanisms such as NfCPB located in pseudopodia [
58].
Nf-actin gene concerned with contact-dependent mechanisms in N. fowleri
Attachment to host cells plays an important role in the cytopathogenicity of
N. fowleri and is the first step in the pathogenesis caused by
N. fowleri infection [
59,
60].
N. fowleri trophozoites invade the central nervous system via the olfactory nerve and subsequently digest neuronal tissues through cytolysis and phagocytosis [
61–
63].
Acanthamoeba can also induce a potent cytopathic effect leading to host cell death following adhesion [
64].
N. fowleri trophozoites employ a contact-dependent mechanism, utilizing food-cup structure or amoebastomes to attach to target cells and induce cell destruction [
30,
65–
67]. The molecules involved in the formation of food cup structures and amoebastomes were studied; using immunofluorescence assays, cytoskeleton proteins (myosin and tubulin) were shown in dispersed cellular location in trophozoites [
53]. Conversely, actin was concentrated in the cytoplasm, pseudopodia, and the food-cup structure. There are numerous reports indicating that cytoskeleton proteins are involved in various cellular functions, including adhesion [
68], motility [
69], and phagocytosis [
69–
70]. With the motility association with cytoskeleton gene in
N. fowleri [
66], the
nf-actin gene cloned with a coding sequence of 1.2 kbp, producing a 50 kDa recombinant fusion protein (rNf-actin) [
53]. We submitted the
nf-actin gene sequence of
N. fowleri to GenBank (no. EU980397) and conducted a comparative analysis of its homology with other actin genes [
53]. It exhibited 82.0% sequence identity with the non-pathogenic
N. gruberi, but there was no identical sequence found in other mammalian and human actin genes. Furthermore, when
N. fowleri was co-cultured with target cells, Nf-actin expression was significantly concentrated in the food-cup structure, indicating its involvement in trogocytosis [
53]. Our study demonstrated that treatment of
N. fowleri with actin inhibitor resulted in reduced food cup formation and in vitro cytotoxicity [
53,
71].
Furthermore, cellular characterization of the actin gene related to contact-dependent mechanisms in
N. fowleri was performed [
71]. A eukaryotic transfection vector was constructed with Ubi-pEGFP-C2/nf-actin which possessed the ubiquitin promoter instead of the CMV promoter [
72]. We analysed the expression level of Nf-actin by western blot and RT-PCR in overexpression or knockdown
N. fowleri.
N. fowleri was transfected with antisense oligonucleotides targeting the
nf-actin gene for the knockdown system [
53]. The Nf-actin knockdown in
N. fowleri showed reduced levels of mRNA and protein [
53].
Adhesion to target cells is a crucial event in the pathogenicity of
N. fowleri infection. Protozoa have been previously reported to be recognized by extracellular matrix (ECM) components [
73–
76]. Specifically,
N. fowleri binds to fibronectin through mediation of a 60 kDa fibronectin binding protein [
22], while
Acanthamoeba binds to laminin1, collagen IV, and fibronectin [
77]. The
nf-actin overexpression or knockdown in
N. fowleri was used to assess the adhesion activity of actin to ECM components [
53]. The
nf-actin overexpressing
N. fowleri exhibited significantly increased adhesion levels to fibronectin and fibrinogen compared to wild type
N. fowleri [
53]. These results indicate that the Nf-actin protein plays a crucial role in adhesion to target cells, contributing to the pathogenicity of
N. fowleri. Additionally, phagocytosis is recognized as a key step in protozoan pathogenesis, with previous studies highlighting the involvement of actin polymerization in bacterial invasion of host cells [
78,
79]. We examined the phagocytic activity using pre-labelled zymosan particles in
nf-actin overexpressing or knocked-down
N. fowleri [
53]. Our results demonstrate a significant increase in phagocytic activity in
nf-actin overexpressing
N. fowleri compared to both
nf-actin knockdown and wild-type strains [
53]. LDH release assay conducted to evaluate the effect of Nf-actin on the cytotoxicity of
N. fowleri showed that
nf-actin overexpression significantly increased cytotoxicity compared to
nf-actin knocked-down and wild-type strains [
53]. These results showed that Nf-actin play an important role in inducing cytotoxic effects on target cells during
N. fowleri pathogenesis [
53]. Moreover,
nf-actin overexpressing
N. fowleri significantly enhances cytotoxicity, cell adhesion, and phagocytosis, highlighting its pivotal role in these cellular processes [
53]. The fact that Nfa1 has a very high homology with myohemerythrin may suggest a relationship with actin in the cytoskeleton. Since the above 2 proteins involved in cytotoxicity, motility, etc. are likely to be related, future research characterizing the relationship will be necessary.
Encystation of N. fowleri induced changes in nfa1 gene expression
Naegleria fowleri undergoes encystation, converting from a trophozoite to a cyst in response to temperature changes and unfavourable conditions. Conversely, in a more hospitable environment, the cyst transforms into a trophozoite through a process called excystation. These processes are important for the survival of amoebae in extreme environments. In particular, encystation represents a significant mechanism that undermines the effectiveness of therapeutic agents. Inhibiting cyst formation may enhance the therapeutic efficacy of agents used to treat amoebic infections.
Sohn et al. [
80] used a modified liquid encystment medium (Buffer. 1) to induce
N. fowleri encystation and abundant pure cysts. The results showed successful induction of amoebic cysts after 48 h with Buffer 1, followed by their recovery as trophozoites in Nelson’s medium [
80]. Furthermore, differential expression levels of the
nfa1 gene [
15] and nf-actin genes (GenBank accession no. EU980397) [
81] were confirmed, with mRNA levels observed to be higher in the trophozoites. A liquid encystment medium, referred to as Buffer 1, has the potential to serve as a replacement for previous methods which involved a solid non-nutrient agar medium with heat-inactivated
Escherichia coli for
N. fowleri encystation. Moreover, Buffer 2 of pH 9 is widely used in various studies and is effective in inducing the formation of
Acanthamoeba cysts [
81–
83]. The
actin gene exhibited overexpression in trophozoites of
A. castellanii and
A. polyphaga, while the
atg8 gene (GenBank accession no. EU935007) showed higher expression in cysts, which is associated with cyst formation in
Acanthamoeba spp [
83].
Transcriptome profiling of N. fowleri revealed several genes involved in cellular mechanisms and immune responses
Understanding the differential gene expression between trophozoites or cysts of
N. fowleri may reveal novel pathogenic factors in amoebic infection, particularly those that contribute to brain infection. A recent RNA-sequencing study investigated the transcriptome for drug treatment of
Balamuthia mandrillaris [
84] and the effect of Hesperidin conjugated with silver nanoparticles in
N. fowleri infection [
85]. We analyzed the transcription profiling performed for the transcriptome analysis of
N. fowleri trophozoites or cysts using RNA-sequencing which provides insights into biological processes [
86]. The transcriptomes of trophozoites or cysts were assembled, resulting in 42,220 contigs containing 11,254 genes (C+G contact of 37.2%) [
86]. A total of 146 and 163 differentially expressed genes (DEGs) were identified in cysts and trophozoites, respectively (2-fold expression) [
86]. Transcriptomic genes associated with cellular motility, growth and death, signal transduction, translation, carbohydrate metabolism, lipid metabolism, and nucleotide metabolism exhibited differential expression levels during both cyst and trophozoite stage [
86]. Gene Ontology (GO) and pathway analysis of the DEGs indicated that ‘cells’, ‘cellular processes’, and ‘catalytic activity’ were the most enriched categories [
86]. These findings are relevant to pathogenesis of
N. fowleri, especially in processes such as amoebic proliferation, differentiation, growth, and death.
We observed increased expression of several genes related to the cytoskeleton in
N. fowleri trophozoites [
86]. The actin-related genes (a total of 38 genes) were classified into categories of cellular processes, including cell motility, growth/death, and cellular community [
86]. There are many reports on the function of the cytoskeleton in relation to pathogenicity of protozoa such as
Entamoeba histolytica [
87–
89],
Toxoplasma gondii [
90], and
Acanthamoeba spp. [
91,
92]. Our results show that profilin gene expression was significantly increased during cyst formation, and unchanged in trophozoites. Profilin gene regulation is known to influence the nucleation rate of actin polymerization and filament elongation [
93,
94]. Moreover, our study identified elevated expression levels of specific genes in cysts, including those related to cytoskeleton and pathogenesis, relative to trophozoites of
N. fowleri [
86]. These findings suggest a significant role of cytoskeleton-related genes in the development of
N. fowleri infection. Although the mechanisms by which
N. fowleri cysts access host cells are not fully understood, our findings suggest the involvement of transporters via the transcriptome. In particular, the functional roles of serine/threonine proteases, kinases, and lipid metabolism-related proteins in regulating
N. fowleri cysts remain elusive but are presumed to contribute to this regulatory process. Our transcriptome data serves as a valuable resource for studying genes implicated in cellular mechanisms and immune responses in other protozoan infections.
Conclusion
The Nfa1 of N. fowleri is one of the proteins that reacts with the serum of laboratory animals infected with N. fowleri. Through functional analysis of the Nfa1 protein, it has been confirmed that inhibition at the nfa1 gene level can suppress the pathogenicity of N. fowleri. In particular, the high expression of Nfa1 protein in pseudopodia may suggest an association with cytoskeleton proteins and proteolytic enzymes. Since N. fowleri causes pathogenicity through contact-dependent and contact-independent mechanisms, it is clear that the nfa1 gene is important. Understanding the pathogenicity of N. fowleri nfa1-related factors will make an important contribution to target-based mechanisms and inhibition.
Notes
-
Author contributions
Conceptualization: Kim JH, Sohn HJ, Walz SE, Jung SY
Data curation: Kim JH, Sohn HJ, Shin HJ, Walz SE, Jung SY
Formal analysis: Kim JH, Sohn HJ, Shin HJ, Walz SE, Jung SY
Investigation: Kim JH, Sohn HJ
Resources: Kim JH, Sohn HJ, Shin HJ, Jung SY
Supervision: Kim JH, Sohn HJ, Jung SY
Validation: Kim JH, Sohn HJ, Jung SY
Writing – original draft: Kim JH, Sohn HJ, Jung SY
Writing – review & editing: Kim JH, Sohn HJ, Shin HJ, Walz SE, Jung SY
-
The authors declare no conflict of interest related to this study.
Fig. 1Expression of a Nfa1 protein in pseudopodia. (A) N. fowleri trophozoite observed by scanning electron microscopy. N. fowleri trophozoites observed by fluorescence microscopy with anti-rabbit Nfa1 polyclonal antibody (B) and anti-mouse Nfa1 monoclonal antibody (C), respectively. (A) and (C) showed a trophozoite with characteristically active pseudopodia movements.
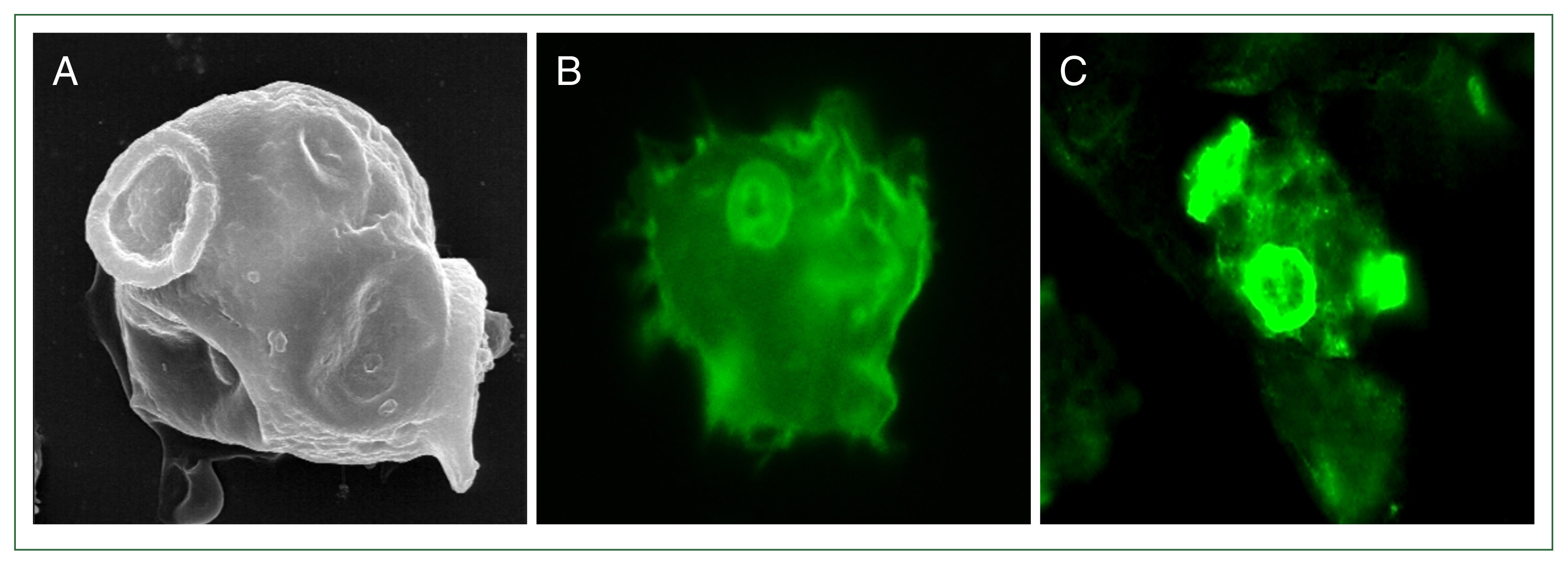
Table 1Major pathogenic factors of
N. fowleri [
10,
11]
Table 1
|
Food-cup |
|
|
Heat-shock protein 70 (HSP70) |
- Involved in regulating the host’s immune system, including protein folding, responses to amoeba survival - Plays a role in amoeba-mediated lysis of target cells
|
|
Naegleriapores (Naegleria pore-forming proteins) |
|
|
Elastase |
|
|
High mobility group protein (HMG) 26S proteasome subunit |
|
|
Nfa1 |
|
|
Cathepsin B |
- Cysteine protease of about 38 kDa - Important role in the proteolysis of immunoglobulin, collagen, fibronectin
|
|
Cytoskeleton (e.g., actin) |
|
References
- 1. Siddiqui R, Ali IKM, Cope JR, Khan NA. Biology and pathogenesis of Naegleria fowleri. Acta Trop 2016;164:375-394.
https://doi.org/10.1016/j.actatropica.2016.09.009
- 2. Jahangeer M, Mahmood Z, Munir N, Waraich UEA, Tahir IM, et al.
Naegleria fowleri: sources of infection, pathophysiology, diagnosis, and management; a review. Clin Exp Pharmacol Physiol 2020;47(2):199-212.
https://doi.org/10.1111/1440-1681.13192
- 3. Hall AD, Kumar JE, Golba CE, Luckett KM, Bryant WK. Primary amebic meningoencephalitis: a review of Naegleria fowleri and analysis of successfully treated cases. Parasitol Res 2024;123(1):84.
https://doi.org/10.1007/s00436-023-08094-w
- 4. The Gurdian. Texas residents warned of tap water tainted with brain-eating microbe[Internet]; Sep. 26. 2020. Available from: https://www.theguardian.com/us-news/2020/sep/26/texas-tap-water-tainted-brain-eating-microbe
- 5. Bellini NK, Santos TM, da Silva MTA, Thiemann OH. The therapeutic strategies against Naegleria fowleri. Exp Parasitol 2018;187:1-11.
https://doi.org/10.1016/j.exppara.2018.02.010
- 6. Herman EK, Greninger A, van der Giezen M, Ginger ML, Ramirez-Macias I, et al. Genomics and transcriptomics yields a system-level view of the biology of the pathogen Naegleria fowleri. BMC Biol 2021;19(1):142.
https://doi.org/10.1186/s12915-021-01078-1
- 7. Serrano-Luna J, Cervantes-Sandoval I, Tsutsumi V, Shibayama M. A biochemical comparison of proteases from pathogenic Naegleria fowleri and non-pathogenic Naegleria gruberi. J Eukaryot Microbiol 2007;54(5):411-417.
https://doi.org/10.1111/j.1550-7408.2007.00280.x
- 8. Cervantes-Sandoval I, Jesús Serrano-Luna J, Pacheco-Yépez J, Silva-Olivares A, Tsutsumi V, et al. Differences between Naegleria fowleri and Naegleria gruberi in expression of mannose and fucose glycoconjugates. Parasitol Res 2010;106(3):695-701.
https://doi.org/10.1007/s00436-010-1727-z
- 9. Son DH, Kim EJ, Matin A, Jung SY. Interaction between Naegleria fowleri and pathogenic Escherichia coli by mannose and changes in N. fowleri protease. Parasitol Res 2022;121(6):1805-1809.
https://doi.org/10.1007/s00436-022-07513-8
- 10. Zysset-Burri DC, Müller N, Beuret C, Heller M, Schürch N, et al. Genome-wide identification of path-ogenicity factors of the free-living amoeba Naegleria fowleri. BMC Genomics 2014;15(1):496.
https://doi.org/10.1186/1471-2164-15-496
- 11. Abo El-Maaty DA, Hamza RS. Primary amoebic meningoencephalitis caused by Naegleria fowleri. Parasitol Uni J 2012;5(2):93-104.
- 12. Oh YH, Jeong SR, Kim JH, Song KJ, Kim K, et al. Cytopathic changes and pro-inflammatory cytokines induced by Naegleria fowleri trophozoites in rat microglial cells and protective effects of an anti-Nfa1 antibody. Parasite Immunol 2005;27(12):453-459.
https://doi.org/10.1111/j.1365-3024.2005.00799.x
- 13. Song KJ, Jeong SR, Park S, Kim K, Kwon MH, et al.
Naegleria fowleri: functional expression of the Nfa1 protein in transfected Naegleria gruberi by promoter modification. Exp Parasitol 2006;112(2):115-120.
https://doi.org/10.1016/j.exppara.2005.10.004
- 14. Jenkins MA. Serum protein electrophoresis. clinical applications of capillary electrophoresis. Method Mol Med 1999;27:11-20.
https://doi.org/10.1385/MB:15:3:201
- 15. Shin HJ, Cho MS, Jung SY, Kim HI, Park S, et al. Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of Naegleria fowleri. J Eukaryot Microbiol 2001;48(6):713-717.
https://doi.org/10.1111/j.1550-7408.2001.tb00211.x
- 16. Takagi T, Cox JA. Primary structure of myohemerythrin from the annelid Nereis diversicolor. FEBS Lett 1991;285(1):25-27.
https://doi.org/10.1016/0014-5793(91)80716-g
- 17. Bailly X, Vanin S, Chabasse C, Mizuguchi K, Vinogradov SN. A phylogenomic profile of hemerythrins, the nonheme diiron binding respiratory proteins. BMC Evol Biol 2008;8(244):1-11.
https://doi.org/10.1186/1471-2148-8-244
- 18. Klippenstein GL, Van Riper DA, Oostrom EA. A comparative study of the oxygen transport proteins of Dendrostomum pyroides: isolation and characterization of hemerythrins from muscle, the vascular system, and the coelom. J Biol Chem 1972;247(18):5959-5963.
- 19. Stenkamp RE, Sieker LC, Jensen LH, McCallum JD, Sanders-Loehr J. Active site structures of deoxyhemerythrin and oxyhemerythrin. Proc Natl Acad Sci 1985;82(3):713-716.
https://doi.org/10.1073/pnas.82.3.713
- 20. Kang SY, Song KJ, Jeong SR, Kim JH, Park S, et al. Role of the Nfa1 protein in pathogenic Naegleria fowleri cocultured with CHO target cells. Clin Diagn Lab Immunol 2005;12(7):873-876.
https://doi.org/10.1128/CDLI.12.7.873-876.2005
- 21. Cho MS, Jung SY, Park S, Kim K, Kim HI, et al. Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic Naegleria fowleri. Clin Diagn Lab Immmunol 2003;10(5):954-959.
https://doi.org/10.1128/cdli.10.5.954-959.2003
- 22. Jeong SR, Kang SY, Lee SC, Song KJ, Im KI, et al. Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria fowleri. Korean J Parasitol 2004;42(1):35-40.
https://doi.org/10.3347/kjp.2004.42.1.35
- 23. Lee YJ, Kim JH, Jeong SR, Song KJ, Kim K, et al. Production of Nfa1-specific monoclonal antibodies that influences the in vitro cytotoxicity of Naegleria fowleri trophozoites on microglial cells. Parasitol Res 2007;101(5):1191-1196.
https://doi.org/10.1007/s00436-007-0600-1
- 24. Agrawal N, Dasaradhi PVN, Mohmmed A, Malhotra P, Bhatnagar RK, et al. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev 2003;67(4):657-685.
https://doi.org/10.1128/MMBR.67.4.657-685.2003
- 25. Motyka SA, Englund PT. RNA interference for analysis of gene function in trypanosomatids. Curr Opin Microbiol 2004;7(4):362-368.
https://doi.org/10.1016/j.mib.2004.06.004
- 26. Bakhetia M, Charlton WL, Urwin PE, McPherson MJ, Atkinson HJ. RNA interference and plant parasitic nematodes. Trends Plant Sci 2005;10(8):362-367.
https://doi.org/10.1016/j.tplants.2005.06.007
- 27. Jung SY, Kim JH, Song KJ, Lee YJ, Kwon MH, et al. Gene silencing of nfa1 affects the in vitro cytotoxicity of Naegleria fowleri in murine macrophages. Mol Biochem Parasitol 2009;165(1):87-93.
https://doi.org/10.1016/j.molbiopara.2009.01.007
- 28. Jung SY, Kim JH, Lee YJ, Song KJ, Kim K, et al.
Naegleria fowleri: nfa1 gene knock-down by double-stranded RNAs. Exp Parasitol 2008;118(2):208-213.
https://doi.org/10.1016/j.exppara.2007.08.008
- 29. Jeong SR, Lee SC, Song KJ, Park S, Kim K, et al. Expression of the nfa1 gene cloned from pathogenic Naegleria fowleri in nonpathogenic N. gruberi enhances cytotoxicity against CHO target cells in vitro. Infect Immun 2005;73(7):4098-4105.
https://doi.org/10.1128/IAI.73.7.4098-4105.2005
- 30. Marciano-Cabral F, Cabral GA. The immune response to Naegleria fowleri amebae and pathogenesis of infection. FEMS Immunol Med Microbiol 2007;51(2):243-259.
https://doi.org/10.1111/j.1574-695X.2007.00332.x
- 31. Cerva L.
Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. J Hyg Epidemiol Microbiol Immunol 1989;33(1):99-103.
- 32. Cursons RT, Keys EA, Brown TJ, Learmonth J, Campbell C, et al. IgA and primary amoebic meningoencephalitis. Lancet 1979;1(8109):223-224.
https://doi.org/10.1016/s0140-6736(79)90635-4
- 33. Park KM, Ryu JS, Im KI. Blastogenic responses of splenic lymphocytes to Naegleria fowleri lysates and T-cell mitogen in mice with primary amoebic meningoencephalitis. Korean J Parasitol 1987;25(1):1-6.
https://doi.org/10.3347/kjp.1987.25.1.1
- 34. Ferrante A. Free-living amoebae: pathogenicity and immunity. Parasite Immunol 1991;13(1):31-47.
https://doi.org/10.1111/j.1365-3024.1991.tb00261.x
- 35. Thong YH, Ferrante A, Rowan-Kelly B, O’Keefe D. Immunization with live amoebae, amoebic lysate and culture supernatant in experimental Naegleria meningoencephalitis. Trans R Soc Trop Med Hyg 1980;74(5):570-576.
https://doi.org/10.1016/0035-9203(80)90141-8
- 36. Reilly MF, White KL, Bradley SG. Host resistance of mice to Naegleria fowleri infections. Infect Immun 1983;42(2):645-652.
https://doi.org/10.1128/iai.42.2.645-652.1983
- 37. Lee YJ, Kim JH, Sohn HJ, Lee J, Jung SY, et al. Effects of immunization with the rNfa1 protein on experimental Naegleria fowleri-PAM mice. Parasite Immunol 2011;33(7):382-389.
https://doi.org/10.1111/j.1365-3024.2011.01296.x
- 38. Kurono Y, Fujiyoshi T, Mogi G. Secretory IgA and bacterial adherence to nasal mucosal cells. Ann Otol Rhinol Laryngol 1989;98(4 Pt 1):273-277.
https://doi.org/10.1177/000348948909800407
- 39. Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, et al. Direct gene transfer into mouse muscle in vivo. Science 1990;247(4949 Pt 1):1465-1468.
https://doi.org/10.1126/science.1690918
- 40. Ahmed SB, Touihri L, Chtourou Y, Dellagi K, Bahloul C. DNA based vaccination with a cocktail of plasmids encoding immunodominant Leishmania (Leishmania) major antigens confers full protection in BALB/c mice. Vaccine 2009;27(1):99-106.
https://doi.org/10.1016/j.vaccine.2008.10.013
- 41. Martínez MB, Rodríguez MA, García-Rivera G, Sánchez T, Hernández-Pando R, et al. A pcDNA-Ehcpadh vaccine against Entamoeba histolytica elicits a protective Th1-like response in hamster liver. Vaccine 2009;27(31):4176-4186.
https://doi.org/10.1016/j.vaccine.2009.04.051
- 42. Khosroshahi KH, Ghaffarifar F, Sharifi Z, D’Souza S, Dalimi A, et al. Comparing the effect of IL-12 genetic adjuvant and alum non-genetic adjuvant on the efficiency of the cocktail DNA vaccine containing plasmids encoding SAG-1 and ROP-2 of Toxoplasma gondii. Parasitol Res 2012;111(1):403-411.
https://doi.org/10.1007/s00436-012-2852-7
- 43. Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques 1989;7(9):980-990.
- 44. Negri DR, Michelini Z, Bona R, Blasi M, Filati P, et al. Integrase-defective lentiviral-vector based vaccine: a new vector for induction of T cell immunity. Expert Opin Biol Ther 2011;11(6):739-750.
https://doi.org/10.1517/14712598.2011.571670
- 45. Kim JH, Lee SH, Sohn HJ, Lee J, Chwae YJ, et al. The immune response induced by DNA vaccine expressing nfa1 gene against Naegleria fowleri. Parasitol Res 2012;111(6):2377-2384.
https://doi.org/10.1007/s00436-012-3093-5
- 46. Kim JH, Sohn HJ, Lee J, Yang HJ, Chwae YJ, et al. Vaccination with lentiviral vector expressing the nfa1 gene confers a protective immune response to mice infected with Naegleria fowleri. Clin Vaccine Immunol 2013;20(7):1055-1060.
https://doi.org/10.1128/CVI.00210-13
- 47. Gurunathan S, Wu C, Freidag BL, Seder RA. DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol 2000;12(4):442-447.
https://doi.org/10.1016/s0952-7915(00)00118-7
- 48. Rappuoli R, Pizza M, Douce G, Dougan G. Structure and mucosal adjuvanticity of cholera and Escherichia coli heat-labile enterotoxins. Immunol Today 1999;20(11):493-500.
https://doi.org/10.1016/s0167-5699(99)01523-6
- 49. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005;11(suppl):S45-S53.
https://doi.org/10.1038/nm1213
- 50. Lee J, Yoo JK, Sohn HJ, Kang HK, Kim D, et al. Protective immunity against Naegleria fowleri infection on mice immunized with the rNfa1 protein using mucosal adjuvants. Parasitol Res 2015;114(4):1377-1385.
https://doi.org/10.1007/s00436-015-4316-3
- 51. Song KJ, Song KH, Na BK, Kim JH, Kwon DH, et al. Molecular cloning and characterization of a cytosolic heat shock protein 70 from Naegleria fowleri. Parasitol Res 2007;100(5):1083-1089.
https://doi.org/10.1007/s00436-006-0404-8
- 52. Song KJ, Song KH, Kim JH, Sohn HJ, Lee YJ, et al. Heat shock protein 70 of Naegleria fowleri is important factor for proliferation and in vitro cytotoxicity. Parasitol Res 2008;103(2):313-317.
https://doi.org/10.1007/s00436-008-0972-x
- 53. Sohn HJ, Song KJ, Kang H, Ham AJ, Lee JH, et al. Cellular characterization of actin gene concerned with contact-dependent mechanisms in Naegleria fowleri. Parasite Immunol 2019;41(8):e12631.
https://doi.org/10.1111/pim.12631
- 54. Kim JH, Yang AH, Sohn HJ, Kim D, Song KJ, et al. Immunodominant antigens in Naegleria fowleri excretory–secretory proteins were potential pathogenic factors. Parasitol Res 2009;105(6):1675-1681.
https://doi.org/10.1007/s00436-009-1610-y
- 55. Klinkert MQ, Felleisen R, Link G, Ruppel A, Beck E. Primary structure of Sm31/32 diagnostic proteins of Schistosoma mansoni and their identification as proteases. Mol Biochem Parasitol 1989;33(2):113-122.
- 56. Brindley PJ, Kalinna BH, Dalton JP, Day SR, Wong JY, et al. Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol 1997;89(1):1-9.
https://doi.org/10.1016/s0166-6851(97)00098-4
- 57. Lee J, Kim JH, Sohn HJ, Yang HJ, Na BK, et al. Novel cathepsin B and cathepsin B-like cysteine protease of Naegleria fowleri excretory–secretory proteins and their biochemical properties. Parasitol Res 2014;113(8):2765-2776.
https://doi.org/10.1007/s00436-014-3936-3
- 58. Seong GS, Sohn HJ, Kang H, Seo GE, Kim JH, et al. Production and characterization of monoclonal antibodies against cathepsin B and cathepsin B-Like proteins of Naegleria fowleri. Exp Parasitol 2017;183:171-177.
https://doi.org/10.1016/j.exppara.2017.09.004
- 59. Alsam S, Sissons J, Dudley R, Khan NA. Mechanisms associated with Acanthamoeba castellanii (T4) phagocytosis. Parasitol Res 2005;96:402-409.
https://doi.org/10.1007/s00436-005-1401-z
- 60. Shin HJ, Im KI. Pathogenic free-living amoebae in Korea. Korean J Parasitol 2004;42(3):93-119.
https://doi.org/10.3347/kjp.2004.42.3.93
- 61. Carter RF. Primary amoebic meningo-encephalitis: clinical, pathological and epidemiológica features of six fatal cases. J Pathol Bacteriol 1968;96(1):1-25.
https://doi.org/10.1002/path.1700960102
- 62. Carter RF. Primary amoebic meningo-encephalitis. An appraisal of present knowledge. Trans R Soc Trop Med Hyg 1972;66(2):193-208.
https://doi.org/10.1016/0035-9203(72)90147-2
- 63. Anderson K, Jamieson A. Agglutination test for the investigation of the genus Naegleria. Pathology 1972;4(4):273-278.
https://doi.org/10.3109/00313027209068953
- 64. De Jonckheere J. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl Environ Microbiol 1980;39(4):681-685.
https://doi.org/10.1128/aem.39.4.681-685.1980
- 65. Cline M, Carchman R, Marciano-Cabral F. Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J Protozool 1986;33(1):10-13.
https://doi.org/10.1111/j.1550-7408.1986.tb05547.x
- 66. Marciano-Cabral F, John DT. Cytopathogenicity of Naegleria fowleri for rat neuroblastoma cell cultures: scanning electron microscopy study. Infect Immun 1983;40(3):1214-1217.
https://doi.org/10.1128/iai.40.3.1214-1217.1983
- 67. Marciano-Cabral F, Cline ML, Bradley SG. Specificity of antibodies from human sera for Naegleria species. J Clin Microbiol 1987;25(4):692-697.
https://doi.org/10.1128/jcm.25.4.692-697.1987
- 68. Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 1996;84(3):345-357.
https://doi.org/10.1016/s0092-8674(00)81279-9
- 69. Bretscher A. Microfilament structure and function in the cortical cytoskeleton. Annu Rev Cell Biol 1991;7(1):337-374.
https://doi.org/10.1146/annurev.cb.07.110191.002005
- 70. Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol 1995;5(3):89-93.
https://doi.org/10.1016/s0962-8924(00)88956-4
- 71. Sohn HJ, Kim JH, Shin MH, Song KJ, Shin HJ. The Nf-actin gene is an important factor for food-cup formation and cytotoxicity of pathogenic Naegleria fowleri. Parasitol Res 2010;106:917-924.
https://doi.org/10.1007/s00436-010-1760-y
- 72. Jentsch S. The ubiquitin-conjugation system. Annu Rev Genet 1992;26(1):179-207.
https://doi.org/10.1146/annurev.ge.26.120192.001143
- 73. Li E, Yang W, Zhang T, Stanley SL Jr. Interaction of laminin with Entamoeba histolytica cysteine proteinases and its effect on amebic pathogenesis. Infect Immun 1995;63(10):4150-4153.
https://doi.org/10.1128/iai.63.10.4150-4153.1995
- 74. Shibayama M, Serrano-Luna JDJ, Rojas-Hernández S, Campos-Rodríguez R, Tsutsumi V. Interaction of secretory immunoglobulin A antibodies with Naegleria fowleri trophozoites and collagen type I. Canadian J Microbiol 2003;49(3):164-170.
https://doi.org/10.1139/w03-023
- 75. Han KL, Lee HJ, Shin MH, Shin HJ, Im KI, et al. The involvement of an integrin-like protein and protein kinase C in amoebic adhesion to fibronectin and amoebic cytotoxicity. Parasitol Res 2004;94(1):53-60.
https://doi.org/10.1007/s00436-004-1158-9
- 76. Rocha-Azevedo BD, Jamerson M, Cabral GA, Silva-Filho FC, Marciano-Cabral F.
Acanthamoeba interaction with extracellular matrix glycoproteins: biological and biochemical characterization and role in cytotoxicity and invasiveness. J Eukaryot Microbiol 2009;56(3):270-278.
https://doi.org/10.1111/j.1550-7408.2009.00399.x
- 77. Gordon VR, Asem EK, Vodkin MH, McLaughlin GL.
Acanthamoeba binds to extracellular matrix proteins in vitro. Investig Ophthalmol Vis Sci 1993;34(3):658-662.
- 78. Coxon PY, Summersgill JT, Ramirez JA, Miller RD. Signal transduction during Legionella pneumophila entry into human monocytes. Infect Immun 1998;66(6):2905-2913.
https://doi.org/10.1128/IAI.66.6.2905-2913.1998
- 79. Khan NA, Wang Y, Kim KJ, Chung JW, Wass CA, et al. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J Biol Chem 2002;277(18):15607-15612.
https://doi.org/10.1074/jbc.M112224200
- 80. Sohn HJ, Kang H, Seo GE, Kim JH, Jung SY, et al. Efficient liquid media for encystation of pathogenic free-living amoebae. Korean J Parasitol 2017;55(3):233-238.
https://doi.org/10.3347/kjp.2017.55.3.233
- 81. Neff R, Ray S, Benton W, Wilborn M. Induction of synchronous encystment (differentiation) in Acanthamoeba sp. Method Cell Biol 1964;1:55-83.
https://doi.org/10.1242/jcs.91.3.389
- 82. Chagla A, Griffiths A. Growth and encystation of Acanthamoeba castellanii. Microbiol 1974;85(1):139-145.
https://doi.org/10.1099/00221287-85-1-139
- 83. Moon EK, Chung DI, Hong YC, Ahn TI, Kong HH.
Acanthamoeba castellanii: gene profile of encystation by ESTs analysis and KOG assignment. Exp Parasitol 2008;119(1):111-116.
https://doi.org/10.1016/j.exppara.2008.01.001
- 84. Phan IQ, Rice CA, Craig J, Noorai RE, McDonald JR, et al. The transcriptome of Balamuthia mandrillaris trophozoites for structure-guided drug design. Sci Rep 2021;11(1):21664.
https://doi.org/10.1038/s41598-021-99903-8
- 85. Siddiqui R, Rajendran K, Abdella B, Ayub Q, Lim SY, et al.
Naegleria fowleri: differential genetic expression following treatment with Hesperidin conjugated with silver nanoparticles using RNA-Seq. Parasitol Res 2020;119:2351-2358.
https://doi.org/10.1007/s00436-020-06711-6
- 86. Sohn HJ, Kim JH, Kim K, Park S, Shin HJ. De novo transcriptome profiling of Naegleria fowleri trophozoites and cysts via RNA sequencing. Pathogens 2023;12(2):174.
https://doi.org/10.3390/pathogens12020174
- 87. Tavares P, Rigothier MC, Khun H, Roux P, Huerre M, et al. Roles of cell adhesion and cytoskeleton activity in Entamoeba histolytica pathogenesis: a delicate balance. Infect Immun 2005;73(3):1771-1778.
https://doi.org/10.1128/IAI.73.3.1771-1778.2005
- 88. Labruyère E, Guillén N. Host tissue invasion by Entamoeba histolytica is powered by motility and phagocytosis. Arch Med Res 2006;37(2):252-257.
https://doi.org/10.1016/j.arcmed.2005.10.005
- 89. Saito-Nakano Y, Wahyuni R, Nakada-Tsukui K, Tomii K, Nozaki T. Rab7D small GTPase is involved in phago-, trogocytosis and cytoskeletal reorganization in the enteric protozoan Entamoeba histolytica. Cell Microbiol 2021;23(1):e13267.
https://doi.org/10.1111/cmi.13267
- 90. Heaslip AT, Dzierszinski F, Stein B, Hu K. TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii. PLoS pathogens 2010;6(2):e1000754.
https://doi.org/10.1371/journal.ppat.1000754
- 91. Bouyer S, Rodier MH, Guillot A, Hechard Y.
Acanthamoeba castellanii: proteins involved in actin dynamics, glycolysis, and proteolysis are regulated during encystation. Exp Parasitol 2009;123(1):90-94.
https://doi.org/10.1016/j.exppara.2009.06.006
- 92. Gonzalez-Robles A, Salazar-Villatoro L, Omana-Molina M, Lorenzo-Morales J, Martinez-Palomo A.
Acanthamoeba royreba: morphological features and in vitro cytopathic effect. Exp Parasitol 2013;133(4):369-375.
https://doi.org/10.1016/j.exppara.2013.01.011
- 93. Didry D, Carlier MF, Pantaloni D. Synergy between actin depolymerizing factor/cofilin and profilin in increasing actin filament turnover. J Biol Chem 1998;273(40):25602-25611.
https://doi.org/10.1074/jbc.273.40.25602
- 94. Ressad F, Didry D, Egile C, Pantaloni D, Carlier MF. Control of actin filament length and turnover by actin depolymerizing factor (ADF/cofilin) in the presence of capping proteins and ARP2/3 complex. J Biol Chem 1999;274(30):20970-20976.
https://doi.org/10.1074/jbc.274.30.20970