Abstract
Schistosomiasis remains one of the most prevalent neglected tropical diseases in Tanzania. World Vision Tanzania, in collaboration with the Ministry of Health through the National Neglected Tropical Diseases Control Programme, implemented school- and community-based mass drug administrations, community-led total sanitation, and community voice and action from 2020 to 2022. This study assessed changes in the prevalence of schistosomiasis in the Itilima district of northwestern Tanzania following the implementation of these integrated interventions. A total of 1,405 students from 22 schools participated in the baseline survey in August to September 2020, and 1,320 in September 2022. Additionally, 368 adults from 8 villages participated in the baseline survey, and 401 in the endline survey. The prevalence difference was calculated to assess changes before and after the integrated interventions. We also investigated risk factors for Schistosoma haematobium infection using endline data. The prevalence difference between 2020 and 2022 was −20.0% (95% confidence interval (CI)=−22.2%–−17.7%, P<0.001) for students and −19.6% (95% CI=−22.2%–−17.7%, P<0.001) for adults. Individuals without a latrine were more likely to have schistosomiasis (adjusted odds ratio=5.9, 95% CI=1.7–21.5, P=0.01) compared to those who had a latrine. The findings indicate substantial changes in schistosomiasis prevalence in the study area following the implementation of integrated interventions. To sustain these achievements in Itilima, a multi-sectorial approach is highly recommended to integrate additional measures for eliminating schistosomiasis as a public health problem.
-
Key words: Schistosomiasis, prevalence, integrated intervention, Tanzania, risk factors
Schistosomiasis remains one of the most prevalent neglected tropical diseases, causing significant morbidity in Sub-Saharan Africa (SSA) [
1,
2]. The most common species causing this disease in the region are
Schistosoma haematobium and
Schistosoma mansoni. Globally, schistosomiasis affects over 290 million people, with more than 779 million residing in areas of high transmission [
2,
3]. Notably, 93% of those infected are in SSA [
1,
2], and about 76% of these individuals live in high-transmission zones. An estimated 120 million people exhibit symptoms related to schistosomiasis, and the disease accounts for over 2.8 million disability-adjusted life years [
2]. School-aged children (SAC) experience the highest prevalence and intensity of schistosome infections, while adults generally show a lower intensity of infection [
2]. Additionally, recent reports indicate that pre-school children in SSA also suffer from high infection intensities [
4].
The strategy for controlling schistosomiasis infections involves mass drug administration (MDA) of praziquantel (PZQ) to children. The World Health Organization (WHO) recommends annual treatment when the baseline prevalence of soil-transmitted helminths (STH) in the community is 20% or higher, or 10% or higher for schistosomiasis [
3]. In cases where endemic communities with a schistosomiasis prevalence of at least 10% do not respond adequately to annual treatment despite sufficient coverage, the WHO advises biannual preventive chemotherapy [
3]. Furthermore, the WHO has established a global goal to eliminate morbidity caused by STH and schistosomiasis infections in children by 2030, targeting treatment for at least 75% of children in affected areas [
3].
Data from the National Neglected Tropical Disease Programme in Tanzania show that the Itilima district was highly endemic for
S. haematobium infection before World Vision implemented its intervention [
5]. The district exhibits varying levels of
S. haematobium endemicity and continues to experience high transmission rates in some villages despite repeated rounds of MDA [
6]. Additionally, the district's environmental conditions are conducive to the transmission of
S. haematobium [
6]. According to data from the National Strategic Plan for controlling schistosomiasis, the overall prevalence of
S. haematobium infection in the district exceeded 50% [
7]. In the most recent baseline surveys, the prevalence of
S. haematobium varied significantly between villages, with Budalabujiga and Sasago recording prevalence of 26.1% and 57.9%, respectively [
8]. The prevalence of
S. mansoni and STHs (
Ascaris lumbricoides,
Trichuris trichiura, and hookworms) was less than 1% [
8].
Against this backdrop, World Vision Tanzania has implemented school- and community-based MDAs, community-led total sanitation (CLTS), and community voice and action (CVA) programs from 2020 to 2022. These initiatives, aimed at combating schistosomiasis and STHs, were carried out in collaboration with the Ministry of Health through the National Neglected Tropical Diseases Control Programme.
The aim of this study was to assess changes in the prevalence of schistosomiasis in the Itilima district of northwestern Tanzania following the implementation of an integrated intervention. Additionally, the study evaluated the risk factors associated with schistosomiasis infection among schoolchildren and adults.
The study was conducted in the Itilima district, which is located at a longitude of −3.73333 and a latitude of 33.48333 in the Simiyu region of northwestern Tanzania. According to the 2022 census, the district has a population of 419,213. Itilima experiences annual rainfall ranging from 930 to 1,200 mm and temperatures typically between 25°C and 28°C. The highest temperatures usually occur from August to October. The primary economic activities of the residents are farming, livestock keeping, and small-scale businesses.
School-based and community-based MDA were undertaken. During the school-based MDA, drugs were distributed to schools by the school head teacher and the health teacher over a period of 2 days. For the community-based MDA, community drug distributors visited homes to distribute PZQ drugs to the respective households. Both the school-based and community-based MDAs were conducted twice between the baseline and endline surveys. CLTS and CVA interventions were also conducted. CLTS aims to sensitize community members to the importance of improved hygiene and sanitation, encouraging local residents to voluntarily build toilets. CVA is a World Vision model designed to empower community members with knowledge, information, and skills about a particular issue, enabling them to speak about the issue, analyze its root causes, and take action. The entire intervention was implemented by World Vision Tanzania with financial support from the Korea International Cooperation Agency.
A school-and-community-based repeated cross-sectional design was employed. The study encompassed SAC aged 5–17 years from selected schools within the district, as well as adults aged 18 years and older residing in the village where these schools were located. The inclusion and exclusion criteria are presented in the
Supplementary Table S1.
We have obtained ethical approval from the Lake Zone Institutional Review Board (MR/53/100/648) for the baseline survey, as well as the National Ethical Committee, Lake Zone Institutional Review Board (MR/53/100/716) for the endline survey. Additionally, permission was sought from the Regional and District Administrative Authorities of Simiyu and Itilima districts. Two days prior to their participation, children were given Kiswahili-translated informed consent forms to take home to their parent(s)/guardian(s). They were also invited to school on the day of screening to provide their consent. An assent form was created for children aged 9–17 years, enabling them to read and understand the study procedures and objectives. For children younger than 8 years, consent to participate in the study was provided by their parents or guardians. All adult participants provided informed consent. To ensure confidentiality, all clinical and demographic data of the study participants were stored in a locked cabinet, and participants were identified using codes or barcoded identification numbers. Any child identified as being infected with either a schistosomiasis or STH by any of the diagnostic tests used in the study received treatment with PZQ (40 mg/kg) or albendazole, in accordance with WHO guidelines [
3].
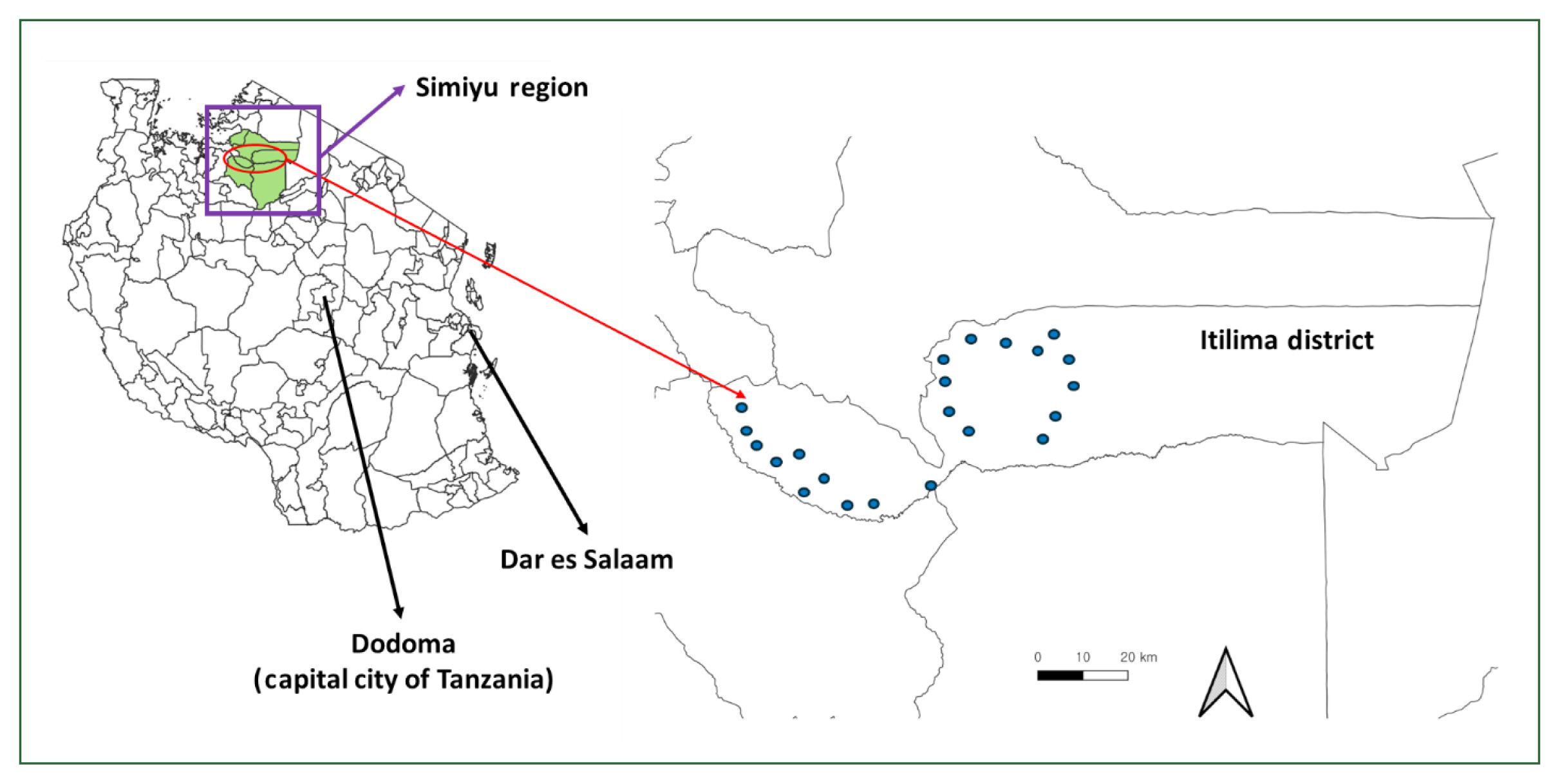
The district has 98 primary schools, 62 of which participated in baseline surveys. For the endline survey, 22 schools were purposefully selected from these 62 based on a baseline
S. haematobium infection prevalence greater than 10% (
Fig. 1). All 98 schools underwent interventions. Additionally, the district contains 102 villages. Of these, 19 and 22 villages were chosen for the baseline and endline assessments.
After selecting the schools, 60 students, which included 30 boys and 30 girls were sampled from each school. The WHO recommends including 50 children from each school in the study [
3]. To accommodate a 16% non-response rate, an additional 10 students were included beyond the WHO’s recommendation. A systematic random sampling technique, as previously described, was used to select children from each school. For community members (adult population), convenience sampling was employed. In each village, 50 individuals—25 females and 25 males—were invited to participate in the study.
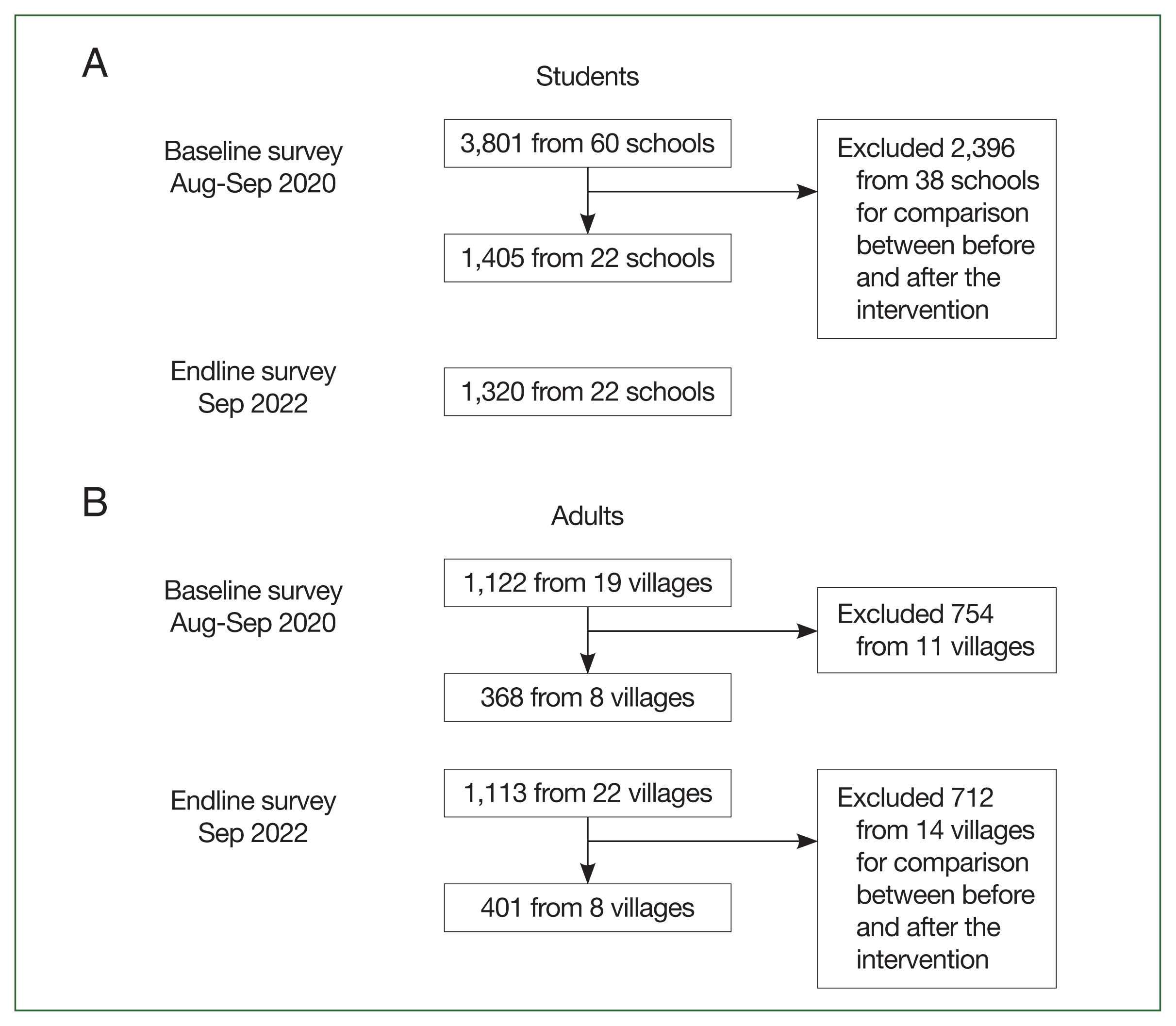
A total of 3,801 students from 60 schools participated in the baseline survey conducted in August to September 2020, and 1,320 students from 22 schools took part in the endline survey in September 2022. From those who participated in the baseline survey, we selected 1,405 students from the same 22 schools involved in the endline survey. This selection was made to facilitate a comparison of their prevalence (
Fig. 2A).
A total of 1,122 adults from 19 villages participated in the baseline survey conducted in August to September 2020, and 1,133 adults from 22 villages took part in the endline survey in September 2022. From those who participated, we selected 368 adults from the baseline and 401 from the endline in the same 8 villages for this study (
Fig. 2B).
Before initiating the research activities, the study team visited the district to hold planning and sensitization meetings with local authorities and leaders at the district, ward, village, and community levels. These key contacts played a crucial role in spreading information about the study. Meetings were also organized in communities and schools to inform parents and guardians about the research activities. The administrations of the schools and villages were briefed on the research activities and the scheduled dates for the visits. The project coordinator from the Catholic University of Health and Allied Sciences collaborated with the district neglected tropical disease coordinators from the departments of primary school education and district health. This partnership aimed to enhance awareness and support the smooth execution of the research activities. At each school, the headteacher and the health education teacher took on the responsibility of informing parents, guardians, and students about the research activities. Parents and guardians were invited to a sensitization meeting at the school, which took place 2 days before the sampling. The meetings aimed to explain the study procedures, treatments, and the significance of obtaining written informed consent for participation in the study.
Face-to-face interviews were conducted with selected children and adults using a pretested questionnaire in both school and community settings. The questionnaire was originally developed in English and then translated into Kiswahili. During field researcher training, the accuracy of this translation was verified, and all question-and-answer options were thoroughly discussed to ensure a uniform understanding among team members. Field supervisors were tasked with identifying the most locally relevant term for schistosomiasis in various localities. They achieved this through discussions with district health staff and community leaders, substituting the local term for the Kiswahili one provided in the questionnaire as necessary. Apart from these substitutions, field researchers read the questions exactly as they were written in the questionnaire. Each question was categorized as either a single- or multiple-response option. Field researchers then assigned one or more responses from a set of predefined answer options that best fit the participant's answers. The questionnaire gathered demographic data from the participants, as well as information on protective practices, including any protective behaviors and participation in MDA both at school and in the community.
A single stool sample was collected from each participant at baseline. Each sample was placed in a sealed, labeled container. The samples were then processed using the Kato-Katz (KK) thick smear technique, with each thick smear containing 41.7 mg of stool. For each sample, 2 thick KK smears were prepared and examined by 2 independent laboratory technicians employing the KK method. To ensure quality assurance, a third laboratory technician, who was blinded to the initial results, re-examined 20% of all positive and negative KK thick smears.
A single urine sample was collected from all participants between 10:00 and 14:00 at both schools and community locations. Each sample was initially examined for macro-hematuria and tested for micro-hematuria using a urine dipstick/urinalysis reagent strip (Mission Expert, ACON, San Diego, CA, USA). To screen for S. haematobium eggs, a urine filtration technique was employed, followed by the examination of the urine filters under light microscopy. For quality control purposes, a senior medical laboratory technician at each school re-examined 20% of the samples, including both positive and negative results, before the conclusion of the field day.
Data were entered into a Microsoft Excel sheet, cleaned, and then exported to Stata version 18 (StataCorp, College Station, TX, USA). The analysis aimed to determine the prevalence of the
S. haematobium,
S. mansoni, and STH. Continuous variables, such as age and egg intensities, were summarized using the mean±standard deviation. The intensity of infection was categorized according to WHO criteria: 1–99 epg, 100–399 epg, and ≥400 epg, defined as low, moderate, and heavy intensities of infection, respectively [
3]. For
S. haematobium infection, the estimated geometric mean egg output was calculated based only on the infected participants. Infection intensities were classified into 2 categories as per WHO recommendations: (i) light infection (<50 eggs/10 ml of urine) and (ii) heavy infection (≥50 eggs/10 ml of urine). The prevalence difference was calculated to assess changes before and after integrated interventions. A logistic regression model was employed to investigate the risk factors for
S. haematobium infection using endline data. In the bivariate analysis, all factors with a
P-value ≤0.2 were considered for multivariate analysis.
The mean age of students was 11.2 (2.6) and 12.0 (1.8) years in the baseline and endline, respectively. The mean age of adults was 30.0 (17.0) and 34.2 (17.4) years in the baseline and endline, respectively. Out of student participants, the proportion of boys were 51.0% (717/1,405) and 48.2% (636/1,320) in the baseline and endline, respectively. The proportion of male participants in the community-based survey were 40.0% (149/367) and 63.1% (253/401) in the baseline and endline, respectively.
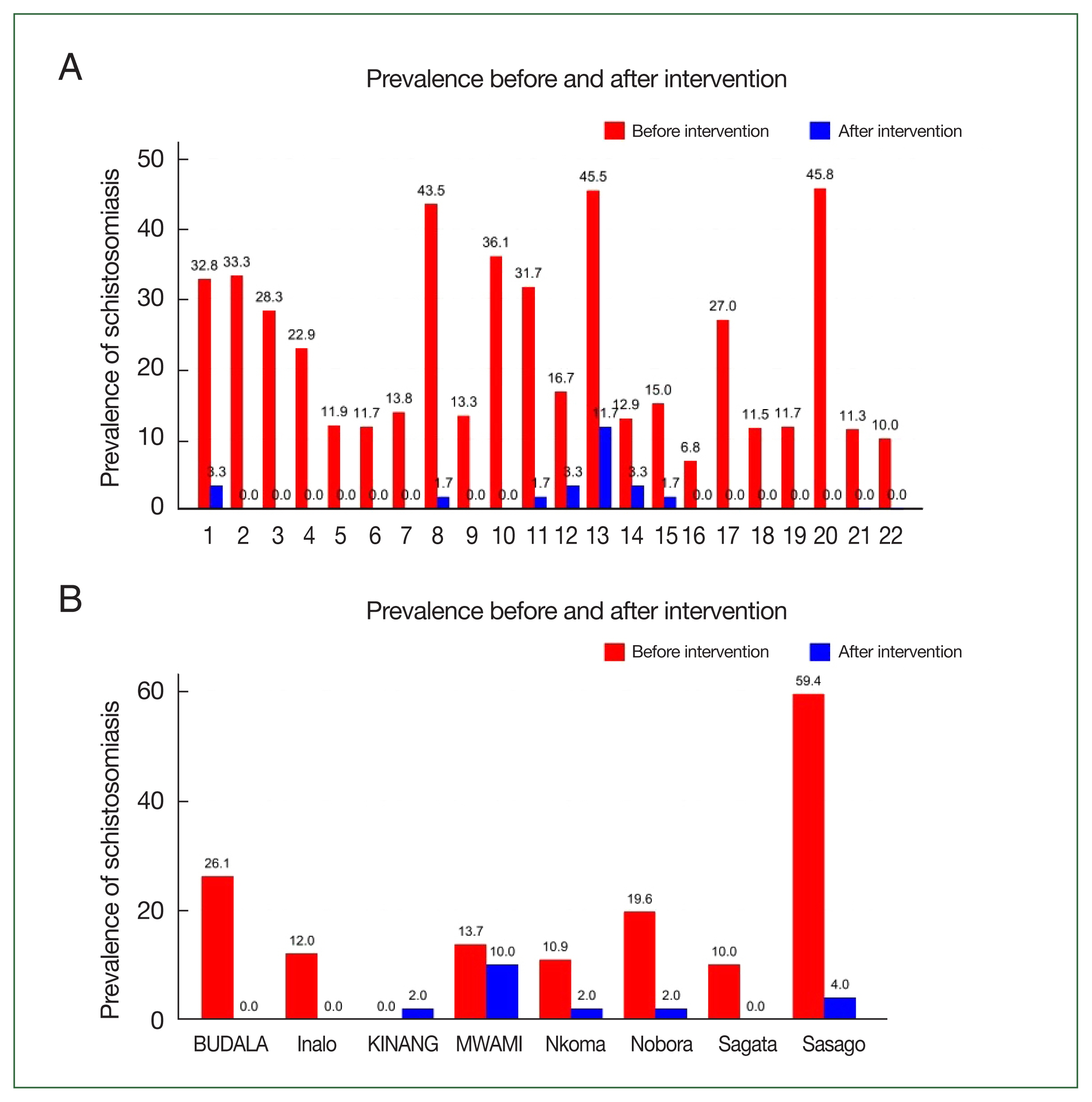
Fig. 3A illustrates the prevalence of schistosomiasis (
S. haematobium or
mansoni) among students before and after the intervention. In the 22 schools selected for this study, the prevalence of schistosomiasis decreased from 21.5% (302 out of 1,405) in 2020 to 1.5% (20 out of 1,320) in 2022. The difference in prevalence between 2020 and 2022 was −20.0% (95% confidence interval (CI)=−22.2%–−17.7%,
P<0.001) among students.
In 2020, the overall prevalence of S. haematobium among children from 60 schools was 10.1%, with 20.3% of those testing positive exhibiting heavy infection intensities (>50 eggs/10 ml). By 2022, the percentage of children with S. haematobium who had heavy infection intensities had decreased to 5.5%. The mean geometric egg infection intensity was recorded at 0.18 eggs/10 ml of urine. Additionally, the overall prevalence of microhematuria stood at 6.4% (84 out of 1,320).
In 2020, the overall prevalence of S. mansoni was found to be 0.3%, with no cases of heavy infection reported. Regarding STH infections, hookworm eggs were detected in 3 children, and 1 child was found to have eggs of T. trichiura. Additionally, another parasitic infection identified was Entamoeba histolytica/Entamoeba dispar, with a prevalence of 25.4%. In 2022, the overall prevalence of S. mansoni was 0.2% (2/1,320). The prevalence of A. lumbricoides was slightly lower at 0.08% (1/1,320). None of the SAC included in this study had infections of hookworm or T. trichiura.
Fig. 3B illustrates the prevalence of schistosomiasis (
S. haematobium or
S. mansoni) among adults before and after the intervention. In the 8 villages selected for this study, the prevalence of schistosomiasis among adults decreased from 22.1% (81/367) in 2020 to 0.0% (0/401) in 2022. The difference in prevalence between 2020 and 2022 was −19.6% (95% CI=−22.2%–−17.7%,
P<0.001) for adults.
In 2020, the overall prevalence of S. mansoni was 0.45%. None of the study participants, including both children and adults, were found to have any species of STH. In 2022, the overall prevalence of S. mansoni increased to 0.7%. Additionally, no eggs from any STH species were detected in the stool samples of the adults included in this study.
Tables 1 and
2 show the associations between schistosomiasis (
S. haematobium) and various risk factors. Among patients with SAC, no variables were found to be associated with schistosomiasis infection, except for a history of PZQ use (
Table 1). Children who had not taken PZQ had higher odds of having schistosomiasis (adjusted odds ratio (AOR)=5.4, 95% CI=1.8–16.5,
P=0.003) than those who had taken the de-wormer.
Among adults, individuals who did not use a latrine were more likely to have schistosomiasis (AOR=5.9, 95% CI=1.7–21.5, P=0.01) than those who did use a latrine regularly. The odds of contracting schistosomiasis for people who had come into contact with water 3 times or more per week were 8.8 times higher than for those who did not come into contact with water (AOR=8.8, 95% CI=1.5–50.8, P=0.02).
The prevalence of schistosomiasis significantly declined after the intervention. In 2020, the overall prevalence of
S. haematobium among those who participated in the survey was 10.1% in SAC and 8.1% in adults. Among those infected with schistosomiasis, 20.3% of SAC and 8.9% of adults, respectively, had heavy infection intensities [
9]. In both populations, the prevalence of STHs (hookworms,
T. trichiura, and
A. lumbricoides) was very low (<1%). After 3 years of comprehensive interventions with school-based and community-based MDA, CLTS, and CVA conducted by World Vision, the prevalence of
S. haematobium declined to 1.36% in SAC and 1.6% among adults in the target areas.
Previous studies have highlighted the significance of incorporating school community-based MDA [
10–
12]. Our findings further emphasize the necessity of integrating additional control measures in endemic countries to advance toward the elimination of schistosomiasis. Relying solely on mass-preventive chemotherapy is inadequate for the elimination of the disease.
The WHO has established criteria aimed at controlling the prevalence and morbidity of schistosomiasis, with the ambitious goal of eliminating it as a public health problem—defined as a heavy intensity infection rate of less than 1%—in all endemic countries by 2030 [
3,
13]. According to these criteria, morbidity control is considered achieved when the proportion of heavy infection intensity for any schistosome species is reduced to below 5% [
3]. In the current study, the proportion of heavy infection intensities declined to 5.5% among SAC and 5.7% among adult community members. To advance towards the elimination phase, the WHO has issued recommendations for administering MDA to either schoolchildren or community members, depending on the local prevalence of schistosomiasis [
3]. For communities in areas where the prevalence of any
Schistosoma species is 10% or higher, it is recommended to conduct a single annual round of MDA using PZQ, with a target coverage of at least 75% across all age groups starting from age 2 to effectively control morbidity [
3]. For communities in areas where the prevalence of any
Schistosoma species infection is below 10%, the WHO recommends either continuing mass preventive chemotherapy at the current frequency or reducing it to interrupt transmission. Alternatively, in areas that have not previously implemented mass preventive chemotherapy, a test-and-treat approach is recommended instead of mass preventive chemotherapy [
3].
To ensure comprehensive treatment coverage, the WHO advises health facilities to provide access to PZQ treatment for controlling morbidity due to schistosomiasis in individuals of all ages [
3]. However, the capacity of primary health facilities in many SSA countries to provide diagnosis and treatment remains uncertain [
3]. Environmental strategies, including water engineering and targeted snail control with molluscicides, along with behavioral modifications such as enhancing water supply, sanitation, and hygiene, are recommended to interrupt the transmission of schistosomiasis in endemic regions [
3]. The findings of this study indicate that schistosomiasis prevalence varies across different areas within the same district, supporting the WHO’s approach of tailoring interventions to local prevalence levels. Consequently, when planning future preventive chemotherapy campaigns, the national control program should evaluate the latest local prevalence data and adjust treatment strategies accordingly [
3].
Alternatively, to interrupt transmission in the future, it is essential to develop a targeted treatment strategy that focuses solely on schools and sub-districts classified as moderate-risk areas, rather than the existing approach that encompasses all schools. However, to facilitate informed decision-making, schistosomiasis must be mapped in all schools within an implementation unit, such as a district or ward.
For STHs (hookworms,
A. lumbricoides, and
T. trichiura) and intestinal schistosomiasis, both baseline and post-intervention data indicate that these infections do not pose a public health problem in the Itilima district. Previous studies support these findings [
14,
15]. In the Victorian Basin, intestinal schistosomiasis is highly endemic among communities residing along the lake’s shore. This prevalence is attributed to the intermediate host,
Biomphalaria snails, which thrive in permanent and large water bodies and do not undergo aestivation [
6]. Therefore,
S. mansoni infection is confined to the shoreline, predominantly affecting school children and adult community members [
6]. Similarly, in the lowland areas of northwestern Tanzania, hookworms are the predominant species for STH infections. They are commonly found along the lake’s shoreline and in the dry areas at the southern end of the lake basin [
14]. In this region, hookworms,
A. lumbricoides, and
T. trichiura are endemic among communities in the highland areas west of Lake Victoria.
Similarly, hookworms were the dominant species for STH infections in the lowland areas of northwestern Tanzania. It is commonly found along the lake shoreline and in the dry areas, which exhibit elevated temperatures conducive to the enhanced survival of its larval forms, at the southern end of the lake basin [
14]. In this region, hookworms
A. lumbricoides and
T. trichiura are endemic to communities living in the highland areas west of Lake Victoria, which are defined by a sub-tropical climate featuring abundant precipitation and relatively lower temperatures year-round, thereby enhancing the viability of parasites [
14]. However, after repeated interventions in Itilima district, northwestern Tanzania, the prevalence of STHs has substantially declined [
8,
9].
Among the components proposed for an integrated intervention are public health initiatives that focus on behavioral changes, health promotion, and the enhancement of clean water supply, sanitation, and hygiene [
16,
17]. The current study observed that a significant number of SAC and adults do not use toilets at home or school, which jeopardizes the progress made in reducing the prevalence and severity of schistosomiasis. These individuals may continue to transmit the disease to both treated and intermediate hosts. Additionally, the study revealed that the availability of clean water in Itilima district remains a significant health concern. Fewer than 50% of the surveyed population reported access to tap water, and other water sources used for domestic purposes could potentially act as vectors for schistosomiasis infection. It is imperative that the health sector collaborates with the water and sanitation sectors to ensure that communities in endemic areas have access to clean and safe water.
In the adult population, the frequency of water contact and irregular toilet use have been linked to schistosomiasis. It is well established that increased water contact, reliance on open water sources, open defecation, and irregular toilet use heightens the risk of schistosomiasis transmission. A previous study Cha et al. identified a correlation between
S. haematobium infections and the absence of toilets at home or school, as well as frequent contact with open water bodies such as rivers, streams, and irrigation canals in Sudan [
18]. They also highlighted the beneficial role of improved toilets in schools and homes [
18]. Our findings, in conjunction with those from other studies, underscore the necessity of combining additional interventions with MDA.
Improved water, sanitation, and hygiene (WASH) is recognized as a crucial factor in achieving the 2030 goal of eliminating schistosomiasis as a public health problem [
13]. Providing a better supply of clean water will decrease the reliance of endemic communities on contaminated water sources that are polluted with human feces and harbor
Schistosoma cercariae. Furthermore, ensuring that the entire community has access to and uses toilets will help to interrupt the transmission cycle of schistosomiasis. The reduction in
S. haematobium infection noted in the current study can be maintained if the WASH components are enhanced across all villages using resources that are locally available. However, the current single health sector approach in Tanzania will not suffice to meet the 2021–2030 goals or to sustain the progress observed in Itilima. A multi-sectorial approach that involves collaboration across various sectors is strongly recommended.
Some of the results presented in this study are based on information reported by the participants, which may be subject to recall bias. Additionally, the parasitological results that were used to define the prevalence and intensities of infection were determined from single urine and stool samples collected from the participants. This approach may lead to an underestimation of the actual values. The cross-sectional design of the study also precluded the collection of data on causality. However, this design allowed us to describe the prevalence and intensity of the targeted infections effectively. Despite these limitations, the findings reported here enable us to draw the following conclusions and make recommendations.
In conclusion, this study demonstrated substantial changes in schistosomiasis prevalence after the implementation of an integrated intervention. In order to sustain the achievements in Itilima, a multi-sectorial approach is highly recommended to incorporate other measures to eliminate schistosomiasis as a public health problem. Integrating water supply, sanitation, and hygiene practices necessitates collaboration among various stakeholders, which is crucial for disrupting the transmission of S. haematobium infection. Furthermore, the influence of social and cultural factors on the transmission of S. haematobium needs to be clearly understood and included in control strategies. Regarding environmental factors, the role of Bulinus species snails should be investigated. In areas where the prevalence remains above 10%, small-scale molluscicidal interventions could be considered as part of the control measures.
Notes
-
Author contributions
Conceptualization: Mazigo H
Data curation: Cho Y, Jin Y
Formal analysis: Mazigo H, Cho Y, Cha S
Investigation: Mazigo H
Methodology: Cho Y, Cha S
Software: Mazigo H
Project Administration: Lee J
Supervision: Mazigo H, Jin Y
Validation: Lee J, Jin Y
Writing – original draft: Mazigo H
Writing – review & editing: Lee J, Cho Y, Cha S, Jin Y
-
Conflict of interest
The authors declare no conflict of interest related to this study.
-
Acknowledgments
This research was supported by the Korea International Cooperation Agency and World Vision Korea under the title of “Neglected Tropical Diseases Elimination Project in Itilima District, Tanzania” between 2020 and 2022 (Letter of Grant Agreement for Korea International Cooperation Agency’s Public-Private Partnership Projects with Global Disease Eradication Fund No.2020-01).
We are grateful to the WV Tanzania Itilima NTD elimination project team, World Vision Tanzania Head Office, and World Vision Korea for their administrative and technical support. We extend our thanks to community members and students in the target areas for their participation in the study.
Supplementary Information
Fig. 1Locations of 22 schools participate in the baseline (August to September 2020) and endline survey (September 2022).
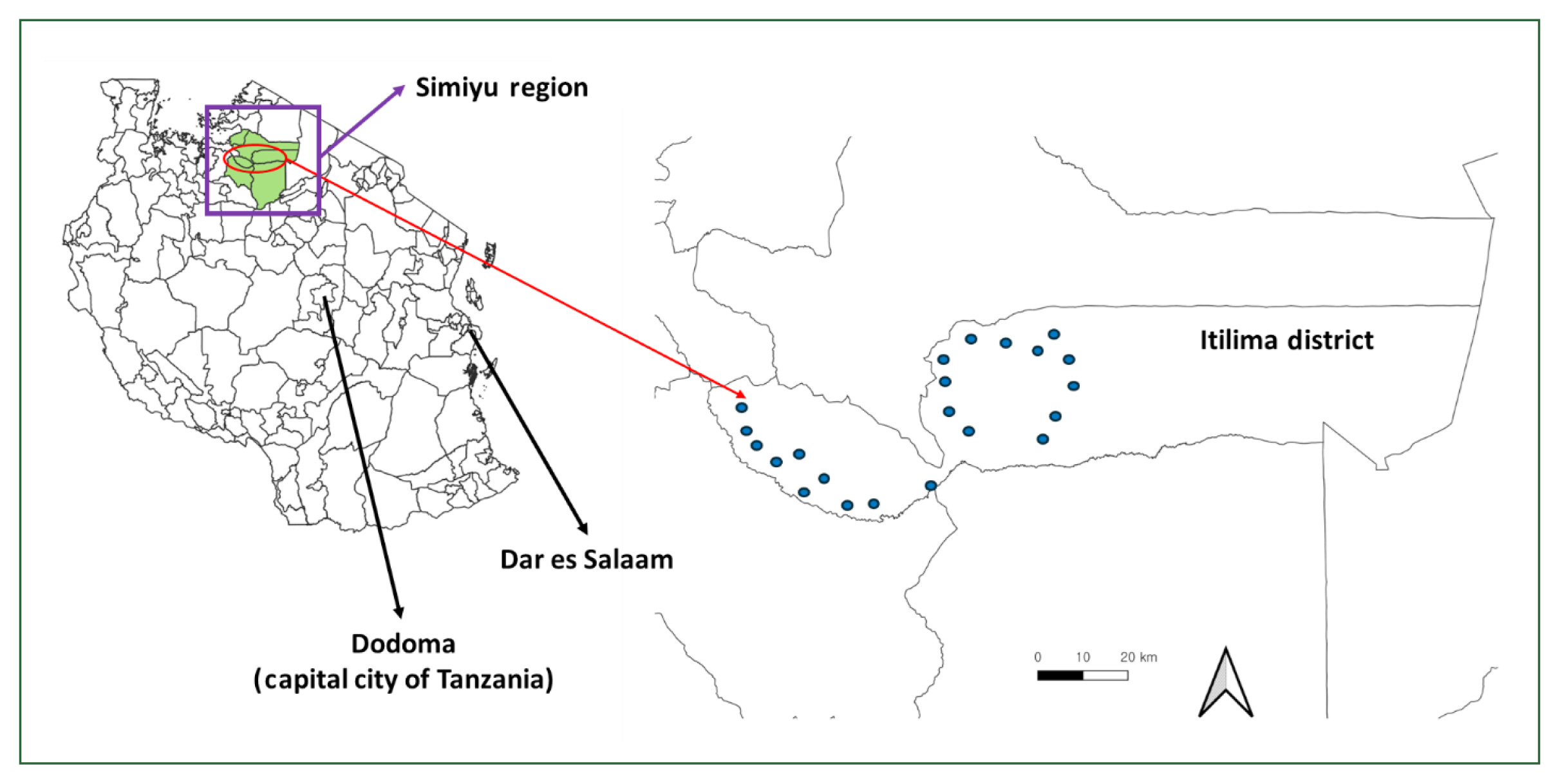
Fig. 2Flow diagram of (A) student and (B) adult selection for comparison between the baseline (August to September 2020) and endline survey (September 2022).
Fig. 3Schistosomiasis (
Schistosoma haematobium or
Schistosoma mansoni) prevalence among (A) students and (B) adults before and after the intervention by village. Name of the schools are indicated in
Supplementary Table S2. BUDALA, Budalabujiga; KINANG, Kinangweri; MWAMI, Mwamigagani.
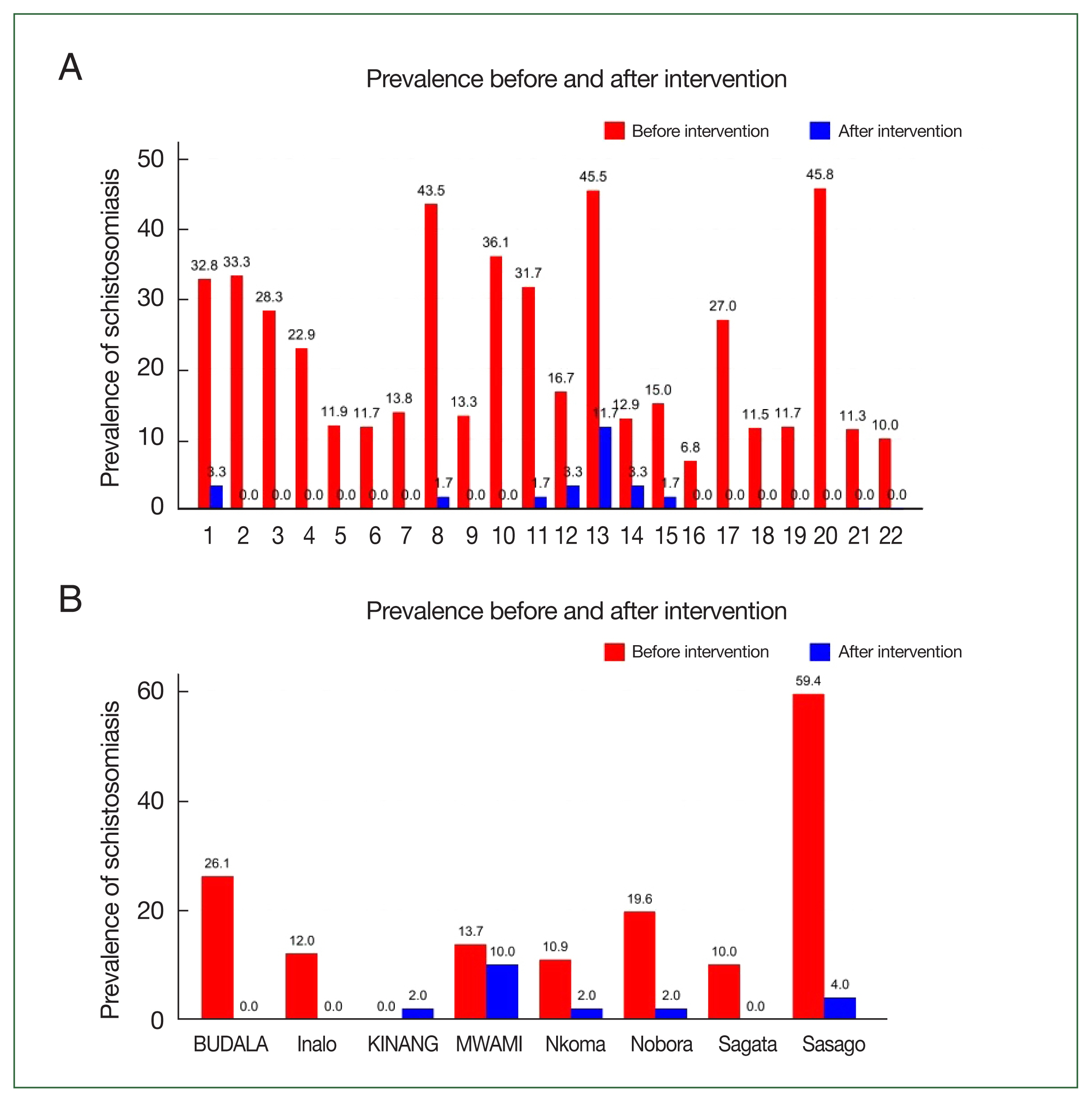
Table 1Factors associated with Schistosoma haematobium infection among school-aged children
Table 1
|
Variable |
|
OR |
95% CI |
P-value |
AOR |
95% CI |
P-value |
|
Age (year) |
5–10 |
0.6 |
0.1–6.9 |
0.7 |
0.6 |
0.1–6.9 |
0.7 |
|
11–14 |
1.5 |
0.2–11.2 |
0.7 |
1.4 |
0.2–11.1 |
0.7 |
|
15–17 |
Reference |
|
|
|
|
|
|
|
Sex |
Male |
1.3 |
0.5–3.4 |
0.5 |
1.6 |
0.6–4.5 |
0.3 |
|
Female |
Reference |
|
|
|
|
|
|
|
Education level of household head |
No education |
0.4 |
0.1–2.1 |
0.3 |
0.4 |
0.1–2.1 |
0.3 |
|
Primary level |
0.9 |
0.3–2.8 |
0.8 |
0.9 |
0.3–2.8 |
0.8 |
|
Secondary level or above |
Reference |
|
|
|
|
|
|
|
Existence of toilet at household |
No |
0.5 |
0.1–3.9 |
0.5 |
0.5 |
0.1–3.9 |
0.5 |
|
Yes |
Reference |
|
|
|
|
|
|
|
Water contact |
Once per week |
2.8 |
0.5–15.9 |
0.2 |
4.8 |
0.8–31.2 |
0.1 |
|
More than once |
1.3 |
0.3–6.1 |
0.6 |
1.7 |
0.3–8.6 |
0.5 |
|
None |
Reference |
|
|
|
|
|
|
|
Experience of taking PZQ |
No |
4.6 |
1.6–13.4 |
0.004 |
5.4 |
1.8–16.5 |
0.003 |
|
Yes |
Reference |
|
|
|
|
|
Table 2Factors associated with Schistosoma haematobium infection among adults
Table 2
|
Variable |
|
OR |
95% CI |
P-value |
AOR |
95% CI |
P-value |
|
Age (year) |
15–19 |
3.4 |
0.1–0.5 |
0.1 |
1.4 |
0.3–6.6 |
0.7 |
|
20–39 |
11.3 |
0.2–5.9 |
0.7 |
0.8 |
0.2–3.8 |
0.8 |
|
40 or above |
Reference |
|
|
|
|
|
|
|
Sex |
Female |
1.7 |
0.7–4.7 |
0.2 |
1.9 |
0.6–5.5 |
0.2 |
|
Male |
Reference |
|
|
|
|
|
|
|
Education level of household head |
No education |
0.4 |
0.1–1.7 |
0.2 |
0.5 |
0.1–2.5 |
0.4 |
|
Primary level |
0.4 |
0.1–1.2 |
0.1 |
0.6 |
0.2–2.0 |
0.4 |
|
Secondary level or above |
Reference |
|
|
|
|
|
|
|
Presence of toilet at household |
No |
2.3 |
0.3–17.7 |
0.4 |
2.3 |
0.3–17.7 |
0.4 |
|
Yes |
Reference |
|
|
|
|
|
|
|
Regular use of toilet |
No |
7.3 |
2.3–23.4 |
0.0001 |
5.9 |
1.7–21.5 |
0.01 |
|
Yes |
Reference |
|
|
|
|
|
|
|
Source of water: surface water |
Yes |
1.8 |
0.4–8.3 |
0.4 |
1.8 |
0.4–8.3 |
0.4 |
|
No |
Reference |
|
|
|
|
|
|
|
Water contact |
Once per week |
12.2 |
2.3–64.1 |
0.003 |
11.1 |
1.9–61.9 |
0.01 |
|
Twice per week |
2.5 |
0.2–27.9 |
0.4 |
2.4 |
0.2–27.6 |
0.4 |
|
Three times per week |
8.7 |
1.6–48.1 |
0.01 |
8.8 |
1.5–50.8 |
0.02 |
|
None |
Reference |
|
|
|
|
|
|
|
Experience of taking PZQ |
No |
1.1 |
0.4–2.9 |
0.004 |
1.1 |
0.4–2.9 |
0.004 |
|
Yes |
Reference |
|
|
|
|
|
References
- 1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396(10258):1204-1222.
https://doi.org/10.1016/S0140-6736(20)30925-9
- 2. Hotez PJ, Kamath A. Neglected tropical diseases in Sub-Saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 2009;3(8):e412.
https://doi.org/10.1371/journal.pntd.0000412
- 3. World Health Organization. WHO Guideline on Control and Elimination of Human Schistosomiasis. World Health Organization; Geneva, Switzerland. 2021, p 12.
- 4. Mduluza T, Mutapi F. Putting the treatment of paediatric schistosomiasis into context. Infect Dis Poverty 2017;6(1):85.
https://doi.org/10.1186/s40249-017-0300-8
- 5. Ministry of Health Tanzania. Report on urinary schistosomiasis, national questionnaire baseline survey in Tanzania mainland 2010. Schistosomiasis Control Initiatives; Dodoma, Tanzania. 2010, pp 1-98.
- 6. Mazigo HD, Nuwaha F, Kinung’hi SM, Morona D, Pinot de Moira A, et al. Epidemiology and control of human schistosomiasis in Tanzania. Parasit Vectors 2012;5:274.
https://doi.org/10.1186/1756-3305-5-274
- 7. Clements AC, Brooker S, Nyandindi U, Fenwick A, Blair L. Bayesian spatial analysis of a national urinary schistosomiasis questionnaire to assist geographic targeting of schistosomiasis control in Tanzania, East Africa. Int J Parasitol 2008;38(3–4):401-415.
https://doi.org/10.1016/j.ijpara.2007.08.001
- 8. World Vision Korea. The baseline report of Itilima neglected tropical disease control project. World Vision Korea; Seoul, Korea. 2020, p 29.
- 9. Lee J, Cha S, Cho Y, Musiba A, Marwa B, et al. Prevalence of schistosomiasis and soil-transmitted helminthiasis and their risk factors: a cross-sectional study in Itilima district, north-western Tanzania. Life (Basel) 2023;13(12):2333.
https://doi.org/10.3390/life13122333
- 10. Trippler L, Ame SM, Hattendorf J, Juma S, Abubakar S, et al. Impact of seven years of mass drug administration and recrudescence of Schistosoma haematobium infections after one year of treatment gap in Zanzibar: repeated cross-sectional studies. PLoS Negl Trop Dis 2021;15(2):e0009127.
https://doi.org/10.1371/journal.pntd.0009127
- 11. Diakité NR, Ouattara M, Bassa FK, Coulibaly JT, Tian-Bi YT, et al. Baseline and impact of first-year intervention on Schistosoma haematobium infection in seasonal transmission foci in the northern and central parts of Côte d’Ivoire. Trop Med Infect Dis 2021;6(1):7.
https://doi.org/10.3390/tropicalmed6010007
- 12. Secor WE, Wiegand RE, Montgomery SP, Karanja DM, Odiere MR. Comparison of school-based and community-wide mass drug administration for schistosomiasis control in an area of western Kenya with high initial Schistosoma mansoni infection prevalence: a cluster randomized trial. Am J Trop Med Hyg 2020;102(2):318-327.
https://doi.org/10.4269/ajtmh.19-0626
- 13. World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. World Health Organization; Geneva, Switzerland. 2021, p 12.
- 14. Lwambo NJ, Siza JE, Brooker S, Bundy DA, Guyatt H. Patterns of concurrent hookworm infection and schistosomiasis in schoolchildren in Tanzania. Trans R Soc Trop Med Hyg 1999;93(5):497-502.
https://doi.org/10.1016/s0035-9203(99)90349-8
- 15. Lwambo NJ. Transmission of urinary schistosomiasis in Sukumaland, Tanzania. 1. Snail infection rates and incidence of infection in school children. J Helminthol 1988;62(3):213-217.
https://doi.org/10.1017/s0022149x00011536
- 16. Grimes JE, Croll D, Harrison WE, Utzinger J, Freeman MC, et al. The roles of water, sanitation and hygiene in reducing schistosomiasis: a review. Parasit Vectors 2015;8:156.
https://doi.org/10.1186/s13071-015-0766-9
- 17. Mazigo HD, Zinga MM, Kepha S, Yard E, McRee-Mckee K, et al. Precision and geographical prevalence mapping of schistosomiasis and soil-transmitted helminthiasis among school-aged children in selected districts of north-western Tanzania. Parasit Vectors 2022;15(1):492.
https://doi.org/10.1186/s13071-022-05547-6
- 18. Cha S, Elhag MS, Lee YH, Cho DS, Ismail HA, et al. Epidemiological findings and policy implications from the nationwide schistosomiasis and intestinal helminthiasis survey in Sudan. Parasit Vectors 2019;12(1):429.
https://doi.org/10.1186/s13071-019-3689-z