Abstract
Hymenolepis nana, commonly known as the dwarf tapeworm, affects 50 to 75 million people worldwide. To date, no studies have explored the disease burden of H. nana infection in Sudan. This study aimed to determine the national prevalence of H. nana across 189 districts and 18 states in Sudan and the number of individuals infected with H. nana who did not receive treatment during the mass drug administration (MDA) campaign targeting schistosomiasis. In addition, the study sought to evaluate the extent of co-infection of H. nana with schistosomiasis and soil-transmitted helminthiasis. This involved a secondary analysis of a nationwide survey conducted in 2017 in Sudan. Binomial family generalized linear models with a logarithmic link function were used to estimate the prevalence ratio of potential risk factors, including sex and water and sanitation conditions in schools and households. For the nationwide survey, a 2-stage sampling method was used, in which 105,167 students were selected from 1,772 schools. A total of 96,679 stool samples were collected, of which 4,706 (4.9%) tested positive for H. nana. Of these, fewer than 1% were co-infected with schistosomiasis (either Schistosoma haematobium or Schistosoma mansoni), and a mere 0.1% had co-infections with soil-transmitted helminths. At an 8% threshold for village-based MDA, approximately 1.1 million infected adults are ineligible to receive praziquantel from the village-based MDA. Children residing in households with improved latrines had a lower odds of H. nana infection than those without improved latrines did (adjusted odds ratio=0.87, 95% confidence interval=0.80–0.94, P=0.001). In countries where H. nana is endemic, such as Sudan, providers making MDA decisions should consider the prevalence of either H. nana or schistosomiasis, rather than focusing solely on the latter.
-
Key words: Hymenolepis nana, mass drug administration, sanitation, Sudan
Hymenolepis nana, which is commonly referred to as the dwarf tapeworm, is the primary parasite responsible for hymenolepiasis, causing 50 to 75 million cases of the disease worldwide [
1]. Although hymenolepiasis is typically asymptomatic, it can sometimes lead to mild clinical symptoms including diarrhea, abdominal pain, anorexia, and nonspecific gastrointestinal manifestations [
2]. In some cases, infection with
H. nana may lead to severe diseases, including life-threatening conditions, particularly in individuals with HIV who are immunosuppressed [
3]. Direct human-to-human transmission is a common route of
H. nana infection [
4,
5]. However, because rodents, such as mice and rats, along with arthropod intermediate hosts, serve as reservoirs of the infection,
H. nana infection is classified as a zoonosis. The significance of each reservoir varies with the environment [
6]. In conditions of poor hygiene and sanitation, especially in densely populated urban areas, transmission risks are particularly high [
7,
8]. Co-infection with other enteric parasites, including soil-transmitted helminths,
Giardia,
Entameba coli,
Blastocystis, and
Chilomastix, is common, with fecal-oral contamination occurring frequently [
9–
12]. Notably, the clinical severity of this polyparasitic infection is primarily intensified by
H. nana [
9].
There are increasing concerns about the invasive nature of
H. nana, which may have been overlooked in many regions and is more pathogenic than previously recognized; furthermore, this disease primarily affects children [
13]. Despite its significance, to the best of my knowledge, no previous studies have investigated the disease burden of
H. nana infection in Sudan. In this region, praziquantel is primarily distributed through mass drug administration (MDA) campaigns that target schistosomiasis. Although praziquantel can treat
H. nana, individuals infected with or at risk of
H. nana have not been considered in these efforts in Sudan.
This study determined the prevalence of
H. nana across 189 districts and 18 states in Sudan. In addition, I sought to analyze the extent of co-infection with schistosomiasis and soil-transmitted helminthiasis. Ultimately, the aim of this study is to provide programmatic recommendations for addressing both schistosomiasis and
H. nana in Sudan. This research involved a secondary analysis of a nationwide survey conducted in Sudan in 2017. Previous reports have documented the prevalence of schistosomiasis and soil-transmitted helminthiasis [
14,
15].
Sudan, the third largest country in Africa, is divided into 18 states and 189 districts. In 2020, the population of the country was approximately 40.2 million [
16]. A 2-stage sampling method was used for the nationwide survey, with 105,167 students selected from 1,772 schools. From this group, 96,679 stool samples were collected. Ethical review and approval were obtained both from the Institutional Review Board of Federal Ministry of Health, Sudan (FMOH/DGP/RD/TC/2016; January 15, 2017) and the Korea Association of Health Promotion (130750–20,164-HR-020; May 16, 2016).
The data collectors visited a school early in the morning, distributed stool and urine containers to selected students, and collected samples on the same day, which were then processed within 24 h. Laboratory technicians used the Kato–Katz technique to examine the eggs in the stool samples. They prepared and examined 2 slides for each student’s stool sample. Two senior supervisors from the Federal Ministry of Health oversaw the quality of the specimen examinations and interviews. Each day, 1 supervisor reexamined 10% of the slides. In addition, independent supervisors from Al Neelain University and the Blue Nile National Institute for Communicable Disease, University of Gezira, were assigned to reexamine 5% of the samples collected during their visits. Fewer than 10 slides had a discrepancy between the findings of the supervisors and the state-level laboratory technicians. To account for this discrepancy, the examination results from the supervisors were considered as the accurate values.
To estimate the prevalence at the state level, I applied sampling weights by state and district, taking into account each district’s sex ratio and population size. For district-level prevalence, I referred only to the sex ratio, as the population size was unknown at levels smaller than the district. Because the survey included only school-aged children (SACs), age was not weighted. I estimated the prevalence and number of infected individuals using the prevalence data for SACs, acknowledging the limitations described. To create a map of H. nana infections, geographical information system software (QGIS v.3.2; QGIS Development Team, Bern and Chur, Switzerland) was used. I gathered information on the MDA target population for schistosomiasis and identified districts that were excluded from the MDA intervention. Based on this information, I estimated the number of individuals infected with H. nana who did not receive praziquantel treatment during the MDA campaign against schistosomiasis. To estimate the prevalence ratio of probable risk factors, including sex and water and sanitation conditions at schools and households, binomial family generalized linear models with a logarithmic link function were used. I used the log-likelihood ratio to assess the inclusion of each variable in the final model. This study reported 95% confidence intervals (CIs), with statistical significance set at P<0.05. Statistical analyses were performed using Stata version 16.0 (College Station, TX, USA).
Supplementary Table S1 presents the number of schools, students, and their ages. Of the participants, 45% were girls. The average age of the students was 11.1 years (SD=2.41) for boys and 10.7 years (SD=2.21) for girls.
Table 1 details the prevalence rates. A survey conducted across 1,722 schools in Sudan collected 96,679 stool samples from 105,167 students, of whom 4,706 (4.9%) tested positive for
H. nana. The state-level weighted prevalence varied, with the lowest being 0.7% (95% CI=0.5%–1.0%) in East Darfur and the highest being 7.2% (95% CI=6.6%–7.9%) in Khartoum. Following closely were the South Darfur and Blue Nile states, with prevalence rates of 6.3% (95% CI=5.9%–6.7%) and 6.1% (95% CI=5.4%–6.9%), respectively.
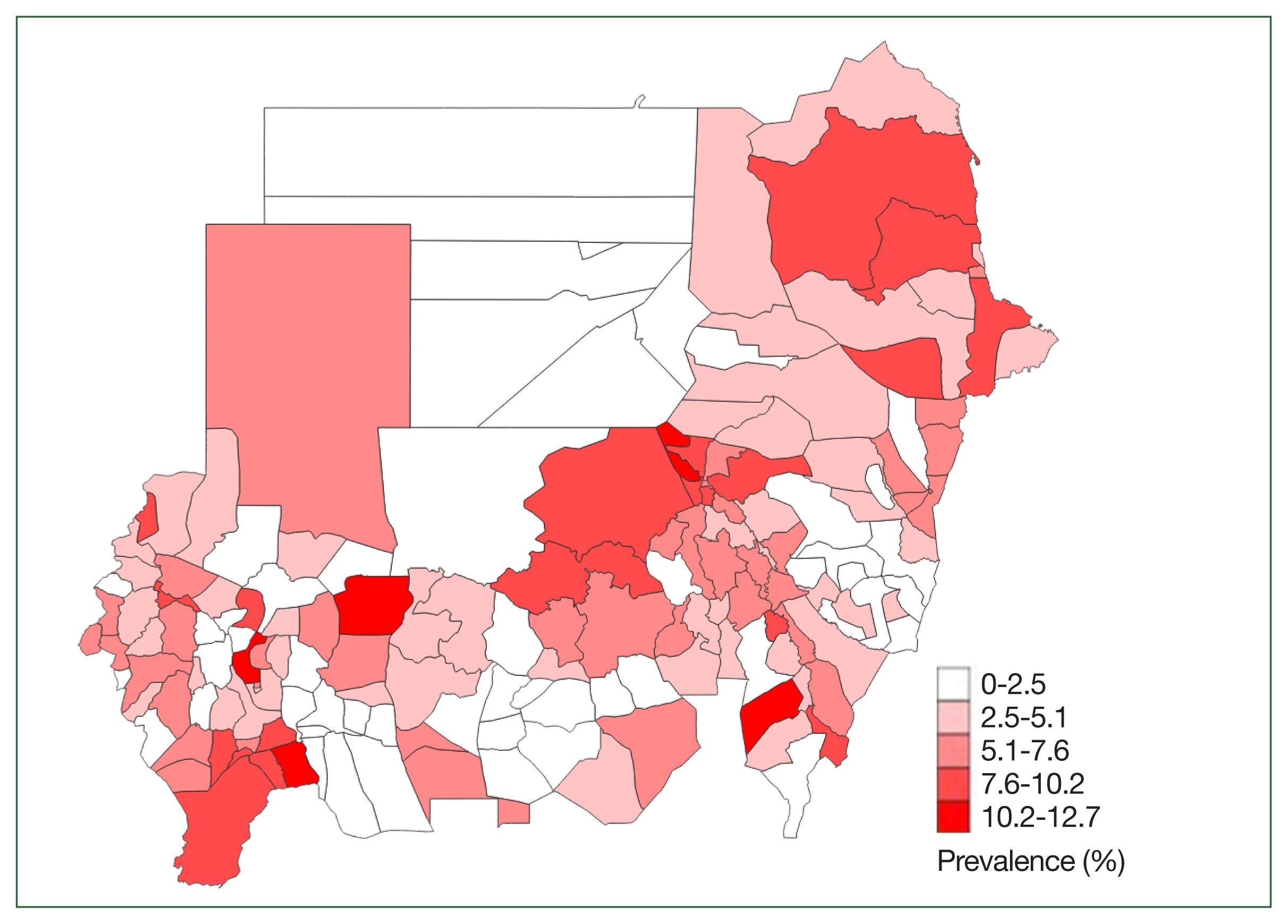
Fig. 1. shows the geographical distribution of
H. nana at the district level. As compared with schistosomiasis,
H. nana was more evenly distributed.
Table 1 also presents the co-infection status of
H. nana with schistosomiasis and soil-transmitted helminthiasis. Among the 4,706 children infected with
H. nana, less than 1% were co-infected with schistosomiasis (either
Schistosoma haematobium or
Schistosoma mansoni). Furthermore, a mere 0.1% of these children had a co-infection with soil-transmitted helminthiasis.
Table 2 displays the number of individuals infected with
H. nana who did not benefit from praziquantel because their districts were excluded from MDA interventions as a result of the low prevalence of schistosomiasis. With an 8% threshold for village-based MDA, approximately 1.1 million infected adults are unable to receive praziquantel. Similarly, under the current school-based MDA program at the 3% threshold for school-based MDA, 360,838 infected students are unable to receive praziquantel.
Table 3 shows the association between
H. nana infection and probable risk factors. Children residing in households equipped with an improved latrine had a lower likelihood of being infected with
H. nana as compared with those in households without such facilities (adjusted odds ratio=0.87, 95% CI=0.80–0.94,
P=0.001). However, when compared with households without a latrine, the presence of any type of latrine in a household did not significantly alter the odds of
H. nana infection (adjusted odds ratio=1.03, 95% CI=0.95–1.10,
P=0.49). In addition, no correlation was found between access to improved water sources, open defecation practices, and
H. nana infection.
This study indicated that approximately 4.2% of the SACs in Sudan were infected with
H. nana. Notably, the prevalence of
H. nana was significantly higher than that of the 3 soil-transmitted helminthiases, which stood at only 0.1%. Unlike schistosomiasis, this disease is more evenly distributed across all 18 states of Sudan [
14,
15]. The observation of the highest prevalence in Khartoum may be associated with the state’s dense population.
Infection with
H. nana can lead to fever, headache, abdominal pain, diarrhea, and anemia. In the absence of treatment, it may result in malnutrition, including stunting and micronutrient deficiencies, due to impaired intestinal permeability, enteritis, or intestinal leakage of micronutrients [
7].
Niclosamide has been proven effective in treating
H. nana infections in humans [
17]. Some studies have reported that both albendazole and praziquantel have successfully eradicated
H. diminuta and adult
H. nana infections [
18].
The most significant finding of this study is that several districts that had a high prevalence of H. nana were excluded from the mass administration of praziquantel because of the low prevalence of schistosomiasis in those areas. It is estimated that about 2 million Sudanese individuals are infected with H. nana, and approximately 1.1 million did not receive praziquantel because they resided in areas not targeted by the MDA intervention. A more effective strategy would likely be addressing both schistosomiasis and H. nana concurrently.
In countries such as Sudan, where
H. nana is endemic, I recommend that decisions regarding MDA be based on the prevalence of either
H. nana or schistosomiasis, rather than exclusively on schistosomiasis. Although further evidence is required to confirm the economic feasibility of this approach, I believe it offers significant cost-effectiveness and a favorable benefit–cost ratio [
1]. Although effective drugs such as praziquantel and albendazole are available to treat
H. nana infections, they are insufficient for controlling the parasite in endemic foci where transmission rates are high [
19]. Reinfection occurs quickly under these conditions, necessitating preventive measures such as education, improved hygiene, and enhanced sanitation. Some studies have suggested that the prevalence of hymenolepiasis could serve as an indicator of the level of hygiene practice and fecal contamination in a village [
20]. This study provides evidence that supports this argument.
With regard to sanitation, a significant association was found between only schistosomiasis and the presence of an improved latrine (at school or household). Merely having any type of latrine at school or in a household did not reduce the odds of hymenolepiasis infection. After adjusting for water and sanitation in the analysis, boys had a higher odds of developing hymenolepiasis than girls did, indicating that they may have other unknown risk factors. A possible explanation for this disparity could be their greater exposure to contaminated fields or soil.
The ingestion of fecal contaminants is a recognized transmission pathway for H. nana. This study shows that simple pit latrines, whether in schools or households, do not provide protection against H. nana in SACs. Furthermore, access to improved water, as defined by the World Health Organization/United Nations Children’s Fund, was not associated with a reduced risk of infection in children.
Although the World Health Organization has emphasized the importance of integrated interventions for neglected tropical diseases (NTDs) by publishing the NTD–water, sanitation, and hygiene (WASH) integrated strategy, many projects aimed at controlling NTDs continue to focus solely on preventive chemotherapy. Communities using the WASH strategy have consistently stressed the significance of eliminating open defecation and ensuring access to household latrines. However, the results of this study demonstrate that simply ending open defecation and possessing a household latrine are not enough; it is crucial to have an improved latrine.
Fortunately, numerous WASH experts have begun to explore the impact of sanitation interventions on disease reduction. To the best of my knowledge, few studies have investigated the relationship between WASH interventions and H. nana infections.
Food hygiene, which encompasses aspects such as food storage and rodent infestation at home, represents a potential risk factor for H. nana infection. These factors were not assessed in this study and thus merit further investigation.
Notes
-
Author contributions
Conceptualization: Jin Y
Data curation: Jin Y
Formal analysis: Jin Y
Investigation: Jin Y
Methodology: Jin Y
Software: Jin Y
Validation: Jin Y
Visualization: Jin Y
Writing – original draft: Jin Y
Writing – review & editing: Jin Y
-
Conflict of interest
The author has no conflicts of interest.
-
Acknowledgments
The project was funded by Korea International Cooperation Agency (P-2015-00145). The author also extends her appreciation to Korea International Cooperation Agency.
The author thanks the project team members for their efforts and contributions in controlling neglected tropical diseases in Sudan. The author extends her appreciation to the village residents, the Ministries of Health of 18 states, and the Federal Ministry of Health, Sudan. Special thanks go to Dr. Hassan Ahmed Hassan Ahmed Ismaeil, the local project manager; Mr. Hoo-Gn Jeoung, Director of the Health Examination Managing Bureau, Korea Association of Health Promotion; and Prof. Sung-Tae Hong, the project manager.
Supplementary Information
Fig. 1
Hymenolepis nana prevalence at district level in Sudan.
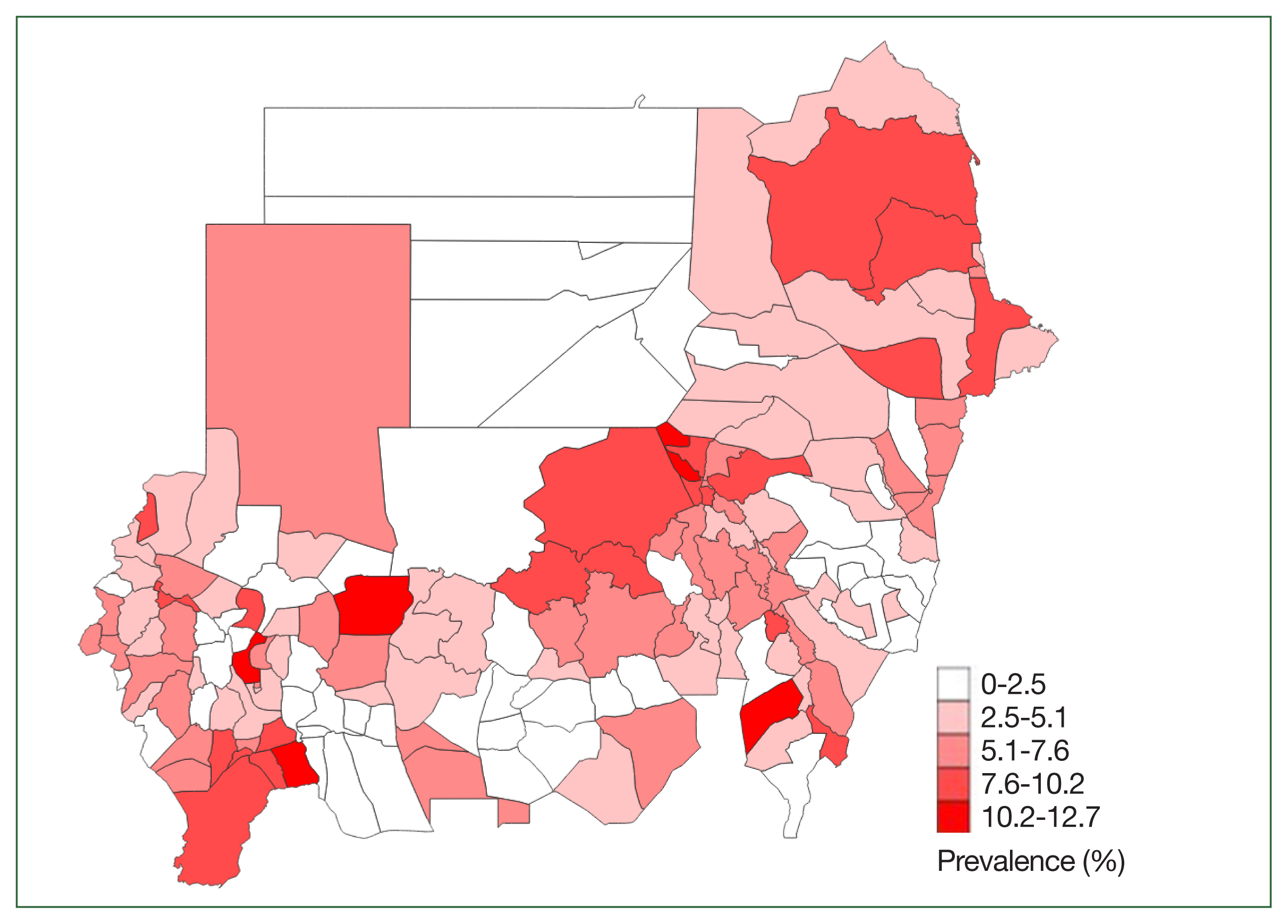
Table 1Prevalence of Hymenolepis nana and number of cases of co-infection
Table 1
|
H. nana
|
No. of cases co-infection |
|
|
|
n/Na
|
Prevalenceb (95% CI) |
H. nana + Schistosomiasis
|
H. nana + Soil-transmitted helminth |
|
Blue Nile |
240/3,709 |
6.1 (5.4−6.9) |
12 |
2 |
|
|
Al Gazeira |
309/5,710 |
4.9 (4.4−5.4) |
9 |
0 |
|
|
Central Darfur |
185/3,175 |
4.3 (3.7−5.0) |
9 |
0 |
|
|
East Darfur |
34/4,252 |
0.7 (0.5−1.0) |
6 |
0 |
|
|
El Gadaref |
169/5,821 |
2.6 (2.3−3.0) |
10 |
0 |
|
|
Al Khartum |
436/5,514 |
7.2 (6.6−7.9) |
12 |
0 |
|
|
North Kordofan |
220/3,578 |
5.9 (5.2−6.7) |
4 |
0 |
|
|
Northern |
34/3,578 |
0.9 (0.6−1.2) |
1 |
1 |
|
|
West Darfur |
178/3,976 |
4.1 (3.5−4.7) |
1 |
0 |
|
|
West Kordofan |
352/10,190 |
3.3 (3.0−3.7) |
18 |
0 |
|
|
Kassala |
165/3,441 |
4.6 (4.0−5.3) |
5 |
0 |
|
|
North Darfur |
311/6,562 |
4.6 (4.1−5.1) |
14 |
0 |
|
|
Red Sea |
198/2,425 |
5.9 (5.2−6.8) |
1 |
0 |
|
|
River Nile |
125/2,988 |
3.4 (2.8−4.0) |
1 |
1 |
|
|
Sinnar |
175/2,763 |
5.7 (4.9−6.6) |
1 |
0 |
|
|
South Darfur |
922/14,048 |
6.3 (5.9−6.7) |
174 |
0 |
|
|
South Kordofan |
324/8,402 |
3.5 (3.2−3.9) |
13 |
0 |
|
|
White Nile |
383/6,579 |
5.1 (4.6−5.6) |
26 |
1 |
|
|
Total |
4,706/96,679 |
4.2 (4.1−4.4) |
317 |
5 |
Table 2Estimated number of people with Hymenolepis nana who would be excluded from MDA if the thresholds are based solely on schistosomiasis prevalence
Table 2
|
State |
Studentsa
|
Adultsb
|
|
Khartoum |
104,613 |
308,283 |
|
Al Gezira |
40,580 |
138,910 |
|
North Kordofan |
23,414 |
78,582 |
|
White Nile |
12,103 |
73,839 |
|
North Darfur |
31,020 |
72,381 |
|
Red Sea |
29,929 |
69,834 |
|
Kassala |
23,202 |
57,348 |
|
Sinnar |
17,449 |
53,963 |
|
South Darfur |
14,205 |
53,322 |
|
South Kordofan |
4,444 |
31,695 |
|
Blue Nile |
13,264 |
30,949 |
|
West Kordofan |
10,371 |
29,426 |
|
El Gadaref |
9,442 |
27,247 |
|
Central Darfour |
5,241 |
25,182 |
|
River Nile |
10,042 |
24,896 |
|
West Darfur |
9,193 |
21,449 |
|
Northern |
1,789 |
4,175 |
|
East Darfur |
540 |
1,837 |
|
Total |
360,838 |
1,103,317 |
Table 3Association between risk factors and Hymenolepis nana
Table 3
|
Variables |
|
|
Crude OR |
95% CI |
P-value |
Adjusted OR |
95% CI |
P-value |
|
Gender |
Male |
4.34 (2,043/47,041) |
0.94 |
0.89−0.99 |
0.04 |
0.91 |
0.86−0.97 |
0.003 |
|
Female |
4.61 (2,657/57,661) |
|
|
Having any type of household latrine |
Yes |
4.52 (3,732/82,479) |
1.04 |
0.97−1.12 |
0.28 |
1.03 |
0.95−1.10 |
0.49 |
|
No |
4.36 (968/22,223) |
|
|
Having an improved household latrine |
Yes |
4.12 (680/16,490) |
0.94 |
0.83−0.98 |
0.02 |
0.87 |
0.80−0.94 |
0.001 |
|
No |
4.54 (4,080/89,961) |
|
|
Open defecation |
Yes |
4.37 (881/20,152) |
0.97 |
0.90−1.04 |
0.45 |
0.98 |
0.91−1.06 |
0.63 |
|
No |
4.49 (3,879/86,599) |
|
|
Having improved water |
Yes |
4.53 (4,310/95,129) |
1.12 |
1.01−1.24 |
0.04 |
1.11 |
0.99−1.23 |
0.06 |
|
No |
4.07 (390/9,573) |
References
- 1. King CH, Mandell GL, Bennet JE, Dolin R. Principles and Practice of Infectious Disease Cestodes (tapeworms). 7th ed. In Livingstone Churchill ed, Elsevier; Philadelphia, USA. 2010.
- 2. Kim BJ, Song KS, Kong HH, Cha HJ, Ock M. Heavy Hymenolepis nana infection possibly through organic foods: report of a case. Korean J Parasitol 2014;52(1):85-87.
https://doi.org/10.3347/kjp.2014.52.1.85.
- 3. Muehlenbachs A, Bhatnagar J, Agudelo CA, Hidron A, Eberhard ML, et al. Malignant transformation of Hymenolepis nana in a human host. N Engl J Med 2015;373(19):1845-1852.
https://doi.org/10.1056/NEJMoa1505892
- 4. Acha PN, Szyfres B. Zoonoses and Communicable Diseases Common to Man and Animals. III Parasitoses. 3rd ed. PanAmerican Health Organization; Washington DC, USA. 2003.
- 5. Macnish MG, Morgan-Ryan UM, Monis PT, Behnke JM, Thompson RCA. A molecular phylogeny of nuclear and mitochondrial sequences in Hymenolepis nana (Cestoda) supports the existence of a cryptic species. Parasitology 2002;125(Pt 6):567-575.
https://doi.org/10.1017/s0031182002002366
- 6. Pakdeenarong N, Siribat P, Chaisiri K, Douangboupha B, Ribas A, et al. Helminth communities in murid rodents from southern and northern localities in Lao PDR: the role of abitat and season. J Helminthol 2014;88(3):302-309.
https://doi.org/10.1017/S0022149X13000187
- 7. Zain SNM, Behnke JM, Lewis JW. Helminth communities from two urban rat populations in Kuala Lumpur, Malaysia. Parasit Vectors 2012;5:47.
https://doi.org/10.1186/1756-3305-5-47
- 8. Sharma D, Joshi S, Vatsya S, Yadav CL. Prevalence of gastrointestinal helminth infections in rodents of Tarai region of Uttarakhand. J Parasit Dis 2013;37(2):181-184.
https://doi.org/10.1007/s12639-012-0158-4
- 9. Thompson RCA, Smith A. Zoonotic enteric protozoa. Vet Parasitol 2011;182(1):70-78.
https://doi.org/10.1016/j.vetpar.2011.07.016
- 10. Conlan JV, Khamlome B, Vongxay K, Elliot A, Pallant L, et al. Soil-transmitted helminthiasis in Lao People’s Democratic Republic: a village-wide cross-sectional study of humans and dogs in a mass drug administration environment. Am J Trop Med Hyg 2012;86(4):624-634.
https://doi.org/10.4269/ajtmh.2012.11-0413
- 11. Mamo H. Intestinal parasitic infections among prison inmates and tobacco farm workers in Shewa Robit, north-central Ethiopia. PLoS One 2014;9(6):e99559.
https://doi.org/10.1371/journal.pone.0099559
- 12. Nxasana N, Baba K, Bhat VG, Vasaikar SD. Prevalence of intestinal parasites in primary school children of mthatha, Eastern Cape Province, South Africa. Ann Med Health Sci Res 2014;3(4):511-516.
https://doi.org/10.4103/2141-9248.122064
- 13. Matthys B, Bobieva M, Karimova G, Mengliboeva Z, Jean-Richard V, et al. Prevalence and risk factors of helminths and intestinal protozoa infections among children from primary schools in western Tajikistan. Parasit Vectors 2011;4:195.
https://doi.org/10.1186/1756-3305-4-195
- 14. Cha S, Hong ST, Lee YH, Lee KH, Cho DS, et al. Nationwide cross-sectional survey of schistosomiasis and soil-transmitted helminthiasis in Sudan: study protocol. BMC Public Health 2017;17(1):703.
https://doi.org/10.1186/s12889-017-4719-4
- 15. Cha S, Elhag MS, Lee YH, Cho DS, Ismail HAHA, et al. Epidemiological findings and policy implications from the nationwide schistosomiasis and intestinal helminthiasis survey in Sudan. Parasit Vectors 2019;12(1):429.
https://doi.org/10.1186/s13071-019-3689-z
- 16. Encyclopædia Britannica. Sudan. Encyclopædia Britannica; Chicago, USA. 2022.
- 17. Schenone H. Praziquantel in the treatment of Hymenolepis nana infection in children. Am J Trop Med Hyg 1980;29(2):320-321.
https://doi.org/10.4269/ajtmh.1980.29.320
- 18. Kline K, McCarthy JS, Pearson M, Loukas A, Hotez PJ. Neglected tropical diseases of Oceania: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis 2013;7(1):e1755.
https://doi.org/10.1371/journal.pntd.0001755
- 19. Mirdha BR, Samantray JC. Hymenolepis nana: a common cause of paediatric diarrhoea in urban slum dwellers in India. J Trop Pediatr 2002;48(6):331-334.
https://doi.org/10.1093/tropej/48.6.331
- 20. Sirivichayakul C, Radomyos P, Praevanit R, Pojjaroen-Anant C, Wisetsing P. Hymenolepis nana infection in Thai children. J Med Assoc Thai 2000;83(9):1035-1038.