Abstract
Trichomonas vaginalis infection causes vaginitis and cervicitis in women, and asymptomatic urethritis and prostatitis in men. Mast cells play a key role in the inflammatory response against T. vaginalis infection. In this study, we examined the signaling pathways involved in mast cell activation induced by T. vaginalis-derived secretory products (TvSP), focusing on IKK2, calcium, MAP kinase (MAPK), and PI3 kinase (PI3K). TvSP stimulation induced phosphorylation and degradation of IκB, indicating NF-κB activation, and triggered phosphorylation of ERK1/2, p38 MAPK, and AKT. TvSP also increased the surface expression of CD63, a marker of exocytosis, which was reduced by IKK inhibition, calcium chelation, or blockade of PI3K and PKC. Furthermore, inhibition of PI3K or MAPKs decreased TvSP-induced interleukin-8 production. These results suggest that IKK2 and calcium are critical for TvSP-induced degranulation, while PI3K and MAPK pathways contribute to interleukin-8 production in mast cells.
-
Key words: Trichomonas vaginalis, mast cells, CD63, IKK2, exocytosis, interleukin-8
Introduction
Trichomonas vaginalis is a flagellated protozoan parasite and the causative agent of trichomoniasis, one of the most common non-viral sexually transmitted infections worldwide [
1-
3]. The World Health Organization estimates 156 million new cases annually, highlighting its public health importance [
4]. Infections are often asymptomatic in men but cause vaginitis, cervicitis, and urethritis in women, increasing the risk of preterm birth, pelvic inflammatory disease, and HIV acquisition [
5].
T. vaginalis secretes virulence factors such as cysteine proteases, lipophosphoglycan, and leukotriene B₄ (LTB₄) [
6-
8], which modulate host innate immunity by promoting local inflammation and inflammatory cell recruitment [
9].
T. vaginalis-derived secretory products (TvSP) activate neutrophils, macrophages, and mast cells [
8-
11], inducing pro-inflammatory cytokines including interleukin-8 (IL-8), a major mediator of neutrophil recruitment and mucosal inflammation [
9]. However, the upstream signaling pathways controlling TvSP-induced IL-8 production remain unclear, particularly the roles of MAP kinase (MAPK) and PI3 kinase (PI3K).
Mast cells are long-lived immune cells abundant in mucosal tissues, where they mediate allergic and antimicrobial responses [
12-
15]. Upon activation, they undergo exocytotic degranulation, releasing histamine, tryptase, and TNF-α, followed by cytokine and chemokine synthesis.
T. vaginalis induces mast-cell activation, promoting β-hexosaminidase release and IL-8, TNF-α, and histamine secretion [
16-
18]. TvSP components such as LTB₄ also enhance NF-κB activation and IL-8 secretion via BLT1 signaling [
19], yet the intracellular mechanisms regulating this response remain largely unknown.
IKK2 (IKKβ) is a central regulator of the canonical NF-κB pathway, phosphorylating IκBα and leading to NF-κB–dependent transcription of pro-inflammatory genes [
20,
21]. While this pathway contributes to mast-cell degranulation and cytokine production, its role in TvSP-induced exocytosis has not been defined. In addition, calcium (Ca²⁺) signaling is crucial for mast-cell activation and function [
13,
22,
23] and interacts with MAPK and PI3K cascades that regulate vesicle fusion, degranulation, and cytokine production [
21,
24].
Therefore, this study aimed to elucidate the intracellular signaling mechanisms underlying mast-cell activation by TvSP, focusing on the roles of IKK2/NF-κB and Ca²⁺ signaling in exocytosis, and MAPK and PI3K pathways in IL-8 production.
Methods
Ethics statement
Not applicable
Reagents
IMD-0354 (MedChemExpress), LTB₄ (Enzo Life Sciences), and platelet-activating factor (PAF, Cayman) were used as stimulants or inhibitors. EDTA, EGTA, wortmannin, LY294002, Ro-31-8220, PD98059, SB203580, and SP600125 were obtained from Sigma-Aldrich. Antibodies against NF-κB (p65), phospho-ERK1/2 (p-p44/42 MAPK), ERK1/2, phospho-p38 (Thr180/Tyr182), p38, phospho-AKT (Ser473), AKT, and β-actin were from Cell Signaling Technology. Anti-phospho-IκBα (S32) antibody was from ABclonal Technology, and anti-IκBα from Santa Cruz Biotechnology. PE-conjugated anti-human CD63 antibody was purchased from BioLegend, and the mouse IgG1 isotype control from Novus Biologicals.
Cultivation of T. vaginalis and preparation of TvSP
T. vaginalis strain T016 was axenically cultured at 37°C in TYM medium supplemented with 10% heat-inactivated horse serum (Gibco). For TvSP preparation, trichomonads (1×10⁷) were washed twice with Hank’s balanced salt solution (Gibco/Invitrogen), resuspended in 1 ml Hank’s balanced salt solution, and incubated for 1 h at 37°C. The supernatant was collected by centrifugation (12,000 rpm, 10 min) and filtration through a 0.22 µm filter. TvSP concentration was determined using a BCA protein assay kit (Thermo).
Human mast cell (HMC-1) culture
The human mast cell line HMC-1 was cultured in Iscove’s Modified Dulbecco’s Medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Corning) and 1% penicillin-streptomycin. Cells were maintained at 37°C in a humidified incubator with 5% CO₂ under sterile conditions.
Stimulation of HMC-1 cells and pretreatment with pharmacological inhibitors
HMC-1 cells (1×10⁵ /well) were seeded in 6-well plates and pretreated with inhibitors before stimulation with TvSP, LTB₄, or PAF. Cells were treated with the IKK2 inhibitor IMD-0354 (0.1–10 µM) for 24 h, or with Ca²⁺ chelators EDTA (20 mM) and EGTA (10 mM) for 30 min. PI3K inhibitor (wortmannin, 2 µM; LY294002 10 or 20 µM), PKC inhibitor (Ro-31-8220, 10 µM), and MAPK inhibitors (PD98059 for ERK, SB203580 for p38, SP600125 for JNK; all 10 µM) were applied for 30 min prior to stimulation. Cells were then stimulated for 1 or 16 h, depending on the experimental design.
CD63 measurement in MHC-1 cells
Surface expression of CD63, a marker of exocytotic degranulation, was analyzed by flow cytometry. HMC-1 cells (1×10⁵/well) were pretreated with inhibitors and stimulated with TvSP, LTB₄, or PAF for up to 60 min. After stimulation, cells were stained at 4°C for 30 min with PE-conjugated anti-human CD63 or an isotype control antibody, washed twice with PBS containing 1% fetal bovine serum, and analyzed using a BD FACSymphony A5 cytometer. CD63 expression was quantified as mean fluorescence intensity from at least 10,000 events per sample.
Immunoblotting
HMC-1 cells (1×10⁶) were stimulated with or without TvSP for the indicated times and lysed on ice for 20 min in buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1 mM PMSF, 1 mM sodium orthovanadate, and protease inhibitors. Lysates were centrifuged (12,000 rpm, 10 min), and supernatants were mixed with SDS-PAGE buffer and heated at 100°C for 3 min. Proteins were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore). Membranes were blocked with 5% skim milk in Tris-Buffered Saline with Tween-20 for 1 h at room temperature, incubated overnight at 4°C with primary antibodies, washed, and then incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h. Bands were visualized using ECL select (Cytiva).
IL-8 ELISA
HMC-1 cells (1×10⁵/well) were seeded in 6-well plates, pretreated with specific signaling inhibitors for 30 min, and stimulated with TvSP for 16 h at 37°C in a 5% CO₂ incubator. Supernatants were collected, and IL-8 levels were measured using a human IL-8 ELISA kit (Thermo Scientific) following the manufacturer’s instructions.
Statistical analysis
Data are presented as mean±SE from at least 3 independent experiments. Statistical comparisons between experimental and control groups were conducted using Student t-test. A P-value of <0.05 was considered statistically significant.
Results
IκB phosphorylation and degradation in HMC-1 cells stimulated with TvSP
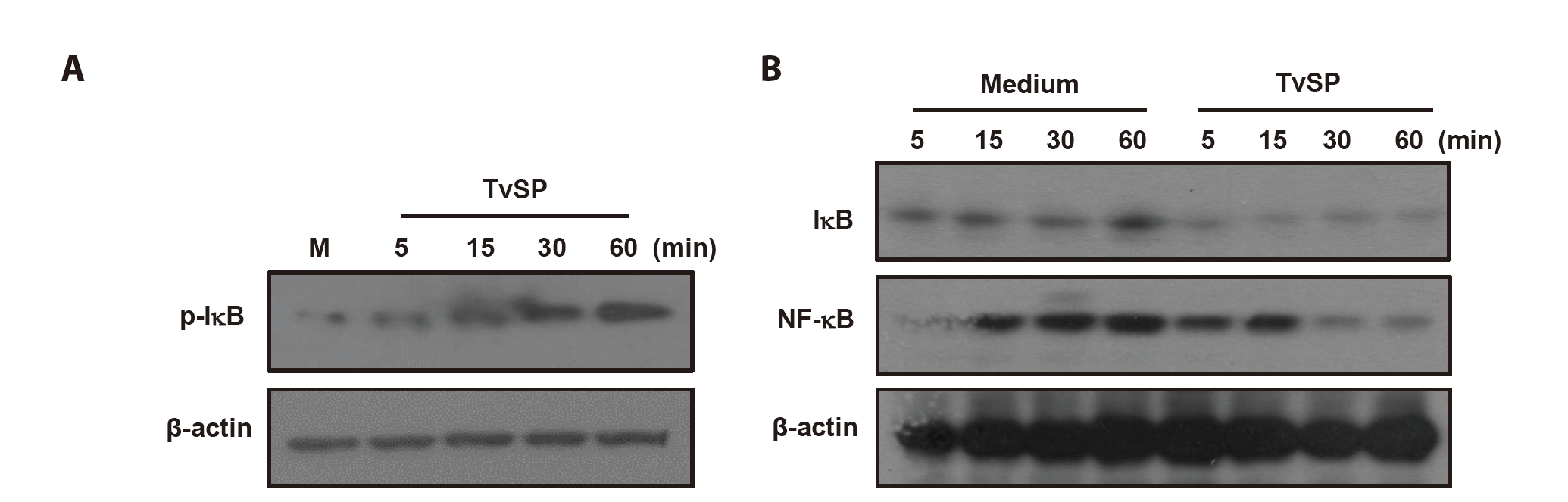
To determine whether TvSP activates the NF-κB pathway in HMC-1 cells, phosphorylation and degradation of IκB and cytosolic NF-κB levels were examined. TvSP induced a time-dependent increase in IκB phosphorylation, detectable at 5 min and maximal at 60 min (
Fig. 1A). Correspondingly, total IκB levels decreased over time, indicating its degradation (
Fig. 1B). Cytosolic NF-κB was strongly detected at 5–15 min but declined at 30–60 min after TvSP stimulation. In contrast, IκB and NF-κB levels remained unchanged in medium-treated control cells (
Fig. 1B)
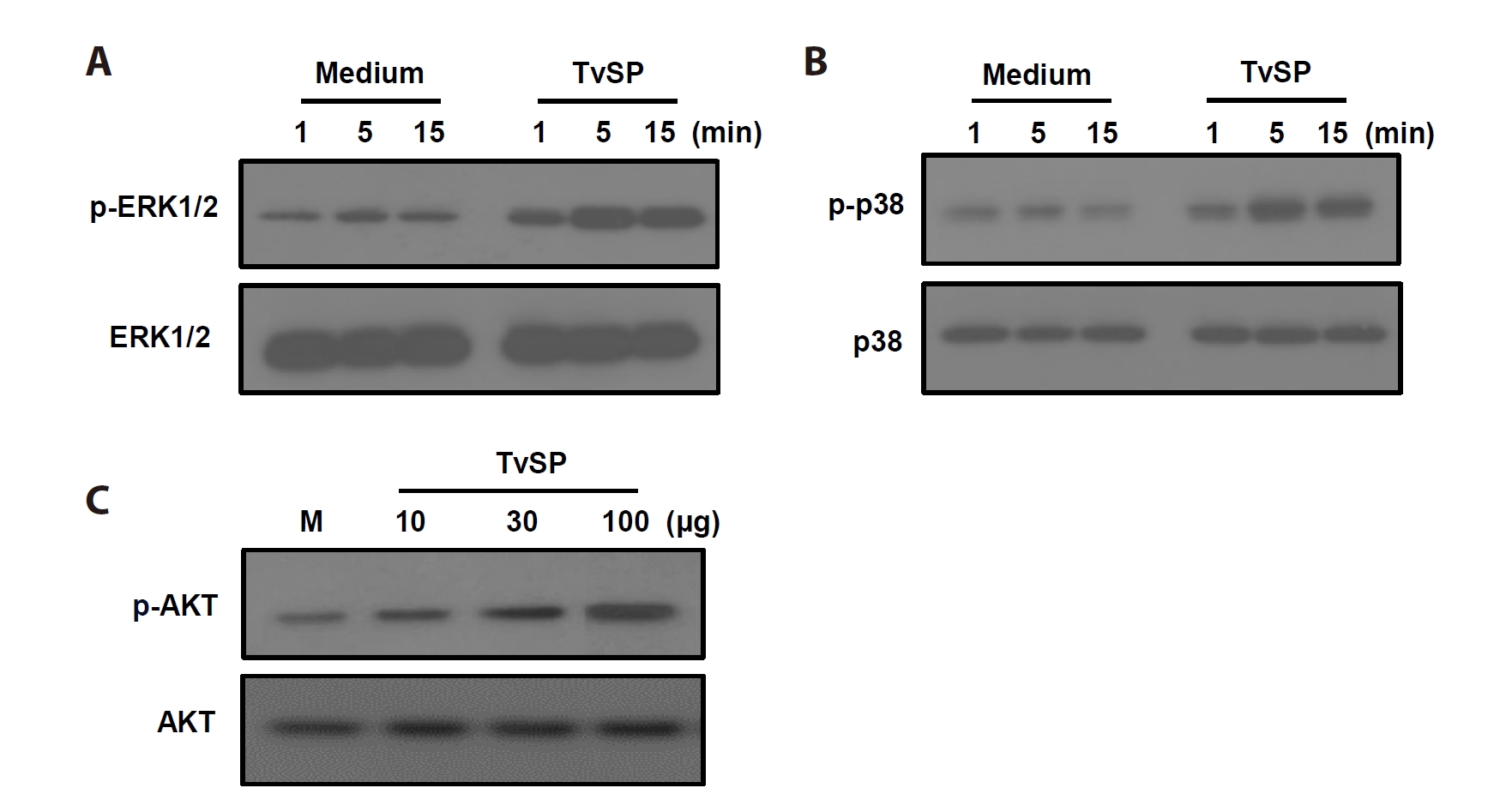
To investigate intracellular signaling activated by TvSP, phosphorylation of key MAPK and AKT pathway proteins was examined. TvSP stimulation rapidly increased ERK1/2 phosphorylation within 5 min, which was sustained up to 15 min, without affecting total ERK1/2 (
Fig. 2A). Similarly, p38 phosphorylation increased in a time-dependent manner, while total p38 remained unchanged (
Fig. 2B). In addition, AKT phosphorylation was enhanced by TvSP in a dose-dependent manner (10–100 µg/ml), with total AKT levels unaffected (
Fig. 2C).
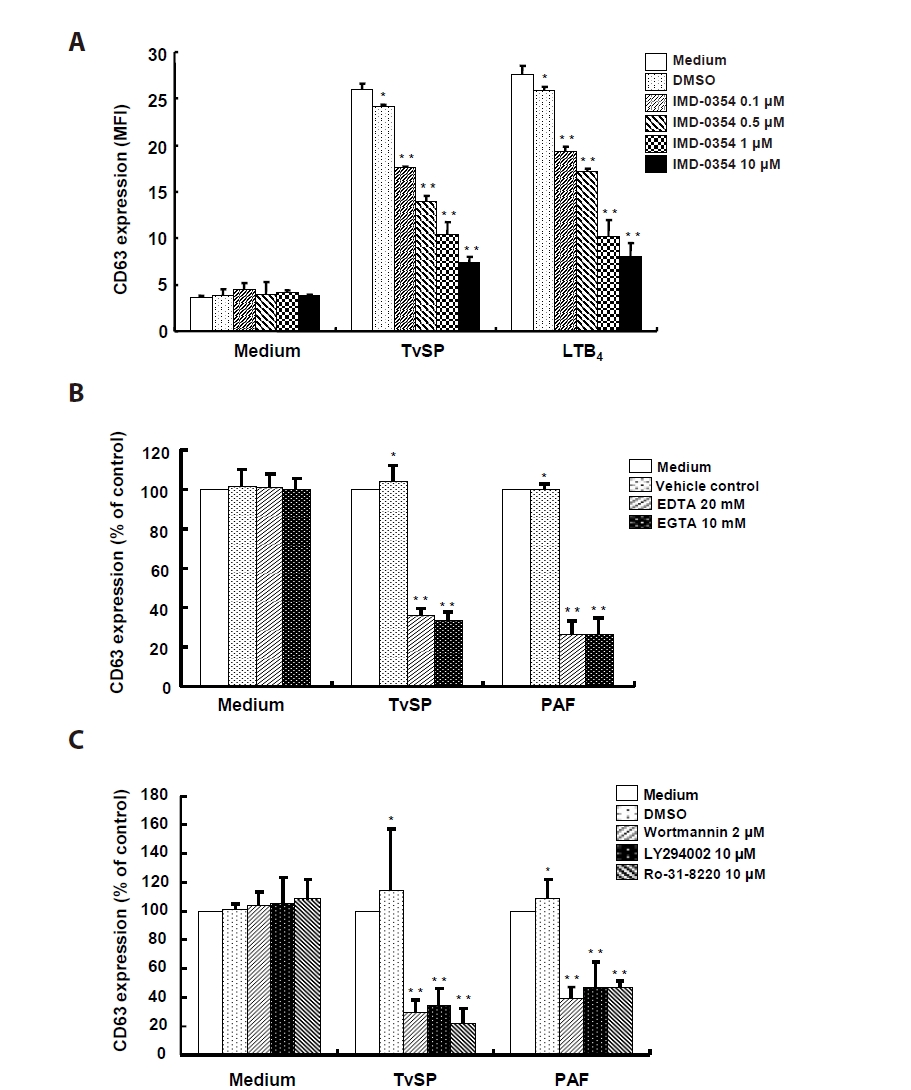
To investigate the signaling mechanisms of TvSP-induced degranulation, HMC-1 cells were pretreated with inhibitors targeting IKK2, Ca²⁺, and PI3K/PKC pathways before stimulation with TvSP or PAF. Pretreatment with the IKK2 inhibitor IMD-0354 suppressed TvSP-induced degranulation in a dose-dependent manner, and a similar inhibition was observed in LTB₄-stimulated cells (
Fig. 3A). Ca²⁺ chelation with EDTA or EGTA markedly reduced CD63 expression in both TvSP- and PAF-stimulated cells compared with control (
Fig. 3B). TvSP stimulation increased CD63 surface expression, whereas pretreatment with PI3K inhibitors (wortmannin, LY294002) or the PKC inhibitor Ro-31-8220 significantly attenuated this response (
Fig. 3C).
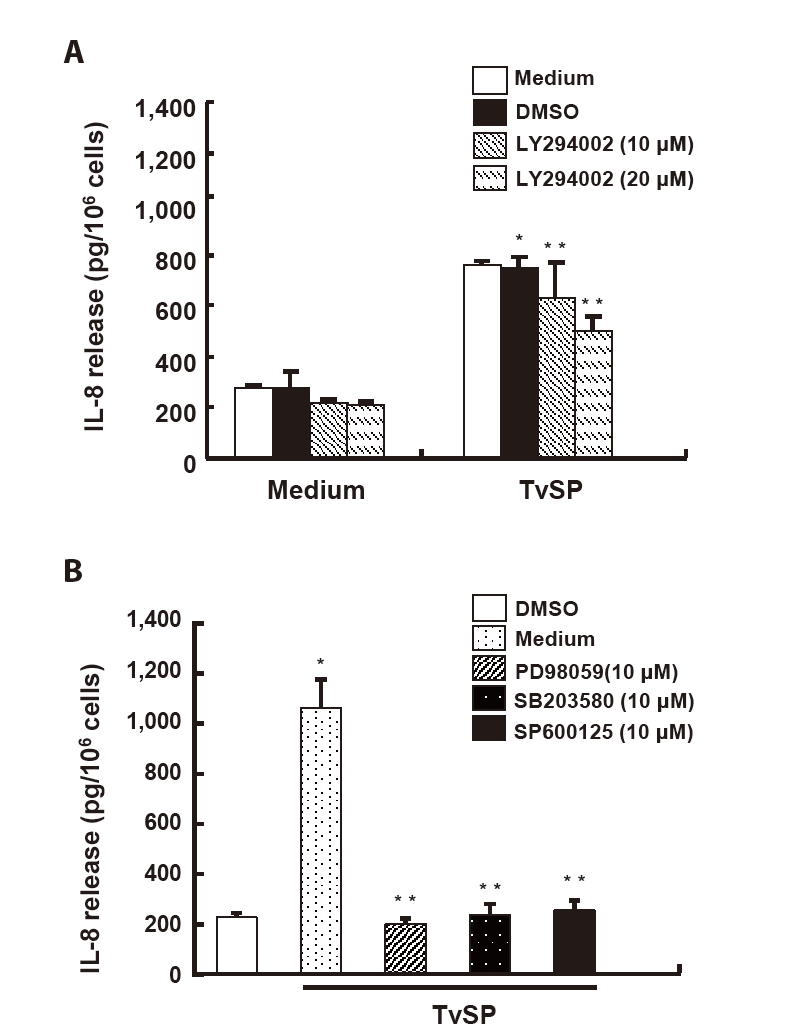
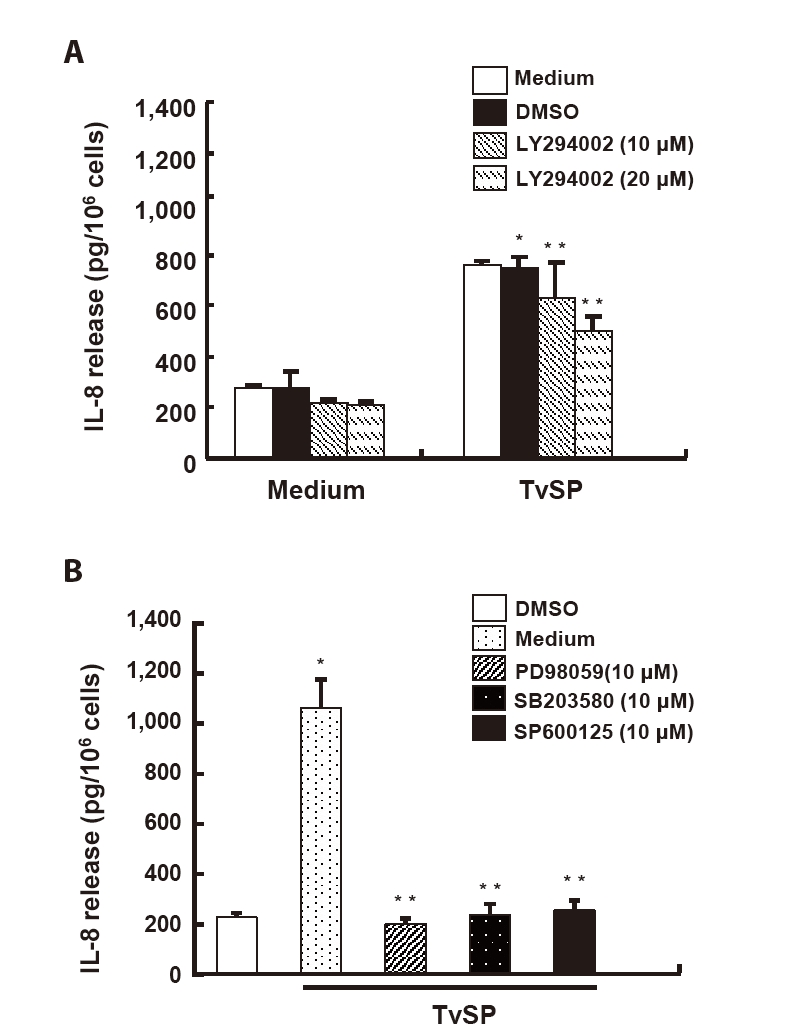
To examine the role of PI3K signaling in TvSP-induced IL-8 production, HMC-1 cells were pretreated with the PI3K inhibitor LY294002. TvSP markedly increased IL-8 secretion compared with the medium control, whereas LY294002 pretreatment significantly reduced IL-8 levels in a dose-dependent manner (
Fig. 4A). Inhibition of MAPK pathways with PD98059 (ERK), SB203580 (p38), or SP600125 (JNK) also significantly suppressed TvSP-induced IL-8 secretion, indicating that ERK, p38, and JNK activation contributes to IL-8 production in response to TvSP (
Fig. 4B).
Discussion
This study demonstrates that TvSP activate mast cells through multiple intracellular signaling pathways regulating exocytotic degranulation and IL-8 production. TvSP stimulation induced phosphorylation and degradation of IκB, indicating activation of the canonical NF-κB pathway via IKK2. TvSP also triggered phosphorylation of ERK, p38 MAPK, and AKT, which contributed to IL-8 production. Inhibition studies revealed that PI3K and PKC regulate degranulation, whereas IL-8 production is mediated by PI3K and MAPK pathways. In addition, IKK2 and Ca²⁺ are essential for TvSP- and LTB₄-induced exocytosis. Collectively, these results highlight IKK2 and Ca²⁺ as central mediators of mast cell activation, with additional input from PI3K, PKC, and MAPK pathways.
IKK2 functions as a critical regulator of mast cell degranulation by phosphorylating IκBα and promoting NF-κB activation [
20]. Loss of IKK2 results in impaired IκBα phosphorylation and defective NF-κB signaling [
20,
25]. Consistent with this, TvSP-induced degranulation was markedly suppressed by IMD-0354, confirming the essential role of IKK2 in mast cell exocytosis. Beyond transcriptional regulation, IKK2 may also mediate non-transcriptional effects, as previous studies reported IKK2-dependent phosphorylation of SNAP23 facilitating vesicle–plasma membrane fusion [
26]. Our earlier work also demonstrated that SNAP23 is crucial for NOX2-mediated degranulation induced by
T. vaginalis-secreted LTB₄ [
26,
27]. Although the direct link between IKK2 and SNAP23 was not examined here, it is plausible that IKK2 contributes to TvSP-induced degranulation through both NF-κB–dependent and SNAP23-related mechanisms during
T. vaginalis–host interactions.
Ca²⁺ plays a pivotal role in mast cell exocytosis. Intracellular Ca²⁺ influx initiates granule–plasma membrane fusion and mediator release [
22,
23,
28,
29]. Consistent with this, Ca²⁺ chelation significantly inhibited TvSP-induced degranulation in HMC-1 cells. Our previous study showed that LTB₄ in TvSP binds to BLT1 receptors on HMC-1 cells [
27], supporting that Ca²⁺ influx through GPCR-mediated signaling is required for degranulation, as seen with other stimuli such as silver nanoparticles or FcεRI activation [
23,
30].
Pharmacological inhibition of PI3K and PKC significantly reduced TvSP-induced degranulation, indicating their importance in exocytosis. PI3K signaling promotes cytoskeletal rearrangement and granule transport via PIP₃ and downstream effectors such as AKT and Rac [
21,
30]. AKT regulates mast cell degranulation and cytokine production [
21]. In this study, TvSP increased AKT phosphorylation without altering total AKT levels. PKC isoforms (PKCα and PKCβ) facilitate granule priming and fusion through phosphorylation of SNARE and Munc proteins [
24,
28]. These findings support a model in which TvSP-induced degranulation is mediated by coordinated activation of IKK2, Ca²⁺, PI3K, and PKC signaling pathways.
IL-8 production in mast cells is regulated by PI3K, PKC, MAPK, and NF-κB pathways [
8,
21,
30]. Activation of PI3K and PKC leads to AKT phosphorylation and NF-κB activation, while ERK and p38 MAPK promote IL-8 gene transcription through AP-1 and CREB [
8,
10,
21]. Consistent with previous reports, TvSP stimulation induced ERK1/2, p38, and AKT phosphorylation, and inhibition of PI3K and MAPK pathways suppressed IL-8 secretion. TvSP also induced IκB phosphorylation and degradation, suggesting NF-κB activation. Although IKK2 inhibition was not directly tested for IL-8 regulation, its suppression of TvSP-induced degranulation implies upstream involvement in cytokine signaling.
In summary, TvSP activates mast cells through an integrated network of IKK2, Ca²⁺, PI3K, PKC, and MAPK pathways. IKK2, Ca²⁺, and PI3K are key regulators of exocytotic degranulation, while PI3K and MAPK pathways are involved in IL-8 production. Although the role of PKC in IL-8 production was not directly examined in this study, our findings that PKC regulates degranulation, together with previous reports, suggest that PKC may also contribute to IL-8 production. IKK2 may mediate mast cell exocytosis through both NF-κB–dependent transcription and a SNAP23-associated non-transcriptional mechanism. These findings enhance understanding of T. vaginalis-induced inflammation and underscore the importance of host–parasite signaling crosstalk in trichomoniasis pathogenesis.
Notes
-
Author contributions
Conceptualization: Lee YA, Shin MH. Funding acquisition: Lee YA, Shin MH. Investigation: Park SH, Lee YA. Methodology: Lee YA. Project administration: Shin MH. Supervision: Shin MH. Writing – original draft: Park SH, Lee YA. Writing – review & editing: Lee YA, Shin MH
-
Conflict of interest
Myeong Heon Shin serves as an editor of Parasites, Hosts and Diseases but had no involvement in the decision to publish this article. No other potential conflicts of interest relevant to this study were reported.
-
Funding
This study was supported by a faculty research grant from the Yonsei University College of Medicine (6-2021-0238) to MH Shin and by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (NRF-2020R1I1A1A01064838) to YA Lee.
Fig. 1.
Trichomonas vaginalis-derived secretory products (TvSP) induces IκB phosphorylation (A) and degradation (B) in HMC-1 cells. HMC-1 cells were treated with or without TvSP collected from 1×10⁷ trichomonads for the indicated time, respectively. After stimulation, whole cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies specific for phospho-IκB (p-IκB), total IκB, total NF-κB, and β-actin. Representative results from 3 independent experiments are shown. M indicates the medium.
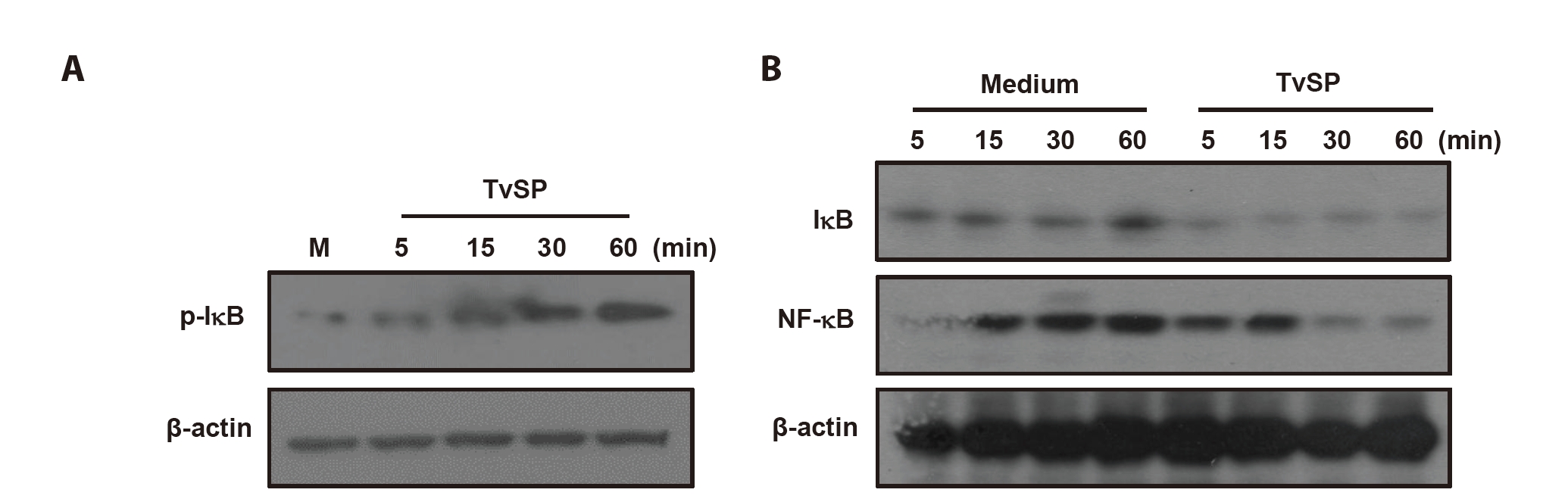
Fig. 2.
Trichomonas vaginalis-derived secretory products (TvSP) induces phosphorylation of ERK (A), p38 MAPK (B), and AKT (C) in HMC-1 cells. HMC-1 cells were stimulated with or without TvSP for the indicated times. Whole cell lysates were analyzed by SDS-PAGE followed by immunoblotting with antibodies specific for phospho-ERK1/2 (p-ERK1/2), total ERK1/2, phospho-p38 (p-p38), total p38, phospho-AKT (p-AKT), or total AKT. Representative results from 3 independent experiments are shown. M indicates the medium.
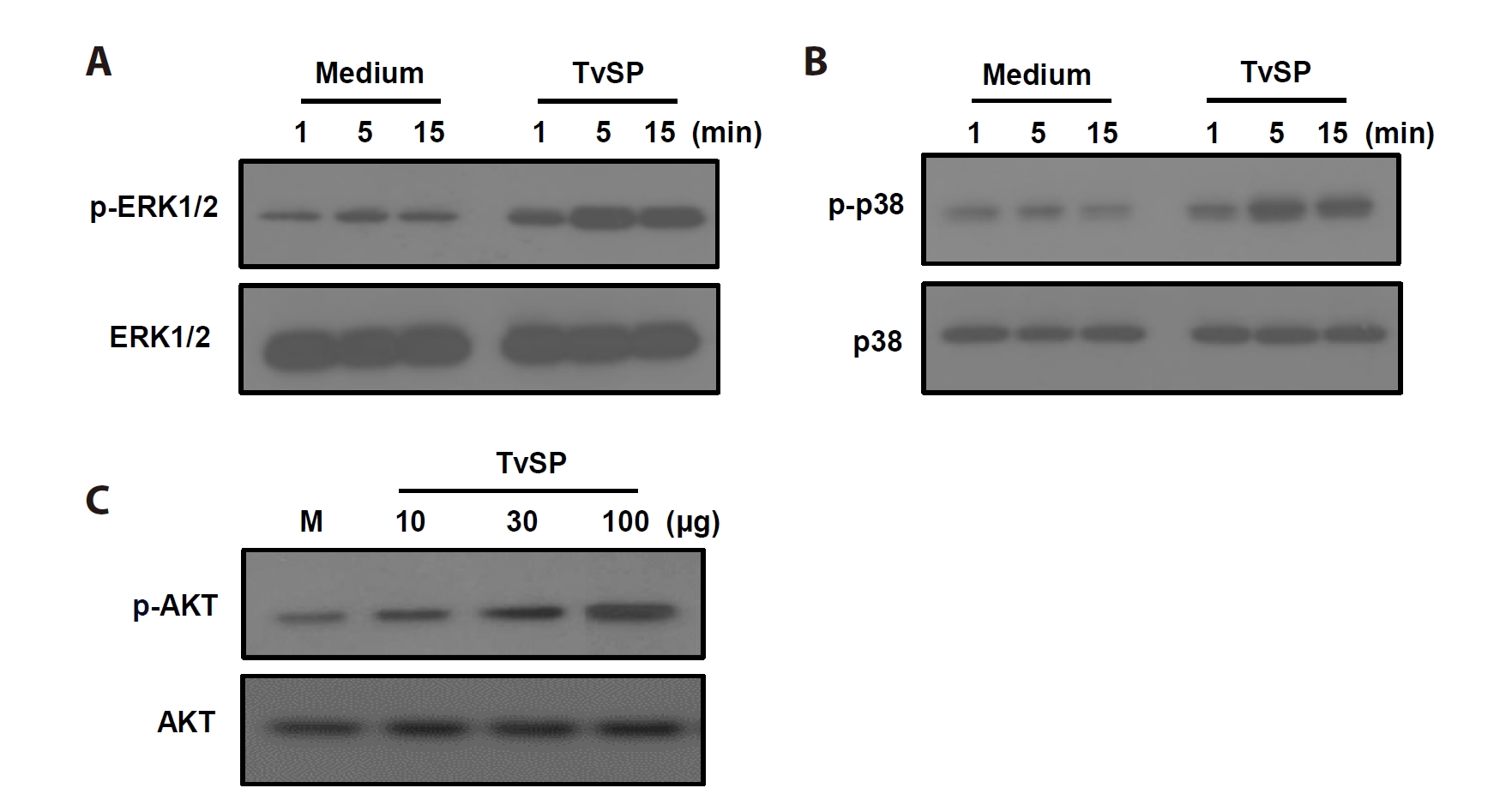
Fig. 3.IKK2, calcium and PI3K/PKC signaling pathway are involved in Trichomonas vaginalis-derived secretory products (TvSP)–induced degranulation in HMC-1 cells. (A) HMC-1 cells were pretreated with IMD-0354 for 24 h, then stimulated with TvSP or leukotriene B₄ (LTB₄, 100 nM) for 1 h. (B) Cells were pretreated with calcium chelators EDTA (20 mM) or EGTA (10 mM) for 30 min, followed by TvSP or platelet-activating factor (PAF) stimulation for 1 h. Values are expressed as percentages against HMC-1 (white bar, % of control) of each group. Basal activity (100% values, mean fluorescence intensity [MFI]) of CD63 expression for each group was 2.09 (medium), 21.89 (TvSP), 31.62 (PAF). (C) Cells were pretreated with PI3K inhibitors (wortmannin, 2 μM; LY294002, 10 μM) or the PKC inhibitor Ro-31-8220 (10 μM) for 30 min, then stimulated with TvSP or PAF for 1 h. Surface CD63 expression was analyzed by flow cytometry and presented as MFI. Values are expressed as percentages against HMC-1 (white bar, % of control) of each group. Basal activity (100% values, MFI) of CD63 expression for each group was 3.47 (medium), 31.71 (TvSP), and 47.01 (PAF). Data represent means±SD from 3 independent experiments *P<0.05, **P<0.01 (compared with the medium control).
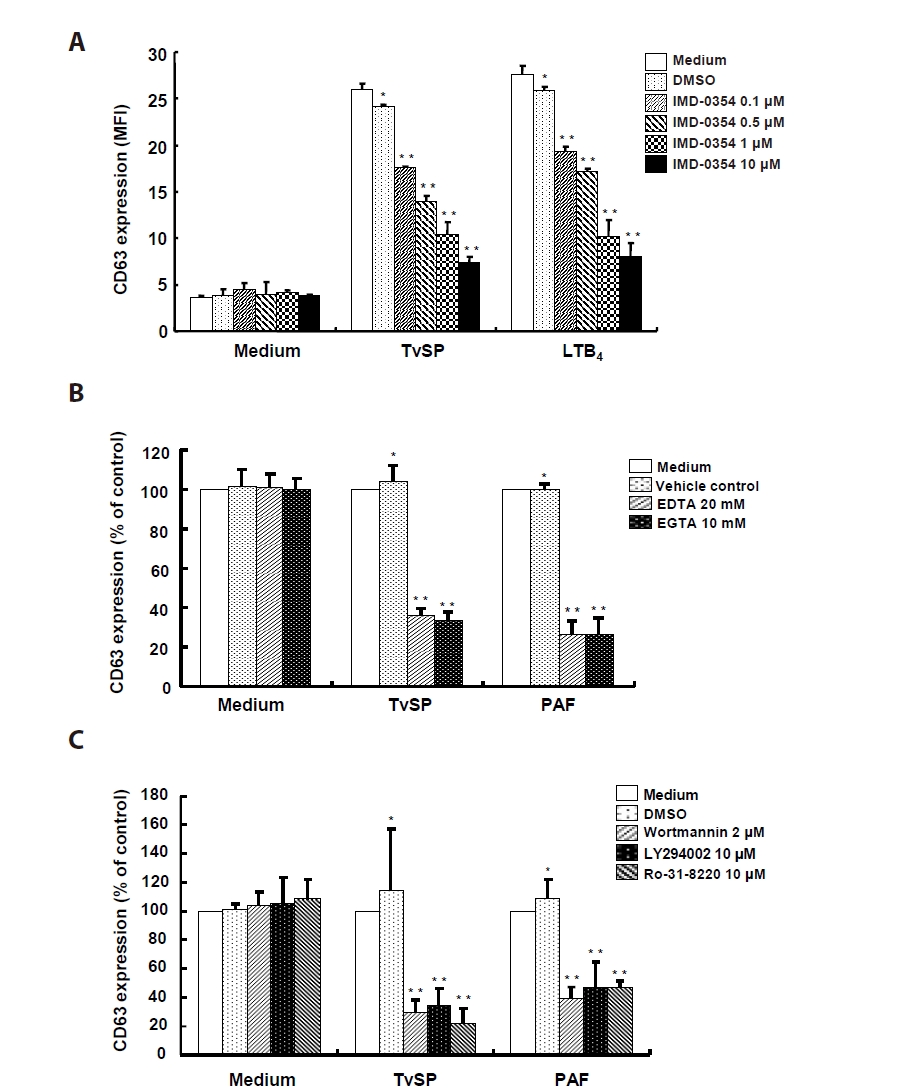
Fig. 4.PI3K, ERK and p38 MAPK contribute to interleukin-8 (IL-8) production in Trichomonas vaginalis-derived secretory products (TvSP)–stimulated HMC-1 cells. (A) For PI3K inhibition, cells were pretreated with LY294002 (10, 20 μM) for 30 min and stimulated with TvSP for 16 h; IL-8 levels were measured by ELISA. (B) To assess MAPK involvement, cells were pretreated for 30 min with PD98059 (10 μM), SB203580 (10 μM), or SP600125 (10 μM) and then stimulated with TvSP for 16 h. Data are mean±SD of 3 independent experiments performed in duplicate *P<0.05, **P<0.01 (compared with the medium control).
References
- 1. Van der Pol B. Trichomonas vaginalis infection: the most prevalent nonviral sexually transmitted infection receives the least public health attention. Clin Infect Dis 2007;44:23-5. https://doi.org/10.1086/509934
- 2. Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ 2019;97:548-62P. https://doi.org/10.2471/BLT.18.228486
- 3. Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis 2015;15:307. https://doi.org/10.1186/s12879-015-1055-0
- 4. World Health Organization. Global health sector strategy on sexually transmitted infections 2016-2021 [Internet]. World Health Organization; 2016. [cited 2025 Sep 1]. Available from: https://www.who.int/publications/i/item/WHO-RHR-16.09
- 5. Ryu JS, Min DY. Trichomonas vaginalis and trichomoniasis in the Republic of Korea. Korean J Parasitol 2006;44:101-16. https://doi.org/10.3347/kjp.2006.44.2.101
- 6. Petrin D, Delgaty K, Bhatt R, Garber G. Clinical and microbiological aspects of Trichomonas vaginalis. Clin Microbiol Rev 1998;11:300-17. https://doi.org/10.1128/CMR.11.2.300
- 7. Fichorova RN, Trifonova RT, Gilbert RO, et al. Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect Immun 2006;74:5773-9. https://doi.org/10.1128/IAI.00631-06
- 8. Nam YH, Min D, Kim HP, et al. Leukotriene B₄ receptor BLT-mediated phosphorylation of NF-κB and CREB is involved in IL-8 production in human mast cells induced by Trichomonas vaginalis-derived secretory products. Microbes Infect 2011;13:1211-20. https://doi.org/10.1016/j.micinf.2011.07.006
- 9. Fichorova RN. Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J Reprod Immunol 2009;83:185-9. https://doi.org/10.1016/j.jri.2009.08.007
- 10. Nam YH, Min D, Park SJ, et al. NF-κB and CREB are involved in IL-8 production of human neutrophils induced by Trichomonas vaginalis-derived secretory products. Korean J Parasitol 2011;49:291-4. https://doi.org/10.3347/kjp.2011.49.3.291
- 11. Kim KS, Moon HS, Kim SS, Ryu JS. Involvement of macrophages in proliferation of prostate cancer cells infected with Trichomonas vaginalis. Korean J Parasitol 2021;59:557-64. https://doi.org/10.3347/kjp.2021.59.6.557
- 12. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med 2012;18:693-704. https://doi.org/10.1038/nm.2755
- 13. Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 2010;10:440-52. https://doi.org/10.1038/nri2782
- 14. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol 2004;4:787-99. https://doi.org/10.1038/nri1460
- 15. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol 2008;9:1215-23. https://doi.org/10.1038/ni.f.216
- 16. Han IH, Park SJ, Ahn MH, Ryu JS. Involvement of mast cells in inflammation induced by Trichomonas vaginalis via crosstalk with vaginal epithelial cells. Parasite Immunol 2012;34:8-14. https://doi.org/10.1111/j.1365-3024.2011.01338.x
- 17. Im SJ, Ahn MH, Han IH, et al. Histamine and TNF-alpha release by rat peritoneal mast cells stimulated with Trichomonas vaginalis. Parasite 2011;18:49-55. https://doi.org/10.1051/parasite/2011181049
- 18. Lee YA, Nam YH, Min A, Shin MH. Trichomonas vaginalis-secreted cysteinyl leukotrienes promote migration, degranulation and MCP-1 production in mast cells. Parasite Immunol 2020;42:e12789. https://doi.org/10.1111/pim.12789
- 19. Lee YA, Shin MH. Dynamin 2-mediated endocytosis of BLT1 is required for IL-8 production in HMC-1 cells induced by Trichomonas vaginalis-derived secretory products. Parasites Hosts Dis 2024;62:281-93. https://doi.org/10.3347/PHD.24049
- 20. Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol 2009;1:a000034. https://doi.org/10.1101/cshperspect.a000034
- 21. Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol 2006;6:218-30. https://doi.org/10.1038/nri1782
- 22. Bell E. The ins and outs of Ca²⁺ signalling in mast cells. Nat Rev Immunol 2008;8:7-15. https://doi.org/10.1038/nri2245
- 23. Chen YC, Chang YC, Chang HA, et al. Differential Ca²⁺ mobilization and mast cell degranulation by FcεRI- and GPCR-mediated signaling. Cell Calcium 2017;67:31-9. https://doi.org/10.1016/j.ceca.2017.08.002
- 24. Blank U, Madera-Salcedo IK, Danelli L, et al. Vesicular trafficking and signaling for cytokine and chemokine secretion in mast cells. Front Immunol 2014;5:453. https://doi.org/10.3389/fimmu.2014.00453
- 25. Nakagomi D, Suzuki K, Nakajima H. Critical roles of IκB kinase subunits in mast cell degranulation. Int Arch Allergy Immunol 2012;158 Suppl 1:92-5. https://doi.org/10.1159/000337800
- 26. Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IκB kinase 2 regulates mast cell degranulation. Cell 2008;134:485-95. https://doi.org/10.1016/j.cell.2008.05.050
- 27. Min A, Lee YA, Kim KA, El-Benna J, Shin MH. SNAP23-dependent surface translocation of leukotriene B4 (LTB4) receptor 1 is essential for NOX2-mediated exocytotic degranulation in human mast cells induced by Trichomonas vaginalis-secreted LTB4. Infect Immun 2016;85:e00526-16. https://doi.org/10.1128/IAI.00526-16
- 28. Hempel N, Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium 2017;63:70-96. https://doi.org/10.1016/j.ceca.2017.01.007
- 29. Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca²⁺ influx and activation of the neutrophil respiratory burst: different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol 2002;71:611-7. https://doi.org/10.1189/jlb.71.4.611
- 30. Alsaleh NB, Persaud I, Brown JM. Silver nanoparticle-directed mast cell degranulation is mediated through calcium and PI3K signaling independent of the high affinity IgE receptor. PLoS One 2016;11:e0167366. https://doi.org/10.1371/journal.pone.0167366