Abstract
A practical and convenient method of rearing Eucyclops serrulatus in a microculture environment is described. A complete life cycle of E. serrulatus was maintained in a narrow space on a microscope slide glass on which a cover glass of 22 x 40 mm in size was mounted at a height of 0.8 mm. The culture medium was constituted by bottled mineral water boiled with grains of Glycine max (soybean). Chilomonas paramecium, a free-living protozoan organism, was provided as live food. Growth of nauplii hatched from eggs to the first stage of copepodite took an average of 7.7 days, and the growth of copepodite 1 to the egg-bearing adult female took an average of 20.1 days in the microculture cell with an average life time of 44.7 days. Continuous passage of copepods was successfully maintained as long as sufficient medium and food were provided. The microculture method enables an in situ microscopic observation on the growth and developmental process of helminth larvae experimentally infected to copepods as well as of copepod itself. Furthermore, it does not require anesthetization and, therefore, minimize the amount of stress exposed to copepods during the handling process.
-
Key words: Eucyclops serrulatus, in situ microculture technique, Chilomonas paramecium
INTRODUCTION
Cyclopoid copepods are one of the most abundant and successful group in freshwater ecosystems and form the first vital link in the food chain from the minute algal cells of the phytoplankton up to the large fish and mammals (
Yoon and Kim, 2000). Furthermore, some species of freshwater cyclopoid genera like
Cyclops, Eucyclops or Mesocyclops serve as the intermediate host for various important helminth parasites of man and animals such as
Dracunculus medinensis,
Gnathostoma spp.,
Diphyllobothrium latum, and
Spirometra spp. The procercoid stage of
Spirometra spp. in copepod hosts, for instance, can cause sparganosis when consumed by humans in which the larva migrates to the subcutis, muscle, eye, scrotum, brain, etc. (
Faust et al., 1929).
Although numerous cases of human sparganosis and researches on the plerocercoid stage of Spirometra have been reported (
Cho et al., 1973,
1975;
Choi, 1984) and experimental life history and culture conditions exploited (
Lee et al, 1990,
Vijerberg, 1989), relatively limited studies have been performed utilizing the procercoid stage in the first intermediate host. Reasons may include difficulties in the handling of copepods due to their characteristic quick and erratic movement. Investigators, therefore, have to anesthetize copepods for an easy handling and microscopic observation, which expose the organisms to considerable stress that often leads them to death. An ideal culture condition would be, therefore, an easy and convenient culture system where minimal handling and stress to the organism is required while an in situ microscopic observation is ensured throughout the entire life cycle of the host animal. In the present study, a microculture method designed for maintaining the complete life cycle of a freshwater cyclopoid copepod,
Eucyclops serrulatus, on a slide glass is described.
MATERIALS AND METHODS
Isolation of Eucyclops serrulatus and the laboratory maintenance
Organisms of E. serrulatus were isolated from water samples collected from a pond in Damyang, Korea in 2002. To secure a pure culture of copepod population, sequential generations of E. serrulatus were started from a single egg-bearing female and was grown to a stock culture.
Preparation of live food and culture medium
Chilomonas paramecium, a free-living protozoan organism that was isolated from a water sample of a rice field at Damyang, Korea, was used as live food for
E. serrulatus. A pure culture of
C. paramecium clone proliferated from a single organism was obtained by two rounds of cloning by limiting dilution (
Freshney, 1987). For the routine laboratory maintenance of
C. paramecium and
E. serrulatus, the culture medium (GM medium) was prepared by boiling 1 liter of bottled mineral water (SoonSoo100, CocaCola Co., Korea) for 10 minutes with 1 g of soybean,
Glycine max. The GM medium was stored at 5℃ until used. Fresh medium was prepared every month. Before each culture was started, the GM medium was supplemented with 1 pre-boiled wheat grain per 10 ml of medium as a nutrient source, as suggested by Kumazawa (
2000). Routine laboratory culture of
E. serrulatus was maintained in disposable petri dishes (87 x 15 mm, Green Cross Medical, Inc. Korea).
Evaluation of various infusion media for the culture of Chilomonas paramecium
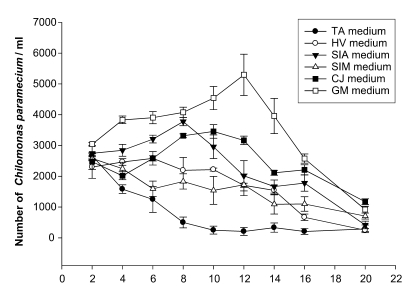
To investigate the growth efficiency of C. paramecium under different infusion media, C. paramecium was cultured in a 24-well flat bottom tissue culture plate (Corning, New York) started at a density of 4 x 103/ml in 2 ml of media prepared with various grains. These infusion media were constituted with 1 liter of bottled mineral water boiled for 10 minutes with 1g grains of either Triticum aestivum (wheat, TA medium), Glycine max (soybean, GM medium), Sesamum indicum (sesamine, SIM medium), Hordeum vulgare (barley, HV medium), Coixlachryma-jobi (Job's tears, CJ medium), or Setaria italica (foxtail millet, SIA medium). The infusion media were stored at 5℃ until used. All cultures were kept at room temperature (20-23℃) and were exposed to 400 lux illumination of fluorescent lighting for 12 hours everyday. The number of C. paramecium was counted using a Neubauer counting chamber after staining the organisms with 0.4% New methylene blue. Each data point represents the average of the triplicated samples.
Preparation of microculture slide
The microculture slide for rearing copepods was prepared using a microscope slide glass (75 x 25 mm, Marienfeld, Germany) on which a cover glass (22 x 40 mm, Marienfeld, Germany) was mounted at a height of 0.8 mm using a couple of polypropylene bars (0.8 x 1.0 x 22 mm) fixed with a silicone-based sealant (
Fig. 1A). The microculture cell space between the slide glass and cover glass held approximately 700 µl of medium. Before a copepod culture was initiated, each microculture cell was filled with the GM medium that contained
C. paramecium at a density of 4 x 10
3/ml. A single egg-bearing female
E. serrulatus was pipetted into the cell space after which the microculture slide was placed in a disposable Petri dish (87 x 15 mm, Green Cross Medical, Inc. Korea,
Fig. 1B). Three layers of wet paper towel with deionized distilled water were placed in the bottom of the petri dish to provide humidity and to minimize the evaporation of culture medium. A couple of wood stick was placed between the wet paper towel and the microculture slide. Petri dishes with microculture slides were kept at room temperature (20-23℃). When multiple slides are used simultaneously, a stainless steel pan with an aluminum slide tray placed on top of three layers of wet paper towel was used. Culture slides were exposed to 400 lux illumination of fluorescent lighting for 12 hours everyday.
Growth and reproduction of E. serrulatus were determined by monitoring the time taken for hatching and the extrusion of egg sacs by in situ observation of copepods individually cultured in microculture slides under the microscope. After all eggs in the first egg sacs of an adult female hatched into nauplii, the female adult copepod was removed from each slide. The duration of developmental stages from egg to nauplius 1, copepodite 1, and adult male and female was monitored and the body length from the rostrum to the end of furcal rami, together with the body width at cephalothorax, was measured. Once nauplii became adults and mating done, they were removed from the cell except for one egg-bearing adult female per slide which was monitored daily thereafter for the number of broods produced and the duration between brood formations. Any nauplius hatched from the subsequent broods produced by the adult female in each microculture cell was removed to avoid the overpopulation which could affect the life span of the adult female. The brood number which was the total number of broods a female produced during its life time was recorded for each animal. The culture was continued until the female copepod in each slide died. Approximately two thirds of media in each microculture cell was replaced daily with fresh GM medium containing C. paramecium at a density of 4 x 103/ml as live food.
RESULTS
Evaluation of various media for the development of Chilomonas paramecium
The proliferation rate of
C. paramecium cultured in six different infusion media is shown in
Fig. 2. Among the tested media, the GM medium provided the highest proliferation rate of
C. paramecium at 12th day of culture at which an average of 5,297/ml of
C. paramecium was recorded. The SIA medium resulted in a peak number of
C. paramecium at the 8th day after culture, while the TA medium showed the lowest growth rate.
Growth parameters of
E. serrulatus from nauplius 1 to adult stages cultured in microcultue slide in terms of size and duration is shown in
Table 1. The average period of eggs hatched from each female copepod to become nauplius 1 in the microculture slide took an average of 1.5 days, while an average of 7.7 days was taken from nauplius 1 to become the copepodite 1 stage. The average period of nauplii 1 hatched from eggs to become egg-bearing adult females took 29.3 days. The average length and width of an nauplius 1 were 80.1 and 120.0 µm which were increased to 283.8 and 845.2 µm in the adult female stage (
Fig. 3). The number of broods produced by an adult female copepod ranged from 1 to 8 (mean, 4.5), and the average life time of copepod was 44.7 days (
Table 2). Continuous passage of copepods was successfully maintained in the microculture environment as long as sufficient medium and food were provided.
DISCUSSION
Results of this study showed that copepods could be successfully cultured in a microculture environment established on a conventional slide glass for microscopic observation. The fact that female copepods lived up to 77 days and produced up to 7 broods of eggs strongly indicates that the relatively convenient and fairly simple design of microculture system described in this study can be a useful research tool in situations where an in situ microscopic observation of copepods is required throughout the entire life cycle. Although cyclopoid copepods can live fairly long (
Maier, 1990,
1992;
Smyly, 1970) and laboratory culture conditions of
E. serrulatus having been established previously (
Yoon and Kim, 2000,
Kumazawa, 2000), the microscopic observation and laboratory handling of these quick and erratic copepods have been cumbersome unless they were narcotized. Narcotization can mount considerable stress to the animals and can cause variation in research data or physiological condition. This in situ culture method of
E. serrulatus, for instance, can be used in the study of human sparganosis, dracunculosis and gnathostomiasis of which cyclopoid copepods serve as the first intermediate host. The procercoid stage of these parasites inhabiting the cyclopoid copepod host can be clearly visible and therefore, an
in situ microscopic observation of the parasite is possible throughout the entire life of the host (data to be published).
The microculture method can also be a useful tool in the ecotoxicology. Calanoid and cyclopoid copepods are by far the most important primary consumers in marine planktonic communities and form the base of virtually all pelagic food chains. Cyclopoids are especially by far the most abundant and successful group of copepods in the freshwater. Among these,
E. serrulatus is one of the most common cyclopoid copepods in various areas of the world and is well adapted to all kinds of aqueous environments (
Kim and Chang, 1989), and can be a good candidate for the test organism to evaluate the potential hazards of eco-environmental toxicants (
Yoon and Kim, 2000). A convenient laboratory culture of this organism, therefore, has broad applications in both ecotoxocology (
Hoffman et al., 1995) and in the study of human sparganosis. Although several investigators provided useful information on the laboratory culture of the organism, an
in situ culture method on a microscope slide has never been tried elsewhere.
The microculture method can also be introduced to various limnological studies and environmental monitoring researches. The accurate identification of zooplanktons including cyclopoid copepods is one of the indispensable prerequisites for limnological study and the various works for nature conservation. The copepods often exist as the pre-maturing copepodite stage IV or V with adults, or as copepodites only in the diapause season when the environmental factors become worse against their favorable conditions (
Lee et al., 2005). However, the developmental study and the morphological taxonomy on the copepodite larvae have been nearly unknown yet. The microculture method will surely contribute for the easy and correct identification of the copepodites of each species.
Moreover, this microculture system is also useful in developing the biomolecular marker. As the morphological characters of freshwater cyclopoid copepods like genera
Eucyclops and
Mesocyclops are so minute and subtle even for the experiencing specialists, so molecular bio-markers for the freshwater cyclopoid copepods like the genetic variations of mitochondrial cytochrome c oxidase subunit I (COI) and the competitive species-specific PCR have been adopted as biomolecular marker for the real-time environmental assessment on the copepods (
Bucklin et al., 1999). This culture system will make it possible to provide the pure material for genetic sequencing durably from an egg-carrying female of each species.
Notes
-
This study was supported by research fund of Chonnam National University in 2001.
References
- 1. Bucklin A, Guarnieri M, Hill RS, Bentley AM, Kaartvedt S. Taxonomic and systematic assessment of planktonic copepods using mitochondrial COI sequence variation and competitive, species-specific PCR. Hydrobiologia 1999;401:239-254.
- 2. Cho SY, Bae JH, Seo BS. Some aspects of human sparganosis in Korea. Korean J Parasitol 1975;13:60-77.
- 3. Cho SY, Hwang KI, Seo BS. On the Sparganum mansoni infection in some Korean terrestrial snakes. Korean J Parasitol 1973;11:87-94.
- 4. Choi WJ. Migration and distribution of spargana in body of experimentally infected mice. Korean J Parasitol 1984;22:229-237. (in Korean).
- 5. Faust EC, Campbell HF, Kellogg CR. Morphological and biological studies on the species of Diphyllobothrium in China. Am J Hyg 1929;9:560-583.
- 6. Freshney RI. Culture of Animal Cells. 1987, 2nd ed. New York, USA. Alan R. Liss; pp 137-144.
- 7. Hoffman DJ, Rattner B, Burton GA Jr, Cairns J Jr. Handbook of Ecotoxicology. 1995, Florida, USA. CRC Press.
- 8. Kim HS, Chang CY. Freshwater Cyclopoid Copepods (Cyclopoida: Cyclopidae) of Korea. Korean J Syst Zool 1989;2:225-256.
- 9. Kozminski Z. Morphometrische und ökologische Untersuchungen an Cyclopiden der strenuus-Gruppe. Int Rev ges Hydrobiol Hydrogr 1936;33:161-240.
- 10. Kumazawa H. Laboratory maintenance of Eucyclops serrulatus (Copepoda: Cyclopoida). Parasitol Int 2000;49:189-193.
- 11. Lee JM, Min GS, Chang CY. Taxonomy on Eucyclops serrulatus species group (Copepoda: Cyclopoida: Cyclopidae) from South Korea. Korean J Syst Zool 2005;21:(in print).
- 12. Lee SH, We JS, Sohn WM, Hong ST, Chai JY. Experimental life history of Spirometra erinacei. Korean J Parasitol 1990;28:161-173.
- 13. Maier G. The effect of temperature on the development, reproduction, and longevity of two common cyclopoid copepods--Eucyclops serrulatus (Fischer) and Cyclops strenuous (Fischer). Hydrobiologia 1990;203:165-175.
- 14. Maier G. The reproductive biology of Cyclops vicinus. J Plankton Res 1992;14:127-135.
- 15. Smyly WJ. Observations on rate of development, longevity and fecundity of Acanthocyclops viridis (Jurine) (Copepoda, Cyclopoida) in relation to type of prey. Crustaceana 1970;18:21-26.
- 16. Vijerberg J. Culture techniques for studies on the growth, development and reproduction of copepods and cladocerans under laboratory and in situ conditions: a review. Freshwater Bio 1989;21:317-373.
- 17. Yoo KI, Lim BJ. Systematic studies on the freshwater Copepoda (Crustacea) in Lake Yongsan, Korea. Korean J Limnol 1989;22:127-146.
- 18. Yoon K, Kim W. Deveopment and reproduction of Eucyclops serrulatus (Copepoda: Cyclopoida) in the laboratory culture. Korean J Limnol 2000;33:1-8.
Fig. 1A: a microculture slide for rearing Eucyclops serrulatus. The microculture slide was prepared using a microscope slide glass (75 x 25 mm) on which a cover glass (22 x 40 mm) was mounted at a height of 0.8 mm using a couple of polypropylene bars (0.8 x 1.0 x 22 mm) fixed with a silicone-based sealant. B: a microculture slide in a petri dish with wet paper towels to provide humidity.

Fig. 2Proliferation curve of Chilomonas paramecium in various media. The infusion media were constituted with mineral water boiled with Triticum aestivum (TA medium), Glycine max (GM medium), Sesamum indicum (SIM medium), Hordeum vulgare (HV medium), Coixlachryma-jobi (CJ medium), or Setaria italica (SIA medium). Each data point represents the average of triplicated samples.

Fig. 3Various stages of Eucyclops serrulatus grown in the microculture slide with GM medium. A: nauplius instars N1; B: nauplius instars N4; C: nauplius instars N6; D: copepodite instar C1; E: copepodite instar C3; F: copepodite instar C4; G: adult male; H: egg-bearing adult female.

Table 1.Relative size and duration of various developmental stages of Eucyclops serrulatus reared in microculture slide
Table 1.
|
Nauplius 1 (n=30)
|
Copepodite 1 (n=28)
|
Adult male (n=13)
|
Adult female (n=15)
|
|
Daya)
|
Widthb)
|
Lengthc)
|
Day |
Width |
Length |
Day |
Width |
Length |
Day |
Width |
Length |
|
Mean |
1.5 |
80.1 |
120.0 |
7.7 |
134.6 |
345.1 |
20.4 |
198.4 |
620.5 |
20.1 |
283.8 |
845.2 |
|
SDd)
|
0.6 |
0.6 |
0.6 |
1.2 |
3.3 |
3.7 |
3.0 |
7.0 |
13.6 |
2.5 |
7.3 |
20.3 |
|
Maxe)
|
2 |
81 |
121 |
9 |
139 |
350 |
25 |
210 |
641 |
25 |
299 |
880 |
|
Minf)
|
0.5 |
79 |
119 |
6 |
127 |
335 |
16 |
184 |
596 |
17 |
272 |
816 |
Table 2.Time intervals for egg sac production by Eucyclops serrulatus in microculture slide
Table 2.
|
Number of egg sacs per female
|
Mean life time (days) |
Mean brood number |
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
No. cyclops alive |
15 |
11 |
11 |
10 |
7 |
7 |
5 |
2 |
|
|
|
Mean (days) |
20.1 |
23.6 |
30.9 |
36.3 |
41.3 |
45.9 |
51.6 |
53.5 |
44.7 |
4.5 |
|
SDa)
|
2.5 |
5.6 |
5.0 |
6.1 |
4.1 |
4.9 |
6.9 |
9.2 |
15.9 |
2.7 |
|
Sizeb): maximum |
25 |
30 |
38 |
45 |
48 |
55 |
62 |
60 |
67 |
8 |
|
minimum |
17 |
11 |
22 |
26 |
36 |
39 |
43 |
47 |
23 |
1 |