Abstract
We investigated the induction of resistance to Haemaphysalis longicornis infestation in rabbits that had been immunized with recombinant H. longicornis P27/30 protein. The success of immunological control methods is dependent upon the use of potential key antigens as tick vaccine candidates. Previously, we cloned a gene encoding 27 kDa and 30 kDa proteins (P27/30) of H. longicornis, and identified P27/30 as a troponin I-like protein. In this study, rabbits that were immunized with recombinant P27/30 expressed in Escherichia coli showed the statistically significant longer feeding duration for larval and adult ticks (P<0.05), low engorgement rates in larval ticks (64.4%), and an apparent reduction in egg weights, which suggest that H. longicornis P27/30 protein is a potential candidate antigen for a tick vaccine. These results demonstrated that the recombinant P27/30 protein might be a useful vaccine candidate antigen for biological control of H. longicornis.
-
Key words: P27/30 protein, Haemaphysalis longicornis, ticks, immunity, tick control
INTRODUCTION
Tick infestation control is difficult, however, it may be effected by integrated pest management, whereby different control methods are adapted in a geographic area against one tick species with due consideration to their environmental effects. Recently, the development of vaccines against
Boophilus spp. has provided new possibilities for the identification of protective antigens for use in vaccines for the control of tick infestations (
Willadsen, 1997;
de la Fuente et al., 1999,
2000;
Willadsen and Jongejan, 1999;
de Vos et al., 2001). Ticks are considered to be second only to mosquitoes as vectors of human diseases, though they are considered to be the most important pathogen vector in North America (
Parola and Raoult, 2001).
The hard tick
Haemaphysalis longicornis Neumann, 1901, is widely distributed in East Asia and Australia, and transmits pathogens that cause deteriorative diseases in human and animals (
Fujisaki, 1978;
Fujisaki et al., 1994). A variety of methods have been employed to suppress tick vector populations, including the application of biological control agents (
Frazzon et al., 2000) and a heavy reliance on the use of chemical acaricides (
Solomon, 1983). Control of ticks by vaccination would avoid environmental contamination and the selection of drug resistant ticks due to repeated acaricide application (
de la Fuente et al., 1998;
Garcia-Garcia et al., 1999). However, some hosts become resistant after multiple infestations and often display immune responses to substances found in tick saliva (
Willadsen, 1980;
Wikel, 1982;
Wikel and Whelen, 1986). Moreover it has been proposed that immunization with tick extracts could induce resistance to infestation resembling the immune protection observed after repeated infestations. The success of such methods is dependent on the identification and cloning of tick molecules involved in the mediation of key physiological roles.
Recently genes for two
Boophilus microplus midgutassociated molecules, Bm 86 and Bm 91, were cloned and expressed (
Rand et al., 1989;
Riding et al., 1994). Others have demonstrated the ability of dogs to develop resistance against
Rhipicephalus sanguineus (
Pogoielyi, 1966;
Inokuma, 1997), and the antigens involved were shown to confer a significant protective immunity against
R. sanguineus infestation in cows (
Rand et al., 1989;
Riding et al., 1994) and in dogs (
Pogoielyi, 1966;
Inokuma, 1997). Host resistance to infestation can be determined by measuring tick feeding and fecundity performance parameters; and several of these parameters have been shown to be affected by hosts resistant to other tick species (
Barriga et al., 1995;
Sahibi et al., 1997). Previously, we cloned the
H. longicornis P27/30 gene and found by immunoblotting that anti-mouse sera against recombinant P27/30 reacts with native 27/30 kDa proteins from
H. longicornis adult lysates, the results obtained suggested that the native P27/30 protein is a troponin I-like protein that has two isoforms (
You et al., 2001). The most striking property of troponin I is its ability to inhibit the magnesium activated ATPase of actomyosin, but not the calcium activated ATPase of actomyosin or myosin (
Zot and Potter, 1987). This indicates that in some way troponin I blocks the interaction between actin and myosin, which is responsible for the activation of MgATPase (
Zot and Potter, 1987).
In the present study, when ticks ingest blood from rabbits immunized with P27/30, tick gut cells lyse via an antibody-mediated mechanism of host defense, thus provoking a reduction in tick number, weight, and reproductive cavity. We describe the effects of immunizing rabbits with recombinant P27/30 protein on tick feeding. In addition, we discuss the possible use of P27/30 as a vaccine candidate for tick control.
MATERIALS AND METHODS
Tick
The parthenogenetic Okayama strain of the tick
H. longicornis (
Fujisaki, 1978) has been maintained for several generations since 2003, in our laboratory on rabbits and mice.
The P27/30 gene encoding H. longicornis P27/30 was inserted into the EcoRI site of pBluescript SK (+) vector. The P27/30 gene in pBluescript SK (+) vector was subcloned into the pGEMEX-2 (Promega, Madison, WI, USA) plasmid of E. coli expression vector after digestion with EcoRI. The resulting plasmid pGEMEX-2/P27/30 was checked for accurate insertion by restriction enzyme analyses. pGEMEX-2/P27/30 was used to transform E. coli (JM109 (DE3), Promega) using standard techniques. Recombinant P27/30 (rP27/30) was expressed as a gene 10 fusion protein, and designated gene 10-P27/30 protein.
Protein determination
Protein concentrations were determined using a BCA protein assay kit (Pierce, Rockford, IL, USA), bovine serum albumin was used as a standard.
Immunization of rP27/30
Twelve female rabbits (2.5-3 kg) were immunized and challenged with H. longicornis. Of the 12 rabbits, six were immunized with rP27/30, and six with control gene 10 proteins. One milligram of rP27/30 and gene 10 proteins were injected into rabbits subcutaneously after emulsifying each with Freund's complete adjuvant (Wako, Tokyo, Japan). First and second booster injections were prepared in the same manner and administered with Freund's incomplete adjuvant (Wako) at 1-month intervals.
Serological analysis
Host immune responses to the recombinant proteins were determined by immunoblot analysis in individual animals using previously described methods (
You et al., 2001) using nitrocellulose strips with transferred rP27/30 and gene 10 proteins. Antibody titers were expressed as the highest dilution showing immune reactive bands.
When antibody titers reached 1:5,000 to 1:8,000, the rabbits were challenged with the different developmental stages of
H. longicornis. Unfed larvae, nymphs and adults of
H. longicornis were fed on shaved ears of rP27/30 or gene 10-immunized rabbits as described elsewhere (
Fujisaki, 1978). Infestations involved 30 adult ticks, 50 nymphs, and 100 larvae per rabbit. Visual examinations of rabbits were performed posttick infestation, and the following parameters were recorded; duration of feeding, engorged rates, engorged weights, molting periods, and egg weights. Once the engorged ticks had been obtained, they were transferred into individual glass flasks in an incubator at 25℃ and 85% RH to allow egg-laying and molting. The time from infestation start to drop-off is referred to as the duration of feeding. Engorged rates were assigned to the rates from infestation to engorgement. Engorged weights were instantly measured after drop-off, and egg weights 3 weeks after drop-off.
All values are expressed as means ± standard error. Comparisons between group means were carried out using the Student's t-test. Differences were considered significant at the 95% confidence level.
RESULTS
The effects of rP27/30 immunization on tick feeding and fecundity are summarized in
Figs. 1,
2 and
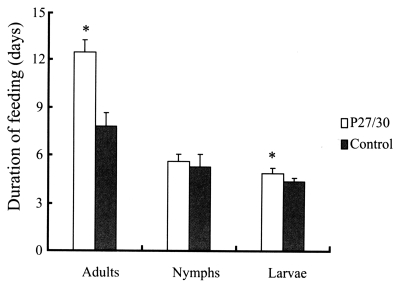
3. No differences in attachment rates were observed during the initial 24 hr after nymphal and adult ticks had been introduced onto rabbit backs. In terms of feeding of larval, nymphal, and adult ticks from infestation to drop, adult ticks that fed on rP27/30-immunized rabbits were observed to feed longer (12.5 ± 0.78 days) compared to the control (7.8 ± 0.83 days) (
P<0.05,
Fig. 1), as did tick larvae (4.9 ± 0.3 days vs. 4.4 ± 0.2 days) (
P <0.05,
Fig. 1). However, no differences were observed in the duration of nymph feeding (5.6 ± 0.45 days) versus the control (5.3 ± 0.76 days) (
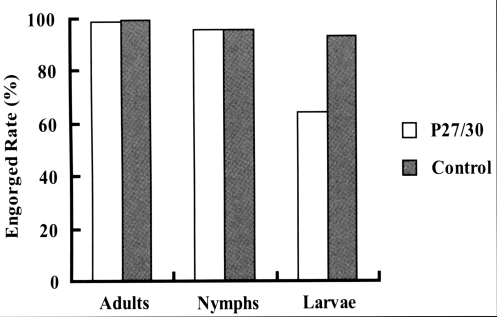
Fig. 1). In terms of engorged rates of larval, nymphal, and adult ticks from infestation to drop-off, larval ticks that fed on rP27/30-immunized rabbits exhibited a lower attachment rate (64.4%) than the control (93.4%). No significant difference was observed for nymphs and adults (
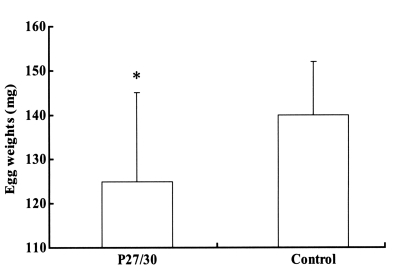
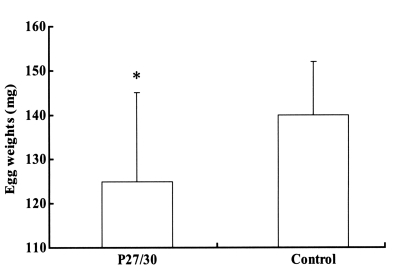
Fig. 2). The engorged body weights of larval, nymphal, and adult ticks following feeding on rP27/30-immunized rabbits were 0.62 ± 0.05, 4.1 ± 0.2, and 257 ± 10.5 mg, respectively, compared to control values of 0.66 ± 0.05, 4.2 ± 0.2, and 263 ± 12.5 mg, none of which were significant. Molting periods of larval and nymphal ticks from drop-off to molting following feeding on rP27/30-immunized rabbits were 9.0 ± 0.2 and 10.3 ± 0.2 days, respectively, versus control values of 9.0 ± 0.3 and 10.2 ± 0.5 days. An apparent reduction in egg weights was observed for adult ticks fed on rP27/30-immunized rabbits (125 ± 20 mg vs a control value of 140 ± 12 mg) (
Fig. 3).
DISCUSSION
Ticks cause direct and indirect damage to livestock and disease in man. Ticks may cause severely toxic conditions, e.g. tick paralysis, various tick toxicoses, irritation, tick bite allergies, immune responses, and economic losses due to blood sucking (
Sonenshine, 1991).
H. longicornis is the most predominant species in East Asian pastures, and is a three-host tick and transmits theileriosis caused by
Theileria sergenti/buffeli/orientalis among grazing cattle (
Fujisaki, 1992;
Fujisaki et al., 1994).
Tick control represents the most important part of the current global strategy to control major tick-borne diseases.
H. longicornis is a vector for rickettsiae causing Q fever, viruses causing Russian spring-summer encephalitis, and for protozoa that cause theileriosis and babesiosis (
Hoogstraal, 1968). Vector control has a vital role in the prevention of malaria and is the only practical option for the control of dengue and Chagas' disease (
Schofield and Dias, 1999). Tick-borne diseases are particularly devastating to livestock, especially cattle, with costs estimated at about $7 billion per annum (
Food and Agricultural Organization, 1995). Moreover, our failure to control ticks and tick-bornedisease is a major factor limiting livestock production world-wide.
The native P27/30 protein of
H. longicornis has been suggested to be a troponin I-like protein that may have two isoforms (
You et al., 2001). Troponin I is a principal component of thin filament-linked system of regulatory proteins. It also binds to actin and inhibits the actin-myosin interaction (
Leavis and Gergely, 1984). This unique role requires that it must interact with each of the proteins involved, i.e., directly with actin, troponin C, and troponin T and possibly indirectly with tropomyosin and myosin (
Perry, 1999). In the present study, we found that recombinant P27/30 protein expressed in
E. coli stimulates a specific protective anti-tick immune response in rabbits, as demonstrated by the statistically significant longer duration of feeding required by the larval and adult forms (
Fig. 1), and the low engorged rates in larval ticks (64.4%,
Fig. 2). These results suggest that rabbits immunized with rP27/30 protein acquired a significant level of resistance against
H. longicornis infestations. No apparent egg weights or molting period difference were observed between adult applied to rP27/30-immunized rabbits versus the controls. Egg weights in ticks fed on rP27/30-immunized rabbits tended to be lower than those of the controls. The findings of the present study are consistent with those of Kemp et al. (
1986) concerning the effects of different vaccines on immature and mature
H. longicornis ticks.
Several previous investigations have indicated potential applications for inducing protective immunity against ticks and analyzed the merits and demerits of using either exposed or concealed antigens as vaccine candidates. A vaccine against
B. microplus, a highly adapted one-host cattle tick, has been developed with the midgut-associated immunogen Bm 86, and was found to reduce the engorged weights and egg laying rates of engorged female ticks (
Willadsen et al., 1995). Tick concealed antigens produce immunity that inhibits tick fecundity, and also prevent tick feeding, whereas tick saliva proteins increase fecundity and facilitate feeding (
Sahibi et al., 1997). Recent studies on tropomyosin have revealed that it may be implicated in host protective responses to microfilariae in onchocerciasis (
Jenkins et al., 1998). In this study, rabbits that were immunized with rP27/30 protein showed considerable resistance to tick infestation, indicating that P27/30 protein implicated in host protective response to
H. longicornis may be identical to tropomyosin. Our data also indicates that P27/30 protein may play an important role during bloodsucking in
H. longicornis, suggesting the possibility that
H. longicornis P27/30 protein is a potential candidate antigen for a tick vaccine.
Previous studies by Riding et al. (
1994) and Willadsen et al. (
1996) provided evidence that antitick immunity induced by a combination of vaccine antigens is more effective than that induced by a single antigen vaccine. Our efforts are currently directed toward cloning other concealed tick molecules for using in vaccine trials in combination with
H. longicornis P27/30 protein.
Notes
-
This study was supported by the Technology Development Program for Agriculture and Forestry, Ministry of Agriculture and Forestry, Republic of Korea.
ACKNOWLEDGEMENTS
We take this opportunity to thank Dr. K. Fujisaki for valuable advice.
References
- 1. Barriga OO, da Silva SS, Azevedo JSC. Relationships and influences between Boophilus microplus characteristics in tick-naive or repeatedly infested cattle. Vet Parasitol 1995;56:225-238.
- 2. de la Fuente J, Rodriguez M, Garcia-Garcia JC. Immunological control of ticks through vaccination with Boophilus microplus gut antigens. Ann NY Acad Sci 2000;916:617-621.
- 3. de la Fuente J, Rodriguez M, Montero C, et al. Vaccination against ticks (Boophilus spp.): the experiencewith the Bm86-based vaccine Gavac. Genet Anal 1999;15:143-148.
- 4. de la Fuente J, Rodriguez M, Redondo M, et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine 1998;16:366-373.
- 5. de Vos S, Zeinstra L, Taoufik O, Willadsen P, Jongejan F. Evidence for the utility of the Bm86 antigen from Boophilus microplus in vaccination against other tick species. Exp Appl Acarol 2001;25:245-261.
- 6. Food and Agricultural Organization. FAO 1994 year book production, 48. 1995, Rome, Italy. FAO; pp 8-12.
- 7. Frazzon AP, da Silva Vaz Junior I, Masuda A, Schrank A, Vainstein MH. In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus. Vet Parasitol 2000;94:117-125.
- 8. Fujisaki K. Development of acquired resistance and precipitating antibody in rabbits experimentally infested with females of Haemaphysalis longicornis(Ixodoidea:Ixodidae). Natl Inst Anim Health Q (Tokyo) 1978;18:27-38.
- 9. Fujisaki K. A review of the taxonomy of Theileria sergenti/buffeli/orientalis group parasites in cattle. J Protozool Res 1992;2:87-96.
- 10. Fujisaki K, Kawazu S, Kamio T. The taxonomy of the bovine Theileria spp. Parasitol Today 1994;10:31-33.
- 11. Garcia-Garcia JC, Gonzalez IL, Gonzalez DM, et al. Sequence variations in the Boophilus microplus Bm86 locus and implications for immunoprotection in cattlevaccinated with this antigen. Exp Appl Acarol 1999;23:883-895.
- 12. Hoogstraal H, Roberts FH, Kohls GM, Tipton VJ. Riview of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae). J Parasitol 1968;54:1197-1213.
- 13. Inokuma H, Tamura K, Onishi T. Dogs develop resistance to Rhicephalus sanguineus. Vet Parasitol 1997;68:295-297.
- 14. Jenkins RE, Taylor MJ, Gilvary NJ, Bianco AE. Tropomyosin implicated in host protective responses to microfilariae in onchocerciasis. Proc Natl Acad Sci USA 1998;95:7550-7555.
- 15. Kemp DH, Agbede RI, Johnston LA, Gough JM. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: feeding and survival of the parasite on vaccinated cattle. Int J Parasitol 1986;16:115-120.
- 16. Leavis PC, Gergely J. Thin filament proteins and thin filament-linked regulation of vertebrate muscle contraction. CRC Crit Rev Biochem 1984;16:235-305.
- 17. Parola P, Raoult D. Tick-borne bacterial diseases emerging in Europe. Clin Microbiol Infect 2001;7:80-83.
- 18. Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem 1999;190:9-32.
- 19. Pogoielyi AI. Immunity to ectoparasites (disease) in animals. Vet Kiev 1966;6:68-75.
- 20. Rand KN, Moore T, Srikantha A, et al. Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc Natl Acad Sci USA 1989;86:9657-9661.
- 21. Riding GA, Jarmey J, McKenna RV, Pearson R, Cobon GS, Willadsen P. A protective concealed antigen from Boophilus microplus: purification, localization and possible function. J Immunol 1994;153:5158-5166.
- 22. Sahibi H, Rhalem A, Barriga OO. Comparative immunizing power of infections, salivary extracts, and intestinal extracts of Hyalomma marginatum marginatum in cattle. Vet Parasitol 1997;68:359-366.
- 23. Schofield CJ, Dias JC. The Southern Cone programme against Chagas disease. Adv Parasitol 1999;42:1-27.
- 24. Solomon KR. Acaricide resistance in ticks. Adv Vet Sci Comp Med 1983;27:273-296.
- 25. Sonenshine DE. Biology of ticks, 1. 1991, London, England. Oxford University Press; pp 99-11.
- 26. Wikel SK, Osburn RL. Immune responsiveness of the bovine host to repeated low-level infestations with Dermacentor andersoni. Ann Trop Med Parasitol 1982;76:405-414.
- 27. Wikel SK, Whelen AC. Ixodid-host interaction, identification and characterization of relevant antigens and tick-induced host immunosuppression. Vet Parasitol 1986;20:149-174.
- 28. Willadsen P. Novel vaccines for ectoparasites. Vet Parasitol 1997;71:209-222.
- 29. Willadsen P. Immunity to ticks. Adv Parasitol 1980;18:293-311.
- 30. Willadsen P, Bird P, Cobon GS, Hungerford J. Commercialization of a recombinant vaccine against Boophilus microplus. Parasitology 1995;110:S43-S50.
- 31. Willadsen P, Jongejan F. Immunology of the tick-host interaction and the control of ticks and tick-borne diseases. Parasitol Today 1999;15:258-262.
- 32. Willadsen P, Smith D, Cobon GS, McKenna RV. Comparative vaccination of cattle against Boophilus microplus with recombinant Bm86 alone or in combinationwith recombinant Bm91. Parasite Immunol 1996;18:241-246.
- 33. You M, Xuan X, Tsuji N, et al. Molecular characterization of a troponin I-like protein from the hard tickHaemaphysalis longicornis. Insect Biochem Mol Biol 2001;32:67-73.
- 34. Zot AS, Potter JD. Structural aspects of troponintropomyosin regulation of skeletal muscle contraction. Annu Rev Biophys Biophys Chem 1987;16:535-559.
Fig. 1Comparison of duration of feeding after applying larval, nymphal, and adult H. longicornis forms to rP27/30-immunized rabbits and gene 10 protein-immunized control rabbits (*P<0.05). Data are presented as means ± SE.
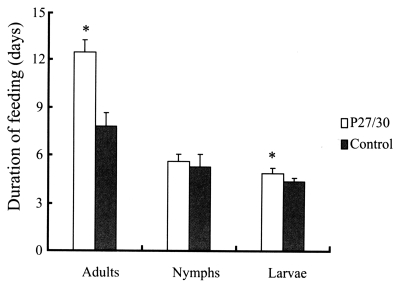
Fig. 2Comparison of engorged rates after the attachment of larva, nymph, and adult H. longicornis to rP27/30-immunized rabbits and gene 10 protein-immunized control rabbits.
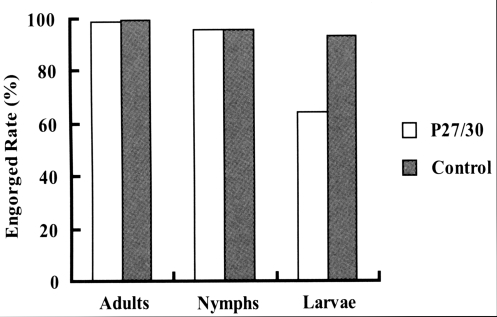
Fig. 3Comparison of egg weights 3 weeks after adult H. longicornis drop-off from rP27/30-immunized rabbits and gene 10 protein-immunized control rabbits (*P<0.05). Data are presented as means ± SE.