Abstract
The developmental features, growth and organogenesis of Macroorchis spinulosus were observed in albino rats. Globular and thick walled metacercariae, possessed a stylet, Y-shaped excretory bladder and extracecal testes. In albino rats, M. spinulosus showed habitat shifting. The majority of M. spinulosus reside in the jejunum for the first four days post infection (p.i.) and migrate to the duodenum at the later stage of infection. M. spinulosus grew rapidly during the first four days and reached full maturity at 14 days p.i. and later reduced in size. The ovary was separated from the genital primodium at one day p.i. The seminal vesicle appeared on the third day and divided into two sacs on the fourth day p.i. and intrauterine eggs and sperm mass were produced on the fourth day. Organogenesis and enlargement of reproductive organs governed the growth of M. spinulosus. The similarity of related species of the genus Macroorchis to M. spinulosus was discussed in consideration to developmental features.
-
Key words: Macroorchis spinulosus, growth, habitat shift, intrauterine sperm mass
INTRODUCTION
Macroorchis spinulosus is a minute trematode possessing large testes which represent generic morphological character.
M. spinulosus has been found in the small intestine of dogs, cats, mice, guinea pigs, rabbits, and albino and house rats (
Ando, 1918,
1921). Metacercariae of
M. spinulosus were identified from intermediate crustacean hosts such as freshwater crabs,
Potamon dehaani and
P. obtusipes (
Ando, 1918) and crayfish,
Cambaroides similis (
Chai et al., 1996). New species of the genus
Macroorchis were proposed in recognition of one or more morphological differences, distinctive intermediate hosts and geographical segregation. These are
M. himizu,
M. chimarrogalus and
M. elongatus (
Machida and Uchida, 1982;
Saito et al., 1982). To supplement the original description, the morphological features of
M. spinulosus were redescribed recently in detail based on adult flukes (
Chai et al., 1996).
Information on the growth and development of M. spinulosus in the final hosts is scarce. It is likely that the excysted metacercariae of M. spinulosus grow up rapidly to ovigerous adult flukes in the small intestine of final hosts. This work was performed to shed light on the growth and development of M. spinulosus in the final host, and may provide knowledge for speciation of probable sibling trematodes.
MATERIALS AND METHODS
The crayfish,
Cambaroides similis, was caught in the streams in Macheon-myeon, Hamyang-gun, Gyeongsangnam-do, Korea from October to December 1993. The crayfish were crushed with a mortar and a pestle, and
M. spinulosus metacercariae were collected from the sediment under a dissecting microscope. Four week old, Charles River strain, Sprague-Dawley, albino rats were each fed with 100 metacercariae (
Table 1).
Rats were sacrificed at predetermined intervals and M. spinulosus were recovered from the duodenum, jejunum and ileum under a dissecting microscope. Recovered flukes were fixed in 10% neutral formalin and stained with Semichon's acetocarmine. Ten flukes were selected randomly from each daily group and measured microscopically. The dimension of organs represents arithmetic mean and one standard deviatio
RESULTS
Metacercariae
A total of 2,905 M. spinulosus metacercariae were collected from 69 crayfishes. Metacercarial cysts were globular, 184.3 ± 10.9 µm in length and 178.4 ± 12.1 µm in width, with clear walls 13.0 ± 2.6 µm thick. Oral sucker was large and armed with a slender stylet. Acetabulum was located in mid-body. The oral sucker and acetabulum were almost equal in size. Excretory bladder was Y-shaped and black and contained numerous excretory granules. Testes were spindle-shape and extra-intestinal ceca.
Fluke recovery
The overall experimental period fluke recovery rate was over 60.7% (
Table 1). Recovery rate of ovigerous flukes, recovered from the duodenum and jejunum only and comprising wholly of flukes older than 4-day-old flukes, increased to 74.3%. During the first four days, the majority of the flukes were recovered from the jejunum, in which the proportion of the recovered flukes decreased from 95.2% at one day p.i. to 77.7% at four days p.i. and dropped to 1.3% at 28 days p.i. During the later stage of infection, the majority of flukes were recovered from the duodenum, increasing from 91.1% at 5 days p.i. to 98.7% at 28 days p.i. (
Table 1).
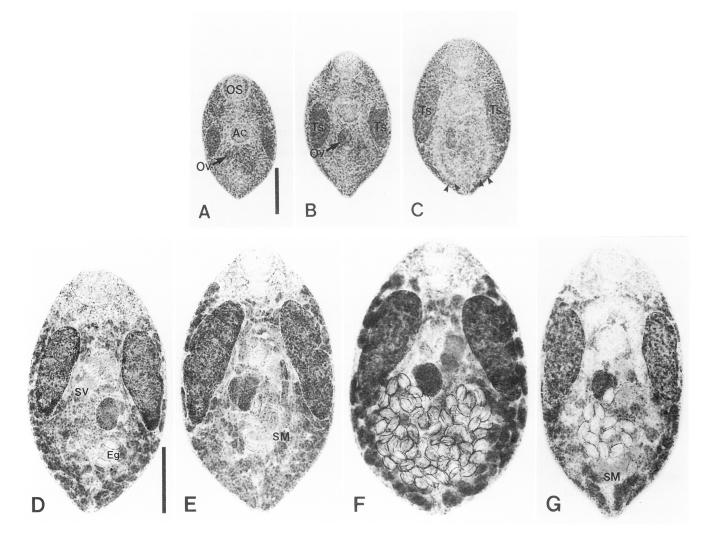
One-day-old flukes were elliptical, 364 µm long and 232 µm wide. The oral sucker was round and ventro-subterminal. Stylet was found in 50% of the oral sucker of flukes observed. Prepharynx was not present. The pharynx was broadened and apple-shaped. The acetabulum was mid-median of the body. Spindle shaped testes were lateral to the acetabulum and extra-cecal at the middle of the body. Anterior margins of the testes and acetabulum appeared at nearly the same level of the body. The intestine was bifurcated immediately after the pharynx into two branches that ran between the testis and the acetabulum, and reached at posterior one-fourth of the body. The ovary, round in shape, was recognised as a separate, round mass from that of primordial complex of genital organs, which was posterodextral to the acetabulum. Excretory pore opened at posterior extremity of the body (
Fig. 1A).
The body of 2-day-old flukes was enlarged compared to that of one-day-old flukes. The ovary became large and stained deep at posterior to the acetabulum. Mehlis' gland appeared to the right of the ovary. The testes were ellipoid and were thick and prominent in this fluke. Eighty percent of the testes contained sperm (
Fig. 1B).
The 3-day-old fluke increased in body area to 1.7 times that of one-day-old fluke. And the oral sucker became wider. The ovary was ovoid and increased in size to two times of that of one-day-old flukes. Primodial seminal vesicle appeared bipartite and sinistral to the ovary in two of ten specimens observed. The testes, elongated and ellipsoid, were bi-lobate in the middle by invagination from the lateral border. All testes produced sperm. Intestinal ceca reached near to the posterior end of the body (
Fig. 1C).
The 4-day-old fluke grew abruptly, 2.1 times that of the 3-day-old flukes in body area. The size of the testes and ovary increased rapidly and were nearly double that of 3-day-old flukes. The testes having anterior margin round and posterior extremity conical or pointed, proceeded to the acetabulum and occupied the mid-lateral regions of the body. Seminal vesicle, bipartited and containing sperms, appeared postero-sinistral or -dextral to the acetabulum. Seminal receptacle was absent. Banana-shaped intrauterine sperm mass was found amongst intrauterine eggs in the posteromedian portion of the body in 60% of flukes. Vitelline glands were follicular and distributed superficially from the posterior end of the body to the level of the pharynx. The vitelline follicles were abundant in the area lateral to the intestinal ceca and confluent in the anterior and posterior areas. Almost all flukes (90%) produced a number of eggs, ranging from 4 to 23. The uterus was convoluted in the portion of the body posterior to the ovary. Genital pore opened adjacent to the posterior margin of the acetabulum (
Fig. 1D).
The size of 7-day-old flukes was not significantly larger than that of 4-day-old flukes. At this stage of infection the flukes grew to 0.778 µm in body length and the anterior one-third line of testes was level to the acetabulum. The areas of the seminal vesicle and intrauterine sperm mass increased for three days by 2.1 and 2.9 times, respectively. Intrauterine sperm mass was found in 90% of specimens. Stylet was observed in 60% of the flukes (
Table 2;
Fig. 1E).
Growth of
M. spinulosus peaked at 14 day p.i in this study, when dimensions of the body and all organs were at a maximum and stained deeply. Intrauterine eggs occupied the posterior half of the body. Growth of testes, a characteristic feature of this genus, was most prominent at this time and showed a 9 fold increase in area compared to that of one-day-old flukes. Seminal vesicle was full of sperm and located in triad formation with the acetabulum, ovary and right testis. Oral sucker was broadly round and as was the acetabulum, and their area was nearly same. Thick tegumental spines were set on the surface of anterior half of the body and became thinner towards the posterior (
Fig. 1F). Intrauterine sperm mass was found infrequently between a large number of intrauterine eggs. The ovulated eggs were broad and golden yellow, thin shelled, had conspicuous operculum and contained yolk cells. Their dimensions were 55.7 ± 2.4 µm long and 36.0 ± 1.5 µm wide.
The 28-day-old fluke was reduced in body width. Dimensions and compactness of reproductive organs were also remarkably decreased (
Table 2;
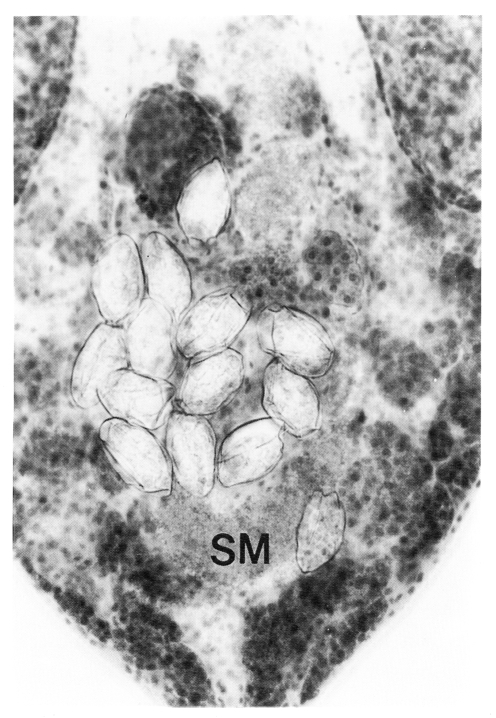
Fig. 1G). The number of intrauterine eggs was also decreased. Seminal vesicle was conspicuously bipartite. Seminal receptacle absent. Banana- or rod-shaped intrauterine sperm mass was observed in all flukes, in space not occupied by uterine eggs, in posterior portion of the body (
Fig. 2).
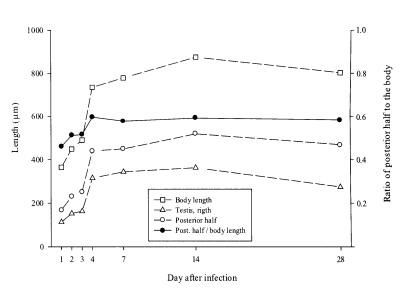
Macroorchis spinulosus grew rapidly during the first four days and measured two times that of one-day-old flukes, and reaching maximum dimensions at 14 days post-infection. The body reduced in size thereafter. Growth was led by an enlargement of the posterior half of the body. The ratio of the posterior half of the body to its overall length increased during the first four days of infection and stayed at plateau thereafter (
Fig. 3). The enlargement of the posterior half of the body was governed by development of reproductive organs such as the testes and ovary and by increase of the amunt of intrauterine eggs (
Table 2).
DISCUSSION
Adult flukes collected in this study were identified as
Macroorchis spinulosus using morphological characteristic such as the presence of bilobed seminal vesicles, anterolateral location of testes, variable location of the ovary, and stylet presence in the oral sucker. These observations are consistent with the original and amended descriptions (
Ando, 1918;
Chai et al., 1996).
For the genus
Macroorchis, the final hosts are reported to be rats, mice, hamsters, dogs, cats, moles and water shrew (
Machida and Uchida, 1982;
Saito et al., 1982;
Chai et al., 1996). Fluke recovery rate was high and maintained thus from albino rats for the infection period in this study. It is considered that albino rats are suitable experimental animal as final hosts for
M. spinulosus.
Habitat shift is an infrequent finding in intestinal trematode distribution in final hosts. In albino rats, the recovery rate of
M. spinulosus from the jejunum decreased dramatically and increased in duodenum after 5 day p.i. without a change in recovery rate. It implies that the distribution of
M. spinulosus was shifted from the jejunum to the duodenum after 5 days following infection. At day 4 p.i.,
M. spinulosus grew to ovigerous adult flukes and needed large amount of nutrient to produce eggs. It is likely that the increased nutritional demand may cause
M. spinulosus to migrate to the more nutrient-rich duodenum. On the other hand, in the case of
Plagiorchis muris, a habitat shift from jejunum to ileum after three days p.i. was noted. It was speculated on this shift that the metacercariae might be excysted in upper or middle part of small intestine and the juvenile flukes be on their ontogenic migration to an favored habitat, the ileum (
Hong et al., 1998). These findings are different from the distribution of
Neodiplostomum seoulense in the small intestine of albino rats. In the early stage of infection,
N. seoulense reside in the upper part of small intestine, with the majority in the duodenum, and are discharged from jejunum as infection period progressed. The change of distribution showing
N. seoulense concentrated in duodenum in later stages of infection (
Hong et al., 1983) suggests that
N. seoulense did not migrate from the jejunum, but rather remained in its initial location.
The bodies of the ovigerous adult flukes of
M. spinulosus were spindle to oval shaped in this study. Enlargement of the testes and an increase of intrauterine eggs distended the body, especially the posterior half, rendering it ovoid. A decrease in the number of intra-uterine eggs caused the body to shrink in 28-day-old
M. spinulosus. Factors affecting the shape of flukes when fixing are freshness and delicacy in handling of the flukes (
Chai et al., 1996). Some flukes collected from dead experimental and wild animals can exhibit unusual shapes after fixation. In this regard, the body shapes of sibling flukes of the genus
Macroorchis - such as the oval shape of
M. himizu and the spindle shape of
M. chimarrogalus - could be intraspecific variations. The proportion of stylet possession in the oral suckers was unrelated to growth and maturity of
M. spinulosus. The highest possession rate was observed in 7-day old flukes and even found in 28-day-old flukes. It is, therefore, unlikely that the absence of stylet from the oral sucker could be a species-specific characteristic for
M. himizu (
Machida and Uchida, 1982).
The dimensions of the oral sucker and the acetabulum of
M. spinulosus observed in this study, were significantly different in juvenile flukes, but similar in ovigerous flukes. The re-description on
M. spinulosus was based on adult flukes possessing an oral sucker and acetabulum that were almost equal in size (
Chai et al., 1996). However, similarity in dimensions of the oral sucker and acetabulum in
M. chimarrogalus was recognized to be a morphological feature distinctive from
M. spinulosus and adopted as a feature in describing a new species (
Saito et al., 1982). Collectively, it is suggested that variations asserted to be specific characteristics for
M. himizu or
M. chimarrogalus could be developmental features of
M. spinulosus.
Ando (
1918) recognized that one of the seminal vesicular sacs acted as a seminal receptacle. Later, flukes of genus
Macroorchis were found lacking a seminal receptacle, but intra-uterine sperm mass occurred along uterine loops (
Saito et al., 1982;
Machida and Uchida, 1982). In this study, intrauterine sperm mass appeared in almost all flukes older than 4-days p.i. The shape of the intra-uterine sperm mass was largely dependent on the amount and location of intra-uterine eggs. Here we add this feature regarding to seminal receptacle to the morphological characteristics of
M. spinulosus.
The crayfish proved to be one of the second intermediate hosts for
M. spinulosus, which was caught from a stream in the mid-eastern mountainous area of the Korean peninsula (
Chai et al., 1996). The stream in which the crayfish were caught in this study is situated in the southern mountainous area of the Korean peninsula. Here we add this area as an ecological focus of
M. spinulosus. This place warrants a survey to determine the final hosts of
M. spinulosus.
Notes
-
This study was supported by a Chung-Ang University Research Grant (1999).
References
- 1. Ando A. On a new trematode with the freshwater crab as its intermediate host. Tokyo Iji Shinshi 1918;2081:1320-1327.
- 2. Ando A. A contribution of the study of a new trematode, Morcroorchis Spinulosus n.g., n. sup., whose intermediate host is the crob. Nippon Biseibutsu Gakkai Zasshi 1921;15:813-826.
- 3. Chai JY, Sohn WM, Huh S, Choi MH, Lee SH. Redescription of Macroorchis spinulosus Ando, 1918 (Digenea: Nanophyetidae) encysted in the fresh water crayfish, Cambaroides similis. Korean J Parasitol 1996;34:1-6.
- 4. Hong SJ, Ahn JH, Woo HC. Plagiorchis muris: recovery, growth and development in albino rats. J Helminthol 1998;72:251-256.
- 5. Hong SJ, Lee SH, Seo BS, Hong ST, Chai JY. Studies on intestinal trematodes in Korea IX. Recovery rate and development of Fibricola seoulensis in experimental animals. Korean J Parasitol 1983;21:224-233.
- 6. Machida M, Uchida A. Some helminth parasites of the Japanese shrew mole from the Izu Peninsula. Mem Natn Sci Mus (Tokyo) 1982;15:149-154.
- 7. Saito Y, Watanabe T, Yamashita T. Two new intestinal trematodes, Macroorchis chimarrogalus n. sp. and Macroorchis elongatus from Japanese water shrew and others, with a description of the metacercaria of Macroorchis chimarrogalus n. sp. (trematoda: Nanophyeditae). Acta Med Biol 1982;30:47-56.
Fig. 1The growth and development of Macroochis spinulosus in albino rats. A, One-day-old fluke shows ovary (Ov) and an undifferentiated primordial mass of genital organs posterior to the acetabulum (Ac); B, Two-day-old fluke shows ovary distinct from the mass of differentiating genital organs; C, Three-day-old fluke. Note intestinal ceca (arrowheads) extending to near posterior end of the body; D, Four-day-old fluke shows intra-uterine eggs (Eg) and seminal vesicle (SV). Vitelline follicles are distributed superficially posterior to the level of the pharynx; E, Seven-day-old fluke shows banana-shaped intra-uterine sperm mass (SM) posterior to the ovary; F, 14-day-old fluke shows a large number of eggs occupying posterior half of the body; G, 28-day-old fluke shows the intra-uterine sperm mass near the posterior end of the body. (OS, oral sucker; Ts, testis. Scale bar for figures A-C is 100 µm and for figures D-G is 200 µm).

Fig. 2A higher magnification of figure 3G shows intrauterine sperm mass (SM).

Fig. 3Growth curves of Macroochis spinulosus. The posterior half of the body was defined as a length between the acetabulum and the posterior end of body.

Table 1.Recovery rate of Macroorchis spinulosus from albino rats infected with metacercariae
Table 1.
|
Days postinfection |
No. of rats |
No. of metacercariae per rat |
No. of flukes recovered from
|
|
duodenum |
jejunum |
ileum |
subtotal (%) |
|
1 |
3 |
100 |
3 |
60 |
0 |
63 (21.0) |
|
2 |
3 |
100 |
12 |
128 |
0 |
140 (46.7) |
|
3 |
3 |
100 |
60 |
81 |
0 |
141 (47.0) |
|
4 |
3 |
100 |
46 |
152 |
0 |
198 (66.0) |
|
5 |
2 |
100 |
112 |
11 |
0 |
123 (61.5) |
|
7 |
3 |
100 |
222 |
41 |
0 |
263 (87.7) |
|
14 |
3 |
100 |
237 |
5 |
0 |
242 (80.7) |
|
28 |
3 |
100 |
224 |
3 |
0 |
227 (75.7) |
|
Total |
23 |
2,300 |
916 |
481 |
0 |
1,397 (60.7) |
Table 2.Measurements (in μm) on the organs of Macroorchis spinulosus recovered from albino rats after experimental infection
Table 2.
|
Organ |
|
Mean ± SD of flukes at day(s) post-infection
|
|
|
1 |
2 |
3 |
4 |
7 |
14 |
28 |
|
Body |
Length |
364.0 ± 46.1 |
448.8 ± 59.3 |
489.8 ± 49.5 |
735.1 ± 75.0 |
778.3 ± 53.5 |
875.0 ± 102.9 |
802.5 ± 67.4 |
|
Width |
232.8 ± 11.6 |
266.8 ± 24.9 |
299.0 ± 17.6 |
421.0 ± 36.2 |
466.1 ± 40.7 |
530.8 ± 32.9 |
414.1 ± 27.8 |
|
Oral sucker |
Length |
80.9 ± 6.1 |
93.6 ± 6.5 |
95.2 ± 4.2 |
95.1 ± 6.4 |
112.9 ± 8.8 |
108.1 ± 5.4 |
112.0 ± 5.7 |
|
Width |
88.0 ± 3.8 |
94.8 ± 6.1 |
98.8 ± 6.2 |
112.1 ± 10.4 |
131.6 ± 6.9 |
137.6 ± 6.1 |
128.1 ± 8.8 |
|
Pharynx |
Length |
35.6 ± 2.9 |
38.5 ± 3.1 |
38.2 ± 4.3 |
44.5 ± 5.6 |
56.4 ± 3.7 |
56.0 ± 3.4 |
52.1 ± 6.7 |
|
Width |
40.9 ± 2.0 |
43.0 ± 3.9 |
37.9 ± 4.2 |
55.2 ± 4.5 |
65.1 ± 4.4 |
67.1 ± 4.9 |
49.7 ± 4.9 |
|
Acetabulum |
Length |
65.3 ± 4.2 |
74.2 ± 7.1 |
78.4 ± 8.9 |
93.9 ± 7.2 |
109.2 ± 6.1 |
118.7 ± 7.5 |
110.1 ± 7.1 |
|
Width |
71.2 ± 4.5 |
76.9 ± 5.5 |
84.8 ± 4.9 |
96.8 ± 6.5 |
109.0 ± 6.5 |
115.1 ± 10.0 |
111.1 ± 6.0 |
|
Ovary |
Length |
39.6 ± 5.5 |
56.1 ± 5.9 |
62.3 ± 5.8 |
107.1 ± 11.3 |
111.6 ± 16.4 |
118.5 ± 16.5 |
104.0 ± 14.1 |
|
Width |
35.0 ± 5.7 |
43.4 ± 5.5 |
45.3 ± 5.8 |
72.4 ± 5.5 |
90.3 ± 12.3 |
97.0 ± 14.1 |
68.3 ± 7.0 |
|
Seminal vesicle |
Length |
N.D.a)
|
N.D. |
44.4 ± 22.1 |
86.1 ± 14.1 |
119.3 ± 19.5 |
141.2 ± 23.8 |
140.6 ± 38 |
|
Width |
N.D. |
N.D. |
18.0 ± 1.7 |
41.3 ± 6.3 |
63.6 ± 20.9 |
75.7 ± 12.3 |
56.6 ± 8.4 |
|
Testis, right |
Length |
113.1 ± 14.7 |
152.5 ± 24.4 |
162.7 ± 24.1 |
314.7 ± 30.3 |
343.2 ± 41.7 |
362.1 ± 32.8 |
273.9 ± 29.2 |
|
Width |
49.2 ± 4.1 |
60.9 ± 11.5 |
68.4 ± 6.0 |
118.4 ± 9.8 |
149.4 ± 18.2 |
146.6 ± 9.3 |
113.2 ± 11.5 |
|
Testis, left |
Length |
108.8 ± 8.5 |
145.3 ± 28.5 |
153.7 ± 21.5 |
293.4 ± 31.1 |
327.7 ± 41.3 |
336.5 ± 28.9 |
260.5 ± 28.1 |
|
Width |
50.8 ± 7.2 |
57.3 ± 8.5 |
70 ± 8.5 |
110.8 ± 11.0 |
143.5 ± 14.6 |
136.7 ± 8.8 |
105.0 ± 8.1 |
|
Intra-uterine sperm mass |
Length |
N.D. |
N.D. |
N.D. |
42.0 ± 15.0 |
62.7 ± 21.5 |
79.8 ± 32.8 |
67.4 ± 21.1 |
|
Width |
N.D. |
N.D. |
N.D. |
43.6 ± 16.9 |
83.7 ± 24.4 |
84.0 ± 50.5 |
78.2 ± 30.4 |
|
Stylet (%) |
|
50 |
40 |
50 |
50 |
60 |
30 |
20 |
|
No. of uterine eggs |
N.D. |
N.D. |
N.D. |
7.8 ± 7.0 |
4.1 ± 2.9 |
36.8 ± 20.9 |
12.2 ± 3.8 |
|
Posterior half/body length |
0.46 ± 0.08 |
0.51 ± 0.04 |
0.52 ± 0.03 |
0.60 ± 0.04 |
0.58 ± 0.01 |
0.59 ± 0.03 |
0.58 ± 0.02 |