Abstract
Glutathione S-transferase (28GST) with molecular mass of 28 kDa is an anti-oxidant enzyme abundant in Clonorchis sinensis. In adult C. sinensis, 28GST was localized in tegumental syncytium, cytons, parenchyma, and sperm tails examined by immunoelectron microscopy. C. sinensis 28GST was earlier found to neutralize bio-reactive compounds and to be rich in eggs. Accordingly, it is suggested that 28GST plays important roles in phase II defense system and physiological roles in worm fecundity of C. sinensis.
-
Key words: Clonorchis sinensis, glutathione S-transferase, immunoelectron microscopy
INTRODUCTION
Glutathione S-transferases (GSTs) of helminths are multifunctional enzymes that neutralize endo- and exogenous bio-reactive free radicals and chemical compounds by catalyzing conjugation of reduced glutathione. GSTs bind non-substrate ligands in cleft formed in the center of subunit dimer and increase their solubility and transfer for excretion (
Sluis-Cremer et al., 1996). Furthermore, nematode GSTs have been suggested to suppress or subvert host immunity, because of its prostaglandin biosynthesis activity (
Meyer et al., 1996).
GSTs are classified into families named as alpha, mu, pi, sigma, and theta, according to the substrate specificity, immunogenic reactivity, and amino acid sequence of their polypeptides (
Brophy and Pritchard, 1994;
Hayes and Pulford, 1995). Biochemical features of helminth GSTs appeared like mosaics of family characters of mammalian GSTs, therefore, it was proposed that helminth GSTs should be classified into three superfamilies, independent of mammalian GST families (
Brophy and Pritchard, 1994).
Of the helminth GSTs, 28 kDa GSTs of trematodes (28GSTs) are localized in tegument, parenchyma and genital organs (
Taylor et al., 1988;
Liu et al., 1996;
Gobert et al., 1998). From its enzymatic activity and tissue localization, 28GST has been shown to play a major role in phase II detoxification system for the fluke's survival (
Brophy and Pritchard, 1994). 28GST cDNA was cloned from
Clonorchis sinensis, and the recombinant protein was produced. Recombinant
C. sinensis 28GST had enzymatic activity and was grouped into class sigma. Immunohistochemical staining revealed localization of 28GST in tegument, parenchyma and intra-uterine eggs of adult
C. sinensis (
Kang et al., 2001). This study was undertaken to further elucidate fine distribution and possible functions of 28GST in adult
C. sinensis.
MATERIALS AND METHODS
Clonorchis sinensis metacercariae were collected from topmouth gudgeons,
Pseudorasbora parva, by artificial digestion. Adult
C. sinensis were recovered from bile ducts of experimental rabbits 7 months after infection with the metacercariae. Flukes were fixed in a solution of 2% paraformaldehyde/0.4% glutaraldehyde, pH 7.4 for an hour at room temperature, and they were dehydrated in a series of alcohol, and embedded in LR gold resin (London Resin Co., UK) and polymerized under UV light. Ultrathin ribbons were cut at 90 nm thickness and mounted on nickel grids. The ultrathin ribbons were immunogold-labeled by the procedure described previously (
Yu and Chai, 1995). Mouse monoclonal antibody (CTHK-17) raised against recombinant Cs28GST was used as primary antibody (
Kang et al., 2001), and goat anti-mouse IgG, 5 nm colloidal gold-conjugated (Sigma), at 1:70 dilution was used as secondary antibody. Stained ribbons were observed under a transmission electron microscope (Jeol 1200EXII, Tokyo, Japan).
RESULTS
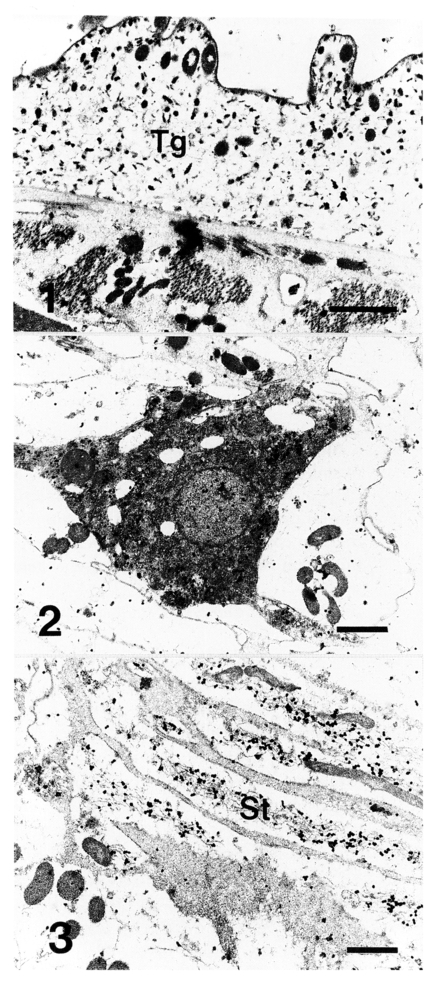
Tegumental syncytium was moderately labeled with gold particles (
Fig. 1), and in tegumental syncytium gold particles were associated with electron dense granules and increased distally toward cytoplasmic membrane. Cytons were irregular shapes and distinguished from subtegumental cells by darker stainability of cytoplasm. Cytons were labeled densely with gold particles (
Fig. 2), and there appeared a tendency that gold particles were more dense and associated with electron dense granules in the periphery of cytons. Mitochondria were abundant around nucleus. Tails of sperms in vas efferens near ovary were labeled heavily with gold particles but sperm body was not (
Fig. 3). Low density of gold particle was found in parenchymal tissues.
DISCUSSION
In adult
C. sinensis, 28GST was abundantly distributed in subtegumental parenchymal tissues and less extent in tegumental syncytium (
Kang et al. 2001). Ultrastructurally, moderate level of 28GST was present in cytons and low level in syncytium. Distribution pattern of
C. sinensis 28GST was similar to that of schistosomes (
Taylor et al., 1988;
Liu et al., 1996;
Gobert et al., 1998). It is known that 28GST is produced in cyton and transferred to tegumental syncytium through cytoplasmic processes. In
Schistosoma japonicum, 28GST is present as a major protein on body surface (
Henkle et al., 1990). Molecules associated with tegument are secreted or released when cytoplasmic membrane is turned over (
McLaren, 1980), and presented to host as stimulatory/reactive bio-compounds. GSTs are one of the anti-oxidant molecules distributed in host-parasite interface, tegument and subtegumental parenchymal tissues (
Mei and LoVerde, 1997;
Hong et al., 2001), and 28GSTs neutralize exogenous bio-reactive compounds as well as endogenous ones (
Kang et al., 2001) and play a key role in defense physiology of trematodes.
In the present study, 28GST was shown to be localized most densely in sperm tails of of
C. sinensis. Isoform of class Mu GST is present in human testes (
Hussey and Hayes, 1993) and abundant in fluid of seminiferous tubules of rat testes (
Mukherjee et al., 1999). Sertoli cells are prime secretors of Mu GSTs in rat testes. 28GST was also shown to be present in male reproductive organs of
S. mansoni (
Liu et al., 1996). Testosterone binds to 28GST of
Schistosoma haematobium and inhibits its enzymatic activity in vitro (
Remoue et al., 2002). Sex-dependent immune response to
S. haematobium 28GST was revealed in infected adult human subjects. These findings imply that 28GST play a key role in metabolism and physiology of reproductive system of flukes. Challenge infection of experimental animals immunized with schistosome GSTs results in decrease of worm fecundity (
Boulanger et al., 1991). In the course of egg formation, fertilization and embedding of oocytes inside the eggs increased expression of 28GST (
Liu et al., 1996). Intra-uterine eggs of
C. sinensis contained large amount of 28GST (
Kang et al., 2001). In life history of
C. sinensis, embryos develop to miracidia in intra-uterine eggs. From the above description, it, therefore, seems that abundancy of 28GST in the eggs may be related to the proliferation of embryonic cells. In conclusion, it is highly possible that 28GST could play a key role in fecundity of
C. sinensis flukes.
Notes
-
This work was supported by the Research Support Program (1998) of Chung-Ang University.
References
- 1. Brophy PM, Pritchard DI. Parasitic helminth glutathione S-transferases: An update on their potential as targets for immuno- and chemotherapy. Exp Parasitol 1994;79:89-96.
- 2. Boulanger D, Reid GD, Sturrock RF, et al. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol 1991;13:473-490.
- 3. Gobert GN, Stenzel DJ, McManus DP. Immunolocalization of the glutathione S-transferases, GST-26 and GST-28, within adult Schistosoma japonicum. Int J Parasitol 1998;28:1437-1443.
- 4. Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 1995;30:445-600.
- 5. Henkle KJ, Davern KM, Wright MD, Ramos AJ, Mitchell GF. Comparison of the cloned genes of the 26- and 28-kilodalton glutathione S-transferase of Schistosoma japonicum and Schistosoma mansoni. Mol Biochem Parasitol 1990;40:23-34.
- 6. Hong SJ, Lee JY, Lee DH, Sohn WM, Cho SY. Molecular cloning and characterization of a mu-class glutathione S-transferase from Clonorchis sinensis. Mol Biochem Parasitol 2001;115:69-75.
- 7. Hussey AJ, Hayes JD. Human mu-class glutathione S-transferase present in liver, skeletal muscle and testicular tissue. Biochim Biophys Acta 1993;1203:131-141.
- 8. Kang SY, Ahn IY, Park CY, et al. Clonorchis sinensis: Molecular cloning and characterization of 28-kDa glutathione S-transferase. Exp Parasitol 2001;97:186-195.
- 9. Liu JL, Fontaine J, Capron A, Grzych JM. Ultrastructural localization of Sm28 GST protective antigen in Schistosoma mansoni adult worms. Parasitology 1996;113:377-391.
- 10. McLaren DJ. In Brown KN ed, Schistosoma mansoni: the parasitic surface in relation to host immunity. Tropical Medicine Research Studies. 1980, vol. 1:New York, USA. Research Studies Press; pp 1-229.
- 11. Mei H, LoVerde PT. Schistosoma mansoni: The developmental regulation and immunolocalization of antioxidant enzymes. Exp Parasitol 1997;86:69-78.
- 12. Meyer DJ, Muimo R, Thomas M, Coates D, Isaac RE. Purification and characterization of prostaglandin-H E-isomerase, a sigma-class glutathione S-transferase, from Ascaridia galli. Biochem J 1996;313:223-227.
- 13. Mukherjee SB, Aravinda S, Gopalakrishnan B, Nagpal S, Salunke DM, Shaha C. Secretion of glutathione S-transferase isofroms in the seminiferous tubular fluid, tissue distribution and sex steroid binding by rat GSTM1. Biochem J 1999;340:309-320.
- 14. Remoue F, Mani JC, Pugniere M, Schacht AM, Capron A, Riveau G. Functional specific binding of testosterone to Schistosoma haematobium 28-kilodalton glutathione S-transferase. Infect Immun 2002;70:601-605.
- 15. Sluis-Cremer N, Naidoo NN, Kaplan WH, Manoharan TH, Fahl WE, Dirr HW. Determination of a binding site for a non-substrate ligand in mammalian cytosolic glutathione S-transferases by means of fluorescence-resonance energy transfer. Eur J Biochem 1996;241:484-488.
- 16. Taylor JB, Vidal A, Torpier G, et al. The glutathione transferase activity and tissue distribution of a cloned Mr28K protective antigen of Schistosoma mansoni. EMBO J 1988;7:465-472.
- 17. Yu JR, Chai JY. Localization of actin and myosin in Cryptosporidium parvum using immunogold staining. Korean J Parasitol 1995;33:155-164.
Figs. 1-3Immunogold localization of 28 kDa glutathione S-transferase in adult Clonorchis sinensis. Fig. 1. Tegument (Tg) shows gold particles dispersed with low density. Fig. 2. Gold particles were localized more densely in a cyton than in near-by cells. Fig. 3. Tails of sperms (St) in vas efferens are densely labeled with gold particles. Bar = 1 µm.

Citations
Citations to this article as recorded by

- Heat-killed Propionibacterium acnes augment the protective effect of 28-kDa glutathione S-transferases antigen against Schistosoma mansoni infection
Ho Yin Pekkle Lam, Ting-Hua Yang, Ting-Ruei Liang, Po-Ching Cheng, Kai-Chih Chang, Shih-Yi Peng
Acta Tropica.2021; 222: 106033. CrossRef - Adult Opisthorchis felineus major protein fractions deduced from transcripts: Comparison with liver flukes Opisthorchis viverrini and Clonorchis sinensis
Mikhail Pomaznoy, Sergey Tatkov, Alexey Katokhin, Dmitry Afonnikov, Vladimir Babenko, Dagmara Furman, Ilya Brusentsov, Pavel Belavin, Alexandr Najakshin, Sergey Guselnikov, Gennady Vasiliev, Anton Sivkov, Egor Prokhortchouk, Konstantin Skryabin, Viatchesl
Experimental Parasitology.2013; 135(2): 297. CrossRef - Identification and biochemical characterization of two novel peroxiredoxins in a liver fluke,Clonorchis sinensis
Y.-A. BAE, S.-H. KIM, E.-G. LEE, W.-M. SOHN, Y. KONG
Parasitology.2011; 138(9): 1143. CrossRef - Progress on the transcriptomics of carcinogenic liver flukes of humans—Unique biological and biotechnological prospects
Neil D. Young, Aaron R. Jex, Cinzia Cantacessi, Bronwyn E. Campbell, Thewarach Laha, Woon-Mok Sohn, Banchob Sripa, Alex Loukas, Paul J. Brindley, Robin B. Gasser
Biotechnology Advances.2010; 28(6): 859. CrossRef - Functional Genes and Proteins of Clonorchis sinensis
Tae Im Kim, Byoung-Kuk Na, Sung-Jong Hong
The Korean Journal of Parasitology.2009; 47(Suppl): S59. CrossRef - Xenobiotic metabolizing enzymes and metabolism of anthelminthics in helminths
Viktor Cvilink, Jiri Lamka, Lenka Skálová
Drug Metabolism Reviews.2009; 41(1): 8. CrossRef - Opisthorchis viverrini: Gene expression profiling of carcinogenic adult liver fluke worms using 5′ SAGE
Nopporn Chutiwitoonchai, Yan Shen, Huajun Zheng, Hui Xiong, Guoping Zhao, Kanokwan Imtawil, Pewpan M. Intapan, Sopit Wongkham, Chaisiri Wongkham
Experimental Parasitology.2008; 120(4): 306. CrossRef - Clonorchiasis: an update
H.-J. Rim
Journal of Helminthology.2005; 79(3): 269. CrossRef