Abstract
The cysteine proteases of Paragonimus westermani metacercariae are involved in metacercarial excystment, host immune modulation, and possibly in tissue penetration. In order to clarify the origin of the enzymes, 28 and 27 kDa cysteine proteases in metacercarial excretory-secretory products were purified through the FPLC system using Mono Q column chromatography. The polyclonal antibodies to the enzymes were produced in BALB/c mice. Immunolocalization studies revealed that both cysteine proteases were distributed at the linings of excretory bladder and excretory concretions of the metacercariae. It was suggested that the excretory epithelium of P. westermani undertake the secretory function of metacercarial cysteine proteases, in addition to its role as a route for eliminating waste products.
-
Key words: Paragonimus westermani, metacercarial excretory-secretory products, cysteine protease, immunolocalization
INTRODUCTION
Parasite proteases play important roles in tissue penetration, nutrient uptake, and evasion from the host immune defense (
Tort et al., 1999;
Sajid and McKerrow, 2002). The cysteine proteases of metacercariae of
Paragonimus westermani also exert pivotal roles in metacercarial excystment (
Chung et al., 1995), host IgG degradation (
Chung et al., 1997b), and evasion from IgG-dependent eosinophil cytotoxicity (
Shin et al., 2001). In the process of excystment,
P. westermani metacercariae secrete two distinct cysteine proteases with molecular masses of 28 and 27 kDa, respectively, which cleave macromolecules of extracellular matrices (
Chung et al., 1995).
Although we understand part of the functional roles of the enzymes, the origin of the 28 and 27 kDa enzymes has not been clarified in the metacercariae. To determine the tissue origin of the enzymes, we purified the enzymes, prepared the polyclonal antibodies reacting to the enzymes, and performed immunolocalization studies.
MATERIALS AND METHODS
Metacercarial excystment and preparation of excretory-secretory product (ESP)
Metacercariae of
P. westermani were obtained from naturally infected freshwater crayfish,
Cambaroides similis, in Bogil Island in Korea. Metacercarial excystment was performed as described previously (
Chung et al., 1995). About 2,000 metacercariae were incubated in carbonate buffer (pH 9, 10 mM) in the presence of 2 mM dithiothreitol. To harvest ESP, newly excysted metacercariae were transferred to 10 ml sterile physiological saline and incubated at 37℃. The ESP was concentrated using a lyophilizer (Hepto-Holten, Denmark) and stored at -70℃ until use.
A small amount of the lyophilized ESP was dissolved in deionized water and dialyzed against 10 mM sodium acetate buffer (pH 6.2). The dialysate was filtered through a 0.2 µm membrane filter and loaded onto the Mono Q HR 5/5 column (Pharmacia-Biotech, Uppsala, Sweden) previously equilibrated with the same buffer. The 28 and 27 kDa cysteine proteases were purified using the FPLC system by increasing the NaCl molarity up to 1 M in a linear gradient fashion. Purity of the enzymes was examined by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Production of polyclonal antibodies
The enzymes, separated on the gels, were cut into fragments and emulsified with Freund's complete adjuvant. The mixture was injected into the peritoneum of BALB/c mice. The mice were additionally immunized twice with the gel fragments mixed with Freund's incomplete adjuvant in a 2 weeks interval. Blood was collected from the tail vein and screened for binding activity against purified proteases by western blot.
Immunohistochemical staining
For immunohistochemical staining, newly excysted metacercariae were fixed in 10% neutral formalin, embedded in paraffin, and cut into ribbons. The polyclonal mice sera, diluted at 1:100, were incubated with deparaffinized worm sections for 3 h at room temperature. The sections were subsequently incubated with peroxidase-conjugated anti-mouse IgG (ICN-Cappel, St. Louis, MO, USA) diluted at 1:500 for 2 h. The reaction was developed with a substrate, 3-amino-9-ethylcarbazole, and quenched with deionized water.
RESULTS
Purification of cysteine proteases
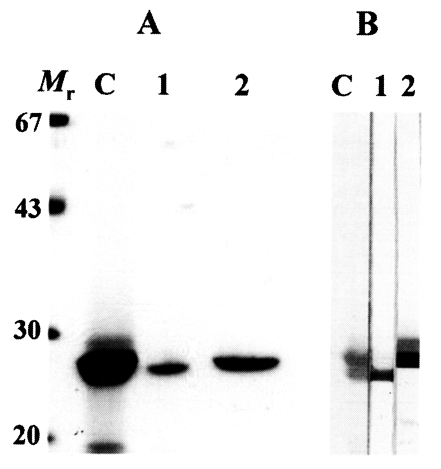
The 27 kDa protease was eluted in a flow-through fraction and 28 kDa protease was eluted through the Mono Q column at 0.2 M concentraion. These two enzymes superimposed each other on gradient SDS-PAGE; therefore, we used 12% separating gels to detect purity and to identify immunoreactivity of polyclonal antibodies against the two enzymes. As shown in
Fig. 1A, the purified cysteine proteases migrated to 28 and 27 kDa on 12% SDS-PAGE analysis, respectively.
On immunoblotting, each of polyclonal antibodies specifically recognized the distinct enzyme (
Fig. 1B). Immunoblotting demonstrated that the polyclonal antibodies produced from the mice had an ability to identify each protease without cross-reaction between the two proteases.
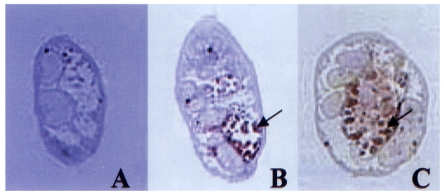
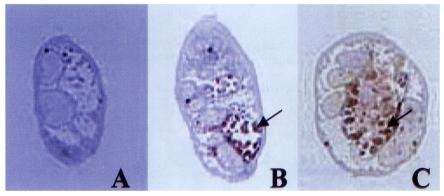
Both polyclonal antibodies to 28 and 27 kDa cysteine proteases reacted against the excretory bladder and excretory concretions in the metacercariae (
Fig. 2). The antibodies did not elicit positive reaction in other organs such as intestine and tegument. Pre-immune sera of the mice did not elicit positive reaction in any of the parasite tissues.
DISCUSSION
As for the origin of the metacercarial cysteine protease, Hamajima et al. (
1985) reported that the thiol protease of
P. westermani metacercariae was localized in epithelia and luminal contents of intestine, and suggested that the protease might play an important role in nutrient digestion. In this study, however, polyclonal antibodies to the 28 and 27 kDa cysteine proteases reacted against the epithelium of the excretory bladders and excretory concretions of the metacercariae. We could not confirm in this study, however, whether the thiol protease of
P. westermani metacercariae (
Hamajima et al., 1985) is the same with the 28 and 27 kDa cysteine proteases.
It has been suggested that the syncytial epithelium of trematodes excretory duct and bladder are involved in a number of physiological activities other than serving as a route for the elimination of metabolic wastes (
Smyth and Halton, 1983). According to Orido (
1990), the syncytial cells of the excretory bladder of
Paragonimus ohirai metacercariae contained a large quantity of excretory materials and were filled with Golgi complexes. These structural characteristics suggested that the excretory bladder in metacercariae exerts active secretory function. The secretion from epithelium of the excretory bladder should contain metacercarial 28 and 27 kDa cysteine proteases for the following two reasons. As shown in this study, polyclonal antibodies against 28 and 27 kDa cysteine proteases reacted against the luminal linings of the excretory bladder and outer linings of the excretory concretions of
P. westermani metacercariae. In addition, ESP of newly excysted metacercariae contained highly active 28 and 27 kDa cysteine proteases (
Chung et al., 1995).
After excystment, calcareous concretions of the excretory bladder of
P. ohirai metacercariae disappeared within 7-10 days in a rat definitive host (
Orido, 1990). The concretions of
P. westermani disappeared in the same period. In this connection, the specific
activities of P. westermani cysteine proteases decline dramatically during maturation stages in the definitive hosts. This phenomenon seems to be mainly due to the decreased secretion of highly active 28 and 27 kDa cysteine proteases and increased secretion of other cysteine proteases of weak activity. The developmentally modulated secretion of cysteine protease may be closely correlated with penetration and migration of the metacercariae within their definitive host (
Chung et al., 1997a). Recently, cruzipain-like 28 kDa cysteine protease was reported as a developmentally regulated cysteine protease of
P. westermani. The enzyme was expressed in a developing adult
P. westermani (
Yun et al., 2000). In situ hybridization study showed that the cruzipain-like protease was localized only in the intestinal epithelium of adult worms. Despite having the same molecular mass, the cysteine protease of metacercariae is distinct from that of an adult worm.
In conclusion, we demonstrated that both 28 and 27 kDa cysteine proteases of newly excysted P. westermani metacercariae come from the excretory bladder.
Notes
-
This study was supported by a grant from the Cheju National University Medical Research Fund (2001).
References
- 1. Chung YB, Kong Y, Joo IJ, Cho SY, Kang SY. Excystment of Paragonimus westermani metacercariae by endogenous cysteine protease. J Parasitol 1995;81:137-142.
- 2. Chung YB, Kong Y, Yang HJ, Kang SY, Cho SY. Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol 1997a;83:902-907.
- 3. Chung YB, Yang HJ, Kang SY, Kong Y, Cho SY. Activities of different cysteine proteases of Paragonimus westermani in cleaving human IgG. Korean J Parasitol 1997b;35:139-142.
- 4. Hamajima F, Yamakami K, Fujino T. Localization of a thiol protease in metacercarial lung fluke. Jpn J Parasitol 1985;34:507-508.
- 5. Orido Y. Development of the excretory bladder of the lung fluke Paragonimus ohirai (Trematoda: Troglotrematidae). J Parasitol 1990;76:205-211.
- 6. Sajid M, McKerrow JH. Cysteine proteases in parasitic organisms. Mol Biochem Parasitol 2002;120:1-21.
- 7. Shin MH, Kita H, Park HY, Seoh JY. Cysteine protease secreted by Paragonimus westermani attenuates effector functions of human eosinophils stimulated with immunoglobulin G. Infect Immun 2001;69:1599-1604.
- 8. Smyth JD, Halton DW. The physiology of trematodes. 1983, 2nd ed. Cambridge. Cambridge University Press; pp 34-38.
- 9. Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Adv Parasitol 1999;43:161-266.
- 10. Yun DH, Chung JY, Chung YB, et al. Structural and immunological characteristics of a 28-kilodalton cruzipain-like cysteine protease of Paragonimus westermani expressed in the definitive host stage. Clin Diagn Lab Immunol 2000;7:932-939.
Fig. 1(A) SDS-PAGE analysis of the purified 28 and 27 kDa cysteine proteases of P. westermani metacercarial ESP. Mr, molecular mass markers in kDa; C, crude ESP; 1, 27 kDa cysteine protease eluted through flow-through fraction; 2, 28 kDa cysteine protease purified in 0.2 M salt concentration. (B) Reactivity of anti-27 and anti-28 kDa cysteine proteases of metacercarial ESP analyzed by immunoblotting. Lanes 1 and 2 denote the reactions with anti-27 and anti-28 kDa antibody, respectively. Other markings are same as in (A).
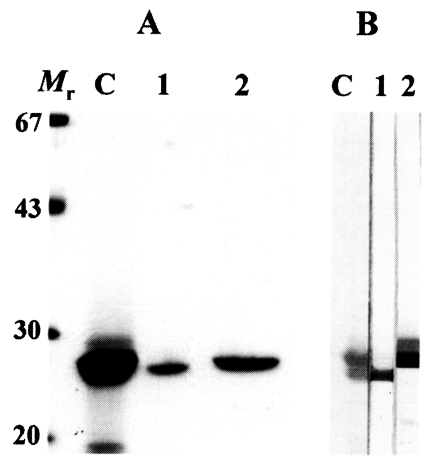
Fig. 2Localization of 28 and 27 kDa cysteine proteases assessed by immunohistochemical staining. Sera from BALB/c mice immunized with respective proteases were used as the source of primary antibodies (1:100 dilution). (A) Negative control. (B) Immunostaining with anti-27 kDa. (C) Staining with anti-28 kDa. Arrows indicate positive reaction on excretory bladder and excretory concretions (red color).
Citations
Citations to this article as recorded by

- Early Cysteine Protease Activity in Excretory Bladder Triggers Metacercaria Excystment of Paragonimus westermani
Y. B. Chung, T. S. Kim, H. J. Yang
Journal of Parasitology.2005; 91(4): 953. CrossRef